Patients with diabetes have better survival from septic melioidosis than patients who without diabetes. This difference was seen only in patients taking glyburide prior to presentation and was associated with an anti-inflammatory effect of glyburide.
Abstract
Background. Patients with diabetes mellitus are more prone to bacterial sepsis, but there are conflicting data on whether outcomes are worse in diabetics after presentation with sepsis. Glyburide is an oral hypoglycemic agent used to treat diabetes mellitus. This KATP-channel blocker and broad-spectrum ATP-binding cassette (ABC) transporter inhibitor has broad-ranging effects on the immune system, including inhibition of inflammasome assembly and would be predicted to influence the host response to infection.
Methods. We studied a cohort of 1160 patients with gram-negative sepsis caused by a single pathogen (Burkholderia pseudomallei), 410 (35%) of whom were known to have diabetes. We subsequently studied prospectively diabetics with B. pseudomallei infection (n = 20) to compare the gene expression profile of peripheral whole blood leukocytes in patients who were taking glyburide against those not taking any sulfonylurea.
Results. Survival was greater in diabetics than in nondiabetics (38% vs 45%, respectively, P = .04), but the survival benefit was confined to the patient group taking glyburide (adjusted odds ratio .47, 95% confidence interval .28–.74, P = .005). We identified differential expression of 63 immune-related genes (P = .001) in patients taking glyburide, the sum effect of which we predict to be antiinflammatory in the glyburide group.
Conclusions. We present observational evidence for a glyburide-associated benefit during human melioidosis and correlate this with an anti-inflammatory effect of glyburide on the immune system.
A recurring theme in the sepsis literature is that the inflammatory response is essential, yet high levels of inflammation correlate with mortality in observational studies of human sepsis [1]. This has led to the notion that containment of an excessive immune response might improve outcome. Glyburide USAN (glibenclamide rINN) is a KATP-channel blocker and broad-spectrum ATP-binding cassette (ABC) transporter inhibitor used to treat type 2 diabetes. The relevant pharmacological drug action in diabetes is inhibition of KATP channels in pancreatic β cells leading to stimulation of insulin secretion, but there is evidence from experimental models that glyburide also has a wide range of antiinflammatory effects. The best described of these is an inhibitory effect on the host inflammasome, an intracellular protein complex present in macrophages that activates caspase 1 and produces active interleukin (IL)-1β and IL-18 when presented with an appropriate inflammatory stimulus [2]. The inflammatory effect of glyburide on outcome from sepsis has not been well studied, but this question is readily addressed, since diabetics commonly take glyburide and are at greater risk than the healthy population of developing bacterial infection.
We report here the effect of glyburide on outcome in a cohort of over 1000 patients infected with a single pathogen (melioidosis, which is Burkholderia pseudomallei infection). Melioidosis is an important clinical model because diabetes is present in up to 50% of patients, manifestations of infection are often severe, mortality is around 40% in northeast Thailand where most cases are diagnosed, and glyburide therapy is prescribed in around half of diabetics in this region. We found that patients with a preadmission diagnosis of diabetes were protected from death but that this survival advantage was confined to those patients taking glyburide prior to the onset of infection. We hypothesized that glyburide was modulating the immune response to B. pseudomallei infection, a hypothesis supported by the findings of a study of the gene-expression profiles of peripheral blood leukocytes in which we compared diabetic patients with melioidosis or otherwise healthy diabetic patients who were or were not taking glyburide.
PATIENTS AND METHODS
Cohort Study
We prospectively identified all patients aged 15 years or older presenting to Sappasithiprasong Hospital, Ubon Ratchathani, northeast Thailand, with culture-confirmed melioidosis between 1 January 2002 and 31 December 2006. Patients on their first admission for culture-confirmed melioidosis were eligible, but patients younger than 15 years of age were excluded because pediatric cases have a different clinical presentation and prognosis. There were no other exclusion criteria.
Patients were classified into 3 groups according to diabetes status at presentation: known diabetes, hyperglycemia, or no diabetes. Patients with a preexisting diagnosis of diabetes mellitus were classified as having known diabetes. The hyperglycemia group comprised patients not previously known to have diabetes, who had either a blood glucose >200 mg/dL (11.1 mmol/L) at any point during the admission or new diabetes diagnosed after recovery as defined by World Health Organization (WHO) criteria. We did not subclassify this group further for the following reasons: Melioidosis is a common first presentation of type 2 diabetes in this region [3], and an associated mortality of 50% (half of which occurs in the first 48 h) means that a new diagnosis of diabetes cannot be made for a significant proportion of patients, and hyperglycemic patients who die are more likely to be classed as having sepsis-induced hyperglycemia whereas patients who survive are more likely to be classed as having a new diagnosis of diabetes. Patients who did not fall into the known diabetes or hyperglycemia groups were classified as having no diabetes. Hemoglobin A1c (HbA1c) values were not available for patients in this cohort. Further definitions are given in the Supplementary Methods.
Statistical Analysis
Analyses were performed using Stata/SE version 9 software (StataCorp). Differences between the 3 patient groups were compared using Fisher exact test for categorical variables and Mann-Whitney U test for continuous variables. Time to death to 28 days was analyzed using the Kaplan-Meier method; patients discharged alive from hospital within 28 days were assumed to have survived, but patients who self-discharged against medical advice were censored on the day of discharge. Logistic regression models were used to adjust for confounders identified using a conceptual framework [4]. Further details are given in the Supplementary Methods.
Gene Expression Study
We compared peripheral white blood cell gene expression in (1) diabetics who were taking glyburide (case) or were not taking this or another sulfonylurea (control) at the time of admission to Sappasithiprasong Hospital with culture-confirmed melioidosis and sepsis and (2) otherwise healthy diabetics attending a routine outpatient clinic who were taking glyburide (case) or were not taking this or another sulfonylurea (control). Eligible cases for both studies were persons aged between 18 and 75 years. A total of 40 patients (10 in each group) were recruited in the period 31 January 2008 to 31 October 2008. Diabetes was defined as an abnormal HbA1c at enrollment (7.8% or greater [5], Bio-Rad D-10 fully automated HbA1c system, Bio-Rad Laboratories) or a previous diagnosis of diabetes. The HbA1c concentration allowed us to identify patients with previously unrecognized diabetes who would otherwise die before we could make a diagnosis of diabetes by WHO criteria.
Blood was collected in PaxGene Blood RNA tubes (PreAnalytiX). Gene expression was assayed using the Illumina HumanWG-6 v3.0 Expression BeadChip (Illumina), and results were confirmed by quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) (see Supplementary Methods).
Gene ontology overrepresentation analysis was performed using InnateDB (www.innatedb.ca) and the P values reported are for the hypergeometric test. Gene lists were supplemented by manual literature searches for genes not curated by InnateDB. Microarray data have been deposited at ArrayExpress, EMBL - EBI (accession number E-TABM--852-n).
Ethics
Approval was obtained from the Ethical and Scientific Review subcommittee of the Thai Ministry of Public Health to use information collected during the cohort study. We obtained approval from the Oxford Tropical Research Ethics Committee and the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, for the gene expression study. Written informed consent was obtained from all subjects by a native Thai speaker. All procedures performed were in accordance with the Helsinki Declaration of 1975 (revised 1983).
RESULTS
Diabetes and Mortality
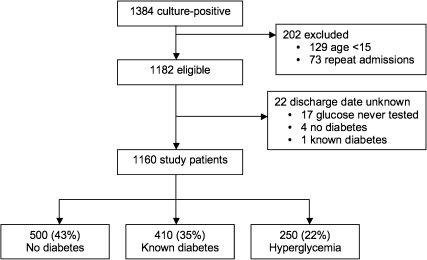
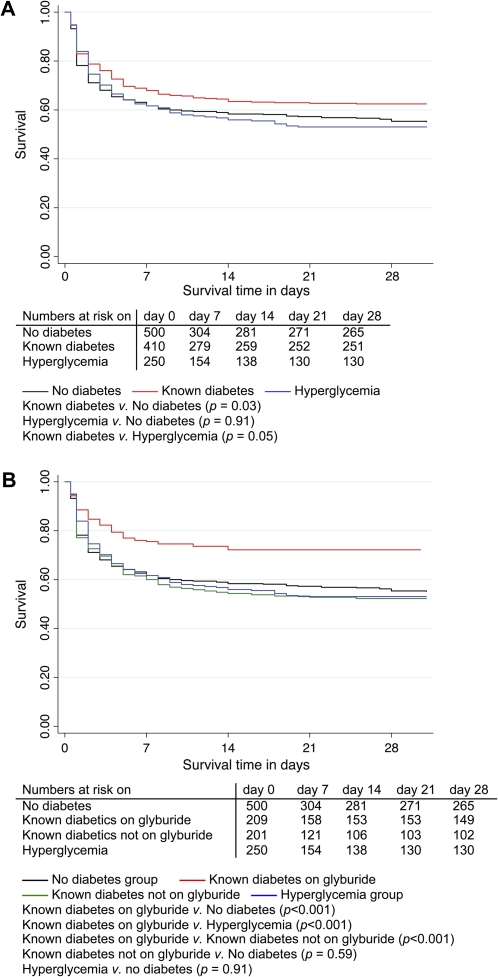
We identified 1384 patients with culture-positive melioidosis, of whom 224 were removed from the final analysis for reasons shown in Figure 1. Of the remaining 1160 patients, 410 (35%) had known diabetes, 250 (22%) had hyperglycemia, and 500 (43%) had no diabetes. Patient characteristics, clinical features of melioidosis, and primary and secondary outcomes were compared between the 3 groups, using the no diabetes group as the comparator for the 2 other groups (Table 1). In-hospital mortality was lower in patients with known diabetes compared with nondiabetics (P = .04), with no difference observed between nondiabetics and patients with hyperglycemia. These findings were reproduced in the survival analysis (Figure 2A). A previous study conducted at the same hospital but in a different (earlier) patient cohort reported an inverse association between diabetes and mortality (unadjusted odds ratio [OR] .49) [6]. We confirmed this observation in our independent cohort (OR .76; adjusted odds ratio [AOR] .78, 95% confidence interval [CI] .59–1.0, P = .07), and extended previous observations that patients in the hyperglycemia group had no survival advantage (OR 1.1; AOR 1.1, 95% CI 0.8–1.5, P = .52) (Table 2, Model A). Thirty-four patients self-discharged against medical advice; results of the logistic regression analyses were not different if these patients were assumed to have lived or died (data not shown).
Figure 1.
Summary of patient recruitment for cohort study.
Table 1.
Patient Characteristics, Clinical Features of Melioidosis, and Outcome
| No Diabetes (n = 500) | Known Diabetes (n = 410) |
Hyperglycemia (n = 250) |
Total (n = 1160) | |||
| No. (%) | No. (%) | P valuea | No. (%) | P valuea | Row total (%) | |
| Female | 165 (33) | 219 (53) | – | 114 (46) | – | 498 (43) |
| Male | 335 (67) | 191 (47) | <.001 | 136 (54) | .001 | 662 (57) |
| Median age (y, IQR) | 52 (39–63) | 51 (42–59) | .40b | 51 (42–59) | .22b | – |
| Rice farmer | 367 (73) | 326 (80) | .04 | 212 (85) | <.001 | 905 (79) |
| Median (IQR) days of infective symptoms prior to presentation | 7 (4–15) | 10 (5–21) | .02b | 8 (5–15) | .03b | 7 (5–20) |
| Diabetes treatmentc | ||||||
| Glyburide | – | 208 (51) | – | 208 (18) | ||
| Metformin | – | 51 (12) | – | 50 (4) | ||
| Insulin | – | 81 (20) | – | 81 (7) | ||
| Other sulphonylurea | – | 10 (2) | – | 10 (.9) | ||
| Unknown oral drug | – | 51 (12) | – | 51 (4) | ||
| No medication | – | 53 (13) | – | 53 (5) | ||
| Risk factors for melioidosis | ||||||
| Chronic kidney disease | 54 (11) | 36 (9) | .32 | 9 (4) | .001 | 99 (9) |
| Nephrolithiasis | 35 (7) | 15 (4) | .03 | 6 (2) | .01 | 56 (5) |
| Corticosteroid use | 21 (4) | 19 (5) | .75 | 9 (4) | .84 | 49 (4) |
| Thalassaemia | 11 (2) | 4 (1) | .19 | 2 (.8) | .24 | 17 (1) |
| Malignancy | 9 (2) | 1 (.2) | .03 | 1 (.4) | .18 | 11 (1) |
| Chronic liver disease | 6 (1) | 5 (1) | 1.00 | 1 (.4) | .43 | 12 (1) |
| Organ involvement | ||||||
| Pneumonia | 190 (38) | 154 (38) | .95 | 117 (47) | .02 | 461 (40) |
| Skin or soft tissue | 86 (17) | 94 (23) | .04 | 42 (17) | .92 | 222 (19) |
| Urinary tract | 78 (16) | 48 (12) | .10 | 15 (6.0) | <.001 | 141 (12) |
| Liver abscess(es) | 33 (7) | 48 (12) | .01 | 27 (11) | .06 | 108 (9) |
| Spleen abscess(es) | 45 (9) | 45 (11) | .37 | 35 (14) | .04 | 125 (11) |
| Septic arthritis | 24 (5) | 40 (10) | .004 | 24 (10) | .02 | 88 (8) |
| Distribution of disease | ||||||
| Bacteremia | 274 (55) | 238 (58) | .35 | 164 (66) | .005 | 676 (58) |
| Single organ disease | 291 (58) | 240 (59) | .95 | 146 (58) | 1.00 | 677 (58) |
| Multiorgan disease | 110 (22) | 102 (25) | .31 | 58 (23) | .71 | 270 (23) |
| Complications | ||||||
| Hypotension | 187 (37) | 135 (33) | .16 | 99 (39) | .58 | 421 (36) |
| Respiratory failure | 179 (36) | 117 (29) | .02 | 101 (40) | .23 | 397 (34) |
| Sepsisd | 392 (78) | 334 (81) | .28 | 209 (84) | .10 | 935 (81) |
| Antibiotic treatment and in-hospital mortality | ||||||
| Effective antibiotic treatment within 24 h of admission | 367 (73) | 359 (88) | <.001 | 212 (85) | <.001 | 938 (81) |
| Died | 225 (45) | 157 (38) | .04f | 117 (47) | .64f | 499 (43) |
| Discharged well | 254 (51) | 245 (60) | – | 128 (51) | – | 627 (54) |
| Outcome unknown (self-discharged) | 21 (4) | 8 (2) | – | 5 (2) | – | 34 (3) |
NOTE. IQR = interquartile range. Anatomical sites for which there were fewer than 20 cases in the 5-year period are omitted from the table. Variables recording organ involvement at 10 sites are not shown here, because there were fewer than 20 events recorded: parotitis, pleural disease, central nervous system disease, peritonitis, pericarditis, osteomyelitis, prostatitis, thyroiditis, ophthalmitis, and cholecystitis. They were, however, taken into account when counting the number of organs involved.
Fisher exact test, except where indicated.
Mann-Whitney U test with No diabetes as the comparator group, because the data were non-normal and could not be transformed to normal.
Numbers do not add up to 410 (100%) because some patients were taking more than 1 type of medication.
Sepsis defined as temperature >38°C or <36°C, heart rate >90 beats per minute, respiratory rate >20 breaths per minute, or total leucocyte count >12 × 109 cells/L.
Patients who took their own discharge were counted as alive.
Figure 2.
Kaplan-Meier survival curves of 1160 patients with melioidosis. The survival curves in panel A show that patients with diabetes have a survival advantage after the development of melioidosis, but the survival curves in panel B indicate that this effect was seen only in the patient group taking glyburide. The P values reported are for the log-rank test. Median duration of follow-up was 6.5 days and total follow-up was 11,845 patient days.
Table 2.
Effect of Diabetes on Mortality
| Parameter | Univariate Analysis |
Logistic Regression Model A (n = 1160) |
Logistic Regression Model B (n = 1109)c |
|||
| OR | (95% CI) | AOR | (95% CI) | AOR | (95% CI) | |
| No diabetesa | 1.0 | — | 1.0 | — | 1.0 | — |
| Known diabetes | .76 | (.58–.99) | .78 | (.59–1.0) | 1.4 | (.89–2.3) |
| Hyperglycaemia | 1.1 | (.79–1.5) | 1.1 | (.81–1.5) | 1.4 | (.98–1.9) |
| Ageb | 1.1 | (1.0–1.2) | 1.1 | (1.0–1.2) | 1.1 | (.99–1.2) |
| Male sex | 1.2 | (.94–1.5) | 1.1 | (.89–1.5) | 1.0 | (.78–1.3) |
| Rice farming | .97 | (.74–1.3) | 1.0 | (.75–1.3) | 1.2 | (.86–1.6) |
| Corticosteroid use | 1.5 | (.86–2.7) | 1.6 | (.87–2.8) | 1.0 | (.62–2.2) |
| Chronic kidney disease | 2.8 | (1.8–4.3) | 2.2 | (1.6–4.0) | ||
| Glyburide treatmentc | .48 | (.35–.67) | .47 | (.28–.74) | ||
| Metformin treatmentc | .61 | (.33–1.1) | 1.1 | (.62–2.4) | ||
| Insulin treatmentc | .79 | (.49–1.3) | .65 | (.37–1.2) | ||
| Effective admission antibiotic treatmentc | .22 | (.16–.31) | .23 | (.17–.34) | ||
NOTE. AOR = adjusted odds ratio; CI = confidence interval; OR = odds ratio (not adjusted). An odds ratio <1 indicates association with survival, whereas an odds ratio >1 indicates association with mortality. The first column describes the contribution of each factor in isolation. The second column (model A) attempts to explain the effect of diabetes by adjusting for several possible confounders for diabetes simultaneously; the third column (model B) adjusts additionally for the effect of diabetes treatment and postadmission antibiotics. Exposures of interest are highlighted in bold.
When considered in isolation, diabetes (OR .76), glyburide treatment (OR .48), and effective admission antibiotic treatment (OR .22) were associated with survival. The effect of diabetes persisted after correcting for confounders for diabetes (AOR .78, model A). When the effect of glyburide treatment and effective admission antibiotic were taken into account, diabetes was no longer associated with survival (AOR 1.4, model B). Sensitivity analysis. We conducted a sensitivity analysis to examine the impact of the missing data. Model A was constructed by assigning the patients who self-discharged as “alive”; but if these patients were instead assigned as “dead”, then the AOR for known diabetes in model A became .71 (.54–.93, P = .01). In model B, assigning the patients who self-discharged to “dead” caused the AOR for known diabetes to become 1.3 (.79–2.1, P = .31), glyburide treatment AOR .48 (.28–.83, P = .008), and effective admission antibiotic treatment AOR .18 (.13–.27, P < .001). Putting the patients on an unknown oral diabetes medication into the glyburide group (n = 1160) in model B meant the AOR for glyburide treatment rose to .59 (.36–.97, P = .04) and effective admission antibiotic treatment became .24 (.17–.34, P < .001). When the patients on an unknown oral diabetes medication were put into the metformin group (n = 1160), the AOR for glyburide treatment became .49 (.30–.78, P = .003) and effective admission antibiotic treatment became .25 (.18–.34, P < .001).
Comparator group.
Number of decades above age 15 years.
The patients on unknown oral diabetes medication were omitted (n = 1109) in model B and in the unadjusted OR for treatment variables.
Glyburide and Mortality
We hypothesized that the association between diabetes and a reduced risk of death was due to the treatment received for diabetes and/or melioidosis. This was tested in a second logistic regression model (Table 2, Model B). We considered drugs prescribed for diabetes (glyburide, metformin, insulin) prior to admission, noting that more than half of known diabetics were receiving glyburide (Table 1). We included the administration of appropriate admission antimicrobial therapy because the association between diabetes and melioidosis is well described and could lead clinicians to prescribe these at an earlier stage in diabetic patients. The AOR for death was lower for patients who received glyburide therapy prior to admission (AOR .47, 95% CI .28–.74, P = .005) and for those receiving effective antimicrobial chemotherapy at admission (AOR .23, 95% CI .17–.34, P < .001) (Table 2, Model B). Neither metformin nor insulin treatment was associated with survival in any analysis. The unadjusted survival curve for patients in the glyburide group is shown in Figure 2B. Patients in the known diabetes group were more likely to receive effective antimicrobial therapy on admission compared with patients without diabetes (88% vs 73%, respectively, P < .001), an association that was seen in each of the treatment groups (glyburide 92%, P < .001; metformin 98%, P < .001; insulin 90%, P = .02), but the association between glyburide therapy and survival was independent of admission antimicrobial therapy (Table 2).
We used logistic regression to evaluate the relationship between diabetes and the secondary outcome measures. We found that diabetes was negatively associated with both hypotension and respiratory failure (AOR .84 [Model C] and AOR .73 [Model E], respectively, see Supplementary Table 1), but that this association did not persist after adjustment for treatment (antimicrobials and antidiabetic treatment) (Models D and F, Supplementary Table 1). However, glyburide was negatively associated with both hypotension (AOR .48, 95% CI .30–.78, P = .007) and respiratory failure (AOR .50, 95% CI .28–.86, P = .01), as was effective antimicrobial therapy within 24 hours of admission (AOR .51, 95% CI .37–.70, P < .001 and AOR .42, 95% CI .31–.58, P < .001, respectively) (Models D and F, Supplementary Table 1). In a sensitivity analysis, if patients taking an unknown oral drug were placed in the glyburide group, then the association between glyburide and hypotension (AOR .65, 95% CI .39–1.1, P = .09) and between glyburide and respiratory failure (AOR .60, 95% CI .36–1.0, P = .05) was no longer significant statistically.
Glyburide and Inflammation
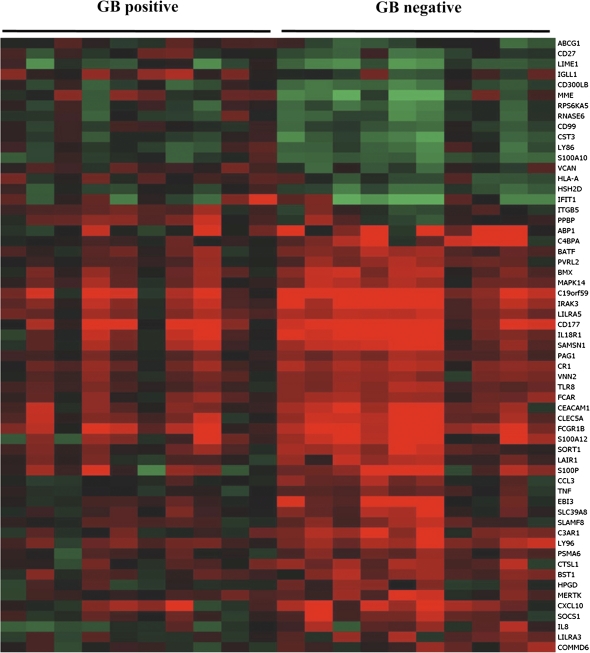
Having observed an association between glyburide and survival, we initially postulated that glyburide was acting as an antimicrobial drug based on its structural homology with the sulfonamides (to which B. pseudomallei is susceptible). However, a solution of glyburide in dimethyl sulfoxide did not inhibit the growth of B. pseudomallei when applied to a lawn of bacteria on solid agar (data not shown). Glyburide is a broad-spectrum ABC transporter inhibitor that alters responses of macrophages to a range of stimuli in vitro and in vivo. We therefore used peripheral leukocyte gene expression as a screening tool to identify global trends in gene function that might be ascribed to the action of glyburide. We compared gene expression profiles in 10 diabetic patients who were taking glyburide at the time of presentation with melioidosis against 10 diabetic melioidosis patients who were not taking this or another sulfonylurea. There were no significant differences in any of the clinical parameters recorded for the 2 patient groups (Supplementary Table 2). The expression of 205 probes (representing 186 distinct genes) were significantly different in patients taking glyburide compared to those who were not (Figure 3). Sixty-three immune-related genes were differentially expressed in melioidosis patients on glyburide (P = .001), the net effect of which would be postulated to be anti-inflammatory (Supplementary Table 3). Prominent among the immune-related genes include those implicated in neutrophil activation, endothelial adhesion, and transmigration. There was no statistical difference in neutrophil, lymphocyte, or monocytes counts between the 2 groups (Supplementary Table 2), indicating that this was not merely an effect of differences in cellular blood constituents. In the microarray analysis, tumor necrosis factor (TNF)-α, IL-18R1, and IL-8 expression were all downregulated; interferon (IFN)-γ showed a modest trend to downregulation. Quantitative RT- PCR corroborated the TNF-α and IL-18R1 results; IFN-γ was down-regulated by this methodology, but the IL-8 results were not confirmed (Supplementary Table 5).
Figure 3.
Differential expression of inflammation-associated genes in diabetic patients with acute melioidosis with or without glyburide (Gb). Genes that are up-regulated are shown in red, down-regulated in green; genes in black are not differentially expressed. The gene symbols used are those assigned by the HUGO gene nomenclature committee.
We sought further evidence for an effect of glyburide on gene expression profiles in otherwise healthy diabetics who were taking glyburide (n = 10) or who were not taking a sulfonylurea drug (n = 10). There were no significant differences in baseline parameters between the 2 groups (Supplementary Table 2). We found that 31 probes (29 distinct genes) were significantly different in patients taking glyburide compared to those who were not, 13 of which were immune related (Supplementary Table 4), again suggesting an anti-inflammatory effect. Of note, genes associated with neutrophil function were down-regulated in the glyburide group (P < .001); however, we found no direct antiinflammatory effect of glyburide on neutrophils in vitro (Supplementary Results).
DISCUSSION
This study of severe gram-negative sepsis has demonstrated a clear association between glyburide and a survival benefit. This second-generation sulfonylurea is widely used to treat type 2 diabetes and acts by inhibiting ATP-sensitive potassium channels (KATP channels) in pancreatic β cells, leading to stimulation of insulin secretion. KATP channels are also expressed in the heart and on vascular smooth muscle, and excessive opening of vascular KATP channels in sepsis (due to a fall in the ATP/ADP ratio, pH or oxygen tension, or a rise in intracellular lactate) leads to hyperpolarization of the cell membrane, a blocking of the calcium influx, and loss of vascular tone (contributing to the “vascular paresis” of septic shock). By blocking vascular smooth muscle KATP channels, glyburide is able to protect from shock in animal models of sepsis, although this did not translate into a detectable effect on cardiovascular parameters in 2 small clinical trials in which glyburide was given at doses normally used to treat diabetes [7, 8]. Neither study was powered to determine an effect on mortality and neither looked at other end points such as respiratory failure.
A retrospective study of 12.5 million sepsis patients, in which patients with diabetes had a lower mortality rate than those without diabetes (18.5% vs 20.6%, respectively, P < .05) ascribed this in part to a lower incidence of acute respiratory failure in diabetics compared to nondiabetics (9% vs 14%, respectively, P < .05) [9]. A second study conducted by the same investigators of 113 intensive care unit patients showed that a preadmission diagnosis of diabetes (but not hyperglycemia) protected patients from acute respiratory distress syndrome (ARDS) [10]. Neither study looked for an effect of diabetes treatment.
Glyburide is known to have a wide range of anti-inflammatory effects, one of which is an inhibitory effect on inflammasome assembly [2]. The inflammasome is an intracellular protein complex present in macrophages that activates caspase 1 and converts pre-formed IL-1β and IL-18 to their active forms when presented with an appropriate inflammatory stimulus [11]. IL-1β and IL-18 levels are high in bronchoalveolar lavage fluid from patients with acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and correlate with mortality [12, 13], which leads us to hypothesize that inhibition of inflammasome assembly may be a mechanism for our finding that glyburide therapy and respiratory failure are inversely associated. It is also unsurprising that fewer genes were found to be differentially expressed in the otherwise healthy diabetic controls than in the patients with melioidosis, as the inflammasome is only assembled as part of the acute inflammatory response and so we expect to see little effect of glyburide when there is no active inflammation.
Neutrophil recruitment to an area of tissue inflammation or infection can also be linked to the inflammasome through the action of IL-1β. While IL-1β is not itself a chemoattractant for neutrophils [14], IL-1β upregulates the expression of endothelial intercellular adhesion molecules essential for the recruit of neutrophils to an area of inflammation [15, 16, 17]. Neutrophils are a critical component of the host response to B. pseudomallei in vivo [18], but may also drive tissue injury and organ damage [19, 20]. The bronchoalveolar fluid of patients with ALI/ARDS is neutrophil-rich and some animal models of ARDS are neutrophil-dependent [20]. Glyburide has been shown to prevent neutrophil extravasation and accumulation in animal models [19], and this may represent indirect evidence that glyburide was responsible for reducing the incidence of respiratory failure in study patients taking this drug. We speculate that conflicting evidence from previous observational studies of the impact of diabetes on outcomes from bacterial sepsis may be explained in part by differences in the management of type 2 diabetes between Europe (metformin has been available in Britain since 1958 and has traditionally been used first-line) and the United States (where sulfonylureas have often been used first-line [21] and metformin has only been available since 1995).
Glyburide has been said to inhibit the inflammasome by blocking KATP channels [22] but this seems unlikely [23] and the actual target remains to be elucidated [2, 24], although the P2X7 receptor is one possibility [25, 26]. The development of inflammasome inhibitors that do not have the hypoglycemic effects of glyburide may depend on unraveling this pathway. Our enthusiasm for this approach is tempered by the finding of one multicenter trial that direct IL-1 blockade does not improve survival in all-cause sepsis [27]. Our study is also unable to provide guidance on whether any benefit would be seen were glyburide started following the onset of infection in nondiabetic patients.
Inflammasome function is not restricted to IL-1β activation and inflammasome inhibition is not the only action of glyburide. Glyburide is a broad-spectrum inhibitor of ABC transporters [28–30], and many members of this family have been implicated in regulating immune function. Glyburide also has a direct effect on neutrophil chemotaxis when administered at a dose 100 times the concentration achievable in humans [31], although we found no direct effect of glyburide on neutrophils in vitro (Supplementary Results).
In seeking confounders for our study, we considered whether glyburide treatment was simply a marker for less advanced diabetes and that this was associated with a survival benefit compared with patients on insulin, which is reserved for patients who fail to achieve adequate diabetic control on oral medication. However, only glyburide treatment and not metformin was associated with an improvement in mortality. We also considered whether thiazolidinediones and statins (which are antiinflammatory and may be required more commonly in diabetics) were confounders, but found that although available, these medications are very rarely used in our setting. This is an observational study in which glyburide was given for a specific indication, namely, the treatment of type 2 diabetes, and it is not possible to exclude unequivocally the possibility of confounding [32]. We are currently seeking to determine the effects of glyburide in directed clinical and animal studies of bacterial sepsis.
Supplementary Material
Acknowledgments
We thank Saowanit Getchalarat, Allen Cheng, Bina Maharajan, and Atchriya Hemachandra, who recruited patients during the study period; Gumphol Wongsuvan, Sukanya Pangmee, and Tanja Kaptein for laboratory support; Maliwan Hongsuvan, Varinthorn Praikaew, and Jintana Suwanapruek for administrative support; Richard J. Maude, Emma K. Nickerson, Anton Ilderton, Alex de Vos, Teunis B. H. Geitenbeek, and Arjan J. Hoogendijk for helpful discussions. We wish also to thank Suthee Suddee at Warin Chamrap Hospital, Ubon Ratchathani, for his help recruiting patients from the diabetes clinic there. We thank the nurses and doctors at Sappasithiprasong Hospital, Ubon Ratchathani, who were responsible for providing all care for the patients in the study.
Financial support. This work was supported by the Wellcome Trust of Great Britain (G.C.K.W.K., R.R.,M.F.S., D.L., V.W., S.J.L., W. Chaowagul, W. Chierakul, N.J.W., N.P.J.D., G.D., and S.J.P.); Foundation for 385 the National Institutes of Health (Grand Challenges in Global Health Initiative to M.F.S.); The Netherlands Organisation for Scientific Research (W.J.W. and T.V.D.P.); Sappasthiprasong Hospital (W.M.); The AMC Research Council, BEGETU, the European Union, de Landsteiner Stichting voor Bloedtransfusie 390 Research, and de Nederlands Astma Fonds (T.v.d.P.); The Bill & Melinda Gates Foundation (N.P.J.D. and N.J.W.); and the National Institutes of Health, Health Protection Agency (UK), European Union, and the UK Clinical Research Collaboration (S.J.P.).
Potential conflicts of interest: G.C.K.W.K. has formerly held shares in GlaxoSmithKline. All other authors: no conflicts of interest.
Supplementary Material
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/).
Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
- 1.Wiersinga WJ, van der Poll T. Is the septic response good or bad? Curr Infect Dis Rep. 2007;9:366–73. doi: 10.1007/s11908-007-0057-5. [DOI] [PubMed] [Google Scholar]
- 2.Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the cryopyrin/NALP3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suputtamongkol Y, Chaowagul W, Chetchotisakd P, et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–13. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 4.Victora CG, Huttly SR, Fuchs SC, Olint MTA. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224–7. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 5.McCane DR, Hanson RL, Charles MA, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ. 1994;308:1323–8. doi: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limmathurotsakul D, Wuthiekanun V, Chierakul W, et al. Role and significance of quantitative urine cultures in diagnosis of melioidosis. J Clin Microbiol. 2005;43:2274–6. doi: 10.1128/JCM.43.5.2274-2276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warrillow S, Egi M, Bellomo R. Randomized, double-blind, placebo-controlled crossover pilot study of a potassium channel blocker in patients with septic shock. Crit Care Med. 2006;34:980–5. doi: 10.1097/01.CCM.0000206114.19707.7C. [DOI] [PubMed] [Google Scholar]
- 8.Morelli A, Lange M, Ertmer C, et al. Glibenclamide dose response in patients with septic shock: effects on norepinephrine requirements, cardiopulmonary performance, and global oxygen transport. Shock. 2007;28:530–5. doi: 10.1097/shk.0b013e3180556a3c. [DOI] [PubMed] [Google Scholar]
- 9.Esper A, Moss M, Martin G. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13:R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28:2187–92. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform trigering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 12.Kikkawa T, Suzuki Y, Makabe H, et al. Assessment of IL-18 values in septic acute lung injury/acute respiratory distress syndrome patients. Crit Care. 2009;13:P368. [Google Scholar]
- 13.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–73. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 14.Koh Y, Hybertson BM, Jepson EK, Cho OJ, Repine JE. Cytokine-induced neutrophil chemoattractant is necessary for interleukin-1-induced lung leak in rats. J Appl Physiol. 1995;79:472–8. doi: 10.1152/jappl.1995.79.2.472. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. J Leukoc Biol. 2005;106:584–92. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996;153:1850–6. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 17.Leff JA, Baer JW, Bodman ME, et al. Interleukin-1-induced lung neutrophil accumulation and oxygen metabolite-mediated lung leak in rats. Am J Physiol Lung Cell Mol Physiol. 1994;266:L2–8. doi: 10.1152/ajplung.1994.266.1.L2. [DOI] [PubMed] [Google Scholar]
- 18.Easton A, Haque A, Chu K, Lukaszewski R, Bancroft GJ. A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J Infect Dis. 2007;195:99–107. doi: 10.1086/509810. [DOI] [PubMed] [Google Scholar]
- 19.Pompermayer K, Amaral FA, Fagundes CT, et al. Effects of the treatment with glibenclamide, an ATP-sensitive potassium channel blocker, on intestinal ischemia and reperfusion injury. Eur J Pharmacol. 2007;556:215–22. doi: 10.1016/j.ejphar.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 20.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 21.Riddle MC. Sulphonylureas differ in effects on ischemic preconditioning—is it time to retire glyburide? J Clin Endocrinol Metab. 2003;88:528–30. doi: 10.1210/jc.2002-021971. [DOI] [PubMed] [Google Scholar]
- 22.Muruve DA, Pétrilli V, Zaiss AK, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–7. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 23.Haskó G, Deitch EA, Németh ZH, Kuhel DG, Szabó C. Inhibitors of ATP-binding cassette transporters suppress interleukin-12 p40 production and major histocompatibility complex II up-regulation in macrophages. J Pharmacol Exp Ther. 2002;301:103–10. doi: 10.1124/jpet.301.1.103. [DOI] [PubMed] [Google Scholar]
- 24.Hamon Y, Luciani MF, Becq F, Verrier B, Rubartelli A, Chimini G. Interleukin-1β secretion is impaired by inhibitors of the ATP binding cassette transporter, ABC1. Blood. 1997;90:2911–5. [PubMed] [Google Scholar]
- 25.Di Virgillo F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci. 2007;28:465–72. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–40. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opal SM, Fisher CJ, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–24. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 28.McNicholas CM, Guggino WB, Schweibert EM, Hebert SC, Giebisch G, Egan ME. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proc Natl Acad Sci U S A. 1996;93:8083–8. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payen L, Delugin L, Courtois A, Trinquart Y, Guillouzo A, Fardel O. The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells. Br J Pharmacol. 2001;132:778–84. doi: 10.1038/sj.bjp.0703863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melin P, Hosy E, Vivaudou M, Becq F. CFTR inhibition by glibenclamide requires a positive charge in cytoplasmic loop three. Biochim Biophys Acta. 2007;1768:2438–6. doi: 10.1016/j.bbamem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Dal-Secco D, Cunha TM, Freitas A, et al. Hydrogen sulfide augments neutrophil migration through enhancement of adhesion molecule expression and prevention of CXCR2 internalization: role of ATP-sensitive potassium channels. J Immunol. 2008;181:4287–98. doi: 10.4049/jimmunol.181.6.4287. [DOI] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31:163–5. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.