Abstract
IFT80, a protein component of intraflagellar transport (IFT) complex B, is required for the formation, maintenance and functionality of cilia. Mutations in IFT80 cause Jeune asphyxiating thoracic dystrophy (JATD) and short rib polydactyly (SRP) type III. Both diseases are autosomal recessive chondrodysplasias and share clinical and radiological similarities, including shortening of the long bones and constriction of the thoracic cage. A murine Ift80 gene-trap line was used to investigate the role of Ift80 during development. The homozygote appears hypomorphic rather than a true null due to low level wild-type transcript production by alternative splicing around the gene-trap cassette. Hypomorphic levels of Ift80 result in embryonic lethality highlighting a key role for Ift80 in development. In rare cases, gene-trap homozygotes survive to postnatal stages and phenocopy both JATD and SRP type III by exhibiting growth retardation, shortening of the long bones, constriction of the ribcage and polydactyly. Mouse embryonic fibroblasts made from this line showed a significant reduction in hedgehog pathway activation in response to Hedgehog analog treatment. This defective signalling was not accompanied by the loss or malformation of cilia as seen in some knockout models of other IFT component genes. Phenotypes indicative of defects in cilia structure or function such as situs inversus, cystic renal disease and retinal degeneration were not observed in this line. These data suggest that there is an absolute requirement for Ift80 in hedgehog signalling, but low level expression permits ciliogenesis indicating separate but linked roles for this protein in formation and function.
INTRODUCTION
Jeune asphyxiating thoracic dystrophy (JATD) and short rib polydactyly type III (SRP type III/Verma–Naumoff syndrome) belong to a spectrum of disorders known collectively as SRP syndromes. Diseases in this spectrum are characterized by a constriction of the thoracic cage which often leads to death perinatally or in infancy due to respiratory insufficiency. Patients also present with other skeletal abnormalities including shortening of the long bones and, in some cases, polydactyly. SRP type III is the more severe of these two disorders and is characterized by early perinatal lethality. It can be accompanied by a range of extra skeletal malformations, including cleft lip or palate, cystic renal disease, gastrointestinal, urogenital, brain and/or cardiac malformations. JATD lies at the milder end of the SRP spectrum, whereby patients often die perinatally or in infancy due to respiratory insufficiency; however, around one-fifth of patients survive beyond this stage. Complications in JATD patients are variable but can include renal, hepatic, pancreatic and retinal abnormalities.
It has been suggested for some time that these two diseases are variants of the same disorder rather than distinct entities owing to the clinically overlapping features and also to the finding of a single family presenting with both JATD and SRPIII (1). More recently, this hypothesis has been verified with the finding of causative mutations for both disorders in two genes IFT80 and DYNC2H1 (2–5). Both the encoded proteins are known to play a role in intraflagellar transport (IFT), the process by which cargo is moved along the ciliary axoneme that is required for the formation and functionality of cilia.
IFT was first described in Chlamydomonas (6) but has since been shown to be an evolutionarily conserved process in all ciliated organisms. During IFT, a pair of multimeric protein complexes (A and B) are transported bidirectionally within the cilium by molecular motors, including kinesin 2 and cytoplasmic dynein. IFT80 has been shown to be a non-core component of IFT complex B (7), whereas DYNC2H1 is a subunit of cytoplasmic dynein, the molecular motor that drives retrograde IFT (8). The combination of these two genes as causative agents suggests that dysfunction of IFT is the underlying mechanism of disease in JATD and SRPIII.
The key phenotypes in all SRP spectrum disorders are by definition the skeletal malformations. In recent years, it has been shown that the process of IFT is essential during skeletogenesis for correct digit patterning, growth plate organization and limb outgrowth (9–11). This requirement has been explained by the finding that cilia and thus IFT are absolutely required for efficient hedgehog signalling (12–17). Components of the signalling pathway including smoothened and the Gli family of transcription factors localize to primary cilia, and IFT is required for processing Gli3 from its activator to repressor form. Previously described IFT mutants including Ift172, Ift88, Kif3A and Dync2H1 itself have been shown to lack or to have morphologically abnormal cilia, and to show defects in hedgehog signalling (11–13,15,17). As Indian hedgehog (Ihh) is one of the essential signalling molecules required for skeletal development, it seems perturbed hedgehog signalling due to defects in IFT could underlie the skeletal malformations seen in JATD and SRPIII.
Here, we focus on the lesser studied of the two JATD/SRPIII genes Ift80. Loss of Ift80 has been shown to lead to a total loss of ciliation in the multiciliate Tetrahymena (2). In Danio rerio, knockdown of ift80 has been shown to reduce the number of cilia present and shorten those that remain (18). These fish have been shown to exhibit classic ciliopathy phenotypes, including curved tail, pronephric cysts and retinal malformations. In this paper, we describe the first murine model for Ift80. This hypomorphic model phenocopies the skeletal phenotypes of SRP syndromes and therefore allows us to investigate the role of Ift80 in skeletal development, including its role in ciliation and hedgehog signalling.
RESULTS
Ift80gt/gt mouse generation and genotyping
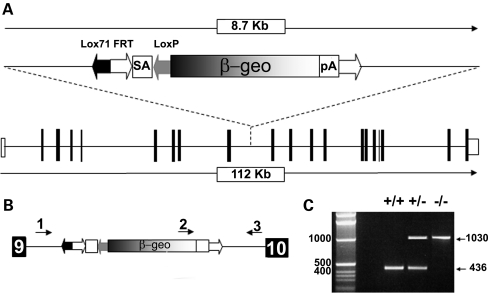
Embryonic stem cell line AN0245 containing gene-trap cassette pGT0lxr in the intronic region downstream of exon 9 in Ift80 (Fig. 1A) was microinjected and founders were bred. To enable genotyping of this line, the position of the gene-trap insertion point was more specifically pinpointed using a series of primer pairs spanning the intron, and found to lie immediately upstream of intronic base G4213. This allowed genotyping primers to be designed flanking the insertion site and within the gene-trap vector (Fig. 1B). Primers flanking the insertion site give a 436 bp fragment from wild-type alleles. A third primer within the gene-trap combines with the intronic reverse primer to give a product of 1030 bp from mutant alleles (Fig. 1C). Mice carrying the gene-trap allele are referred to as Ift80gt.
Figure 1.
Generation of Ift80gt/gt mice. (A) Wild-type Ift80 and the gene-trap cassette pGT0lxr showing the insertion point between exons 9 and 10. (B) Genotyping primer location. 1 = Ift80_gtgt_5′, 2 = Ift80_gtgt_3′, 3 = Ift80_gtgt_int. Sequences are available in the methods. (C) PCR products from tail-tip genotyping. Primers 1and 3 create a 436 bp product from a wild-type allele and primers 2 and 3 produced a 1030 bp product from a gene-trapped allele.
Ift80gt/gt offspring from this line are hypomorphs expressing variable levels of wild-type Ift80 transcript due to alternative splicing around the gene-trap cassette
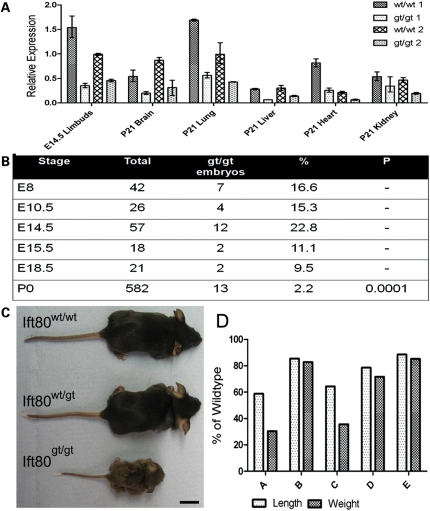
To ensure functionality of the gene-trap, RT–PCR primers were designed flanking the region of mRNA conceptually interrupted by the gene-trap insertion and within the β-geo of the gene-trap cassette. RNA extracted from wild-type and Ift80gt/gt tissue was reverse transcribed and RT–PCR was carried out on the resulting cDNA. Both sets of primers amplified products from the Ift80gt/gt cDNA showing that both wild-type and gene-trap fusion products were being produced from the trapped allele. The wild-type transcript contained no mutations showing normal splicing from exons 9 to 10 instead of using the splice acceptor of the gene-trap cassette. This inefficient splicing to the acceptor of the trap cassette leading to wild-type transcript production means that all Ift80gt/gt animals from this line should be described as hypomorphs rather than null. To quantify the levels of wild-type transcript being expressed from the Ift80gt/gt allele, real-time primers were designed against the murine sequence of Ift80 across the exon 14–15 boundary downstream of the gene-trap insertion site. Only wild-type transcript should contain this sequence. Comparison of the levels of this sequence showed that all Ift80gt/gt examined exhibited variably reduced levels of the wild-type Ift80 transcript, but not a total loss (Fig. 2A). Expression of β-gal from the Ift80gt/gt alleles was assessed in whole-mount embryos. Although the gene trap is inefficient, a fusion protein is produced. At E7.5, expression is seen mainly in the node, the only population of ciliated cells in the embryo at this stage (Supplementary Material, Fig. S1A). Later at E11.0, expression can be seen in the limb buds, brain, neural tube and eye (Supplementary Material, Fig. S1B).
Figure 2.
Ift80gt/gt animals are hypomorphs and most die perinatally but a subset is viable although growth retarded. (A) qRT–PCR data showing levels of wild-type Ift80 transcript expression in a range of issue types. Two Ift80gt/gt animals and wild-type littermate controls are shown at each stage. Data were normalized to GAPDH and are expressed as arbitrary units showing expression relative wt/wt2 E14.5 limb buds. All Ift80gt/gt animals show hypomorphic levels of expression from the gene-trapped allele in all tissues tested. (B) Numbers of Ift80gt/gt offspring observed at different developmental stages. No statistically significant losses are seen until postnatal day 0 suggesting death is perinatal. (C) Comparative size of Ift80gt/gt animals at P21. No phenotype is seen in Ift80wt/gt animals, whereas Ift80gt/gt show growth retardation. Scale bar = 2 cm. (D) Analysis of the level growth retardation of five Ift80gt/gt animals at P21. The crown/rump length and weight were measured and are expressed as a percentage of wild-type littermate controls. Growth retardation is variable and likely correlates with wild-type Ift80 expression levels.
Hypomorphic levels of Ift80 are embryonic lethal
Although all Ift80gt/gt offspring expressed hypomorphic levels of Ift80, the percentage surviving to birth was <3% highlighting an essential role for Ift80 in normal embryonic development (Fig. 2B). The specific timing of this lethality is yet to be determined but is probably variable and likely correlates with the expressed level of the wild-type Ift80 transcript. At E14.5, the numbers of Ift80gt/gt embryos do not differ significantly from expected values showing that early development occurs relatively normally. Statistically significant losses were only observed at postnatal stages suggesting that the majority of the loss was perinatal in line with the human JATD phenotype.
Hypomorphic mice phenocopy JATD and SRPIII
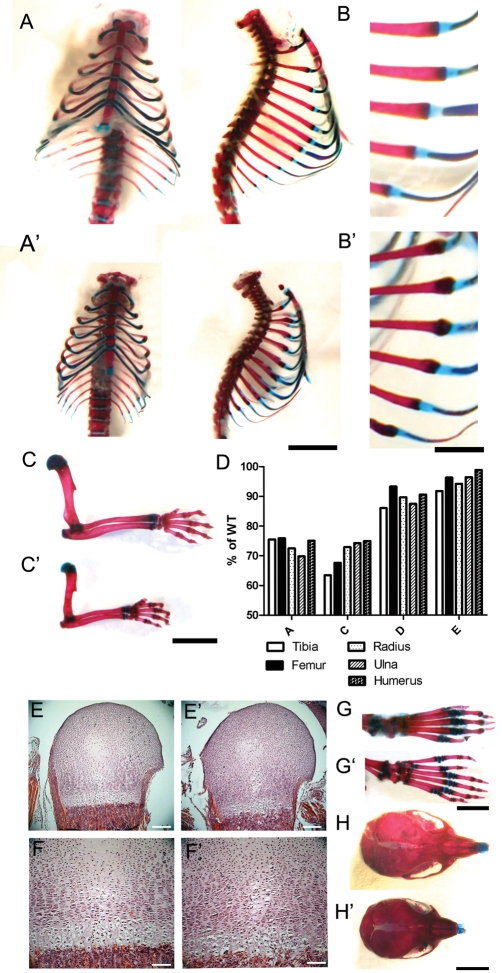
To investigate whether the reduction in the level of Ift80 in these mice recapitulated the human diseases with mutated Ift80, the phenotype of postnatal survivors was carefully analysed. No discernable phenotype was observed in Ift80wt/gt animals. At birth, hypomorphic Ift80gt/gt animals were slightly smaller than wild-type littermates; however, they failed to thrive and over the next few days became markedly growth retarded in comparison (Fig. 2C and D). Alcian blue and alizarin red staining of skeletal preparations at P21 showed a range of skeletal malformations characteristic of SRP syndromes. In Ift80gt/gt mice, thoracic cages were constricted and ribs showed an irregular margin between bone and cartilage (Fig. 3A, A′, B, B′). The long bones were significantly shortened compared with wild-type littermates (Fig. 3C and C′). This shortening was slightly more severe in all distal rather than proximal limb elements (Fig. 3F). Growth plates of P2 Ift80gt/gt animals showed defects in organization and changes in cellularity compared with wild-type littermates (Fig. 3G, G′, H, H′). In addition, all Ift80gt/gt animals observed demonstrated a characteristic bilateral pre-axial polydactyly of the hind limbs in which a single extra digit is formed that was markedly longer than any of the other digits (Fig. 3D and D′). The skull was of a reduced size and was flattened in the anterior posterior axis (Fig. 3E and E′).
Figure 3.
Skeletal phenotypes of Ift80gt/gt recapitulate SRP syndromes. (A–C′, G–H′) Alizarin red and Alcian blue staining of skeletal preparations from Ift80gt/gt and wild-type littermates at P21. (A and A′) Wild-type (A) and Ift80gt/gt (A′) ribcages showing constriction of the thoracic cage. Scale bar = 500 µm. (B and B′) An enlargement of the wild-type (B) and Ift80gt/gt (B′) showing an irregular margin between cartilage and bone in the Ift80gt/gt ribs. Scale bar = 2500 µm. (C and C′) Wild-type (C) and Ift80gt/gt (C′) forelimb bones showing correct patterning but shortening of the long bones in Ift80gt/gt. Scale bar = 5000 µm. (D) An analysis of the lengths of limb bones in four of the five P21 Ift80gt/gt animals described in Figure 1. Lengths are expressed as a percentage of a wild-type littermate control. In all cases, the distal long bones show increased shortening compared with the proximal bones. (E–F′) Haematoxylin and eosin staining of the growth plates of Ift80gt/gt and wild-type littermates at P2. Growth plates in the humerus of Ift80gt/gt and (E′ and enlargement F′) show disorganization of chondrocyte layers. Cells in the hypertrophic zone are particularly poorly organized and appear smaller than those in the wild-type. Scale bars = 400 µm (E and E′) and 100 µm (F and F′). (G and G′) Staining of hind limb digits showing the pre-axial polydactyly common to all Ift80gt/gt hind limbs (G′). Scale = 4000 µm. (H and H′) Staining of skulls showing a compression of the anterior posterior axis in Ift80gt/gt (H′). Scale = 5000 µm.
Hedgehog signalling is disrupted in Ift80 hypomorphs
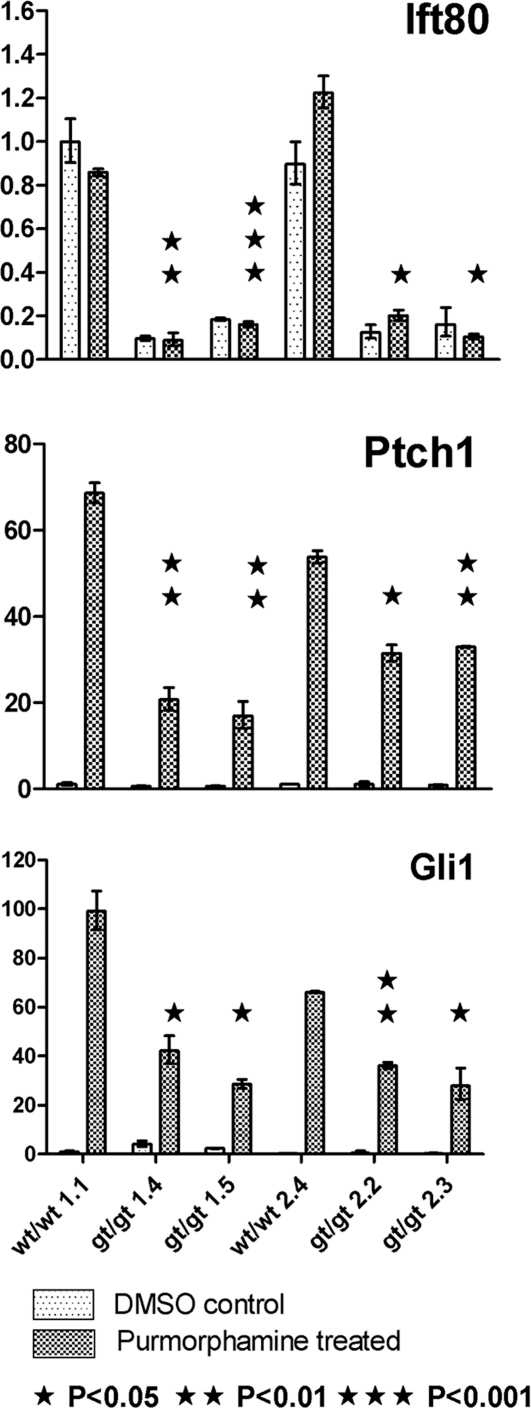
As the skeletal phenotype of these mice was very reminiscent of multiple other lines with defects in hedgehog signalling, we next investigated possible signalling defects in our mice. Mouse embryonic fibroblasts (MEFs) were established from wt and Ift80gt/gt E14.5 embryos to enable direct study of hedgehog pathway activation using the smoothened agonist purmorphamine. Real-time PCR of both Patched 1 and Gli1, direct transcriptional readouts of hedgehog signalling, showed a reduction in upregulation of signalling following purmorphamine treatment in all four Ift80gt/gt animals. The Patched1 and Gli1 levels were statistically significantly different from wild-type littermate controls in all four Ift80gt/gt animals to a P-value of at least 0.05. The reduction in signalling correlated with the level of wild-type Ift80 expression (Fig. 4).
Figure 4.
Hedgehog signalling is disrupted in Ift80gt/gt MEFs. qRT–PCR data showing levels of wild-type Ift80, Ptch1 and Gli1 expression after treatment with DMSO alone or with purmorphamine. Four null lines and two corresponding wild-type littermate controls are shown. In all MEF lines, expression of Ptch1 and Gli1 is upregulated by purmorphamine treatment; however, this upregulation is significantly reduced in all Ift80gt/gt MEFs. Significance levels are indicated on the graphs with asterisks. This reduced expression correlates with the level of Ift80 expression. Data were normalized to GAPDH and are expressed as arbitrary units showing expression relative to wt/wt1.1 untreated.
Phenotypes observed in this line are not due to a lack of ciliation
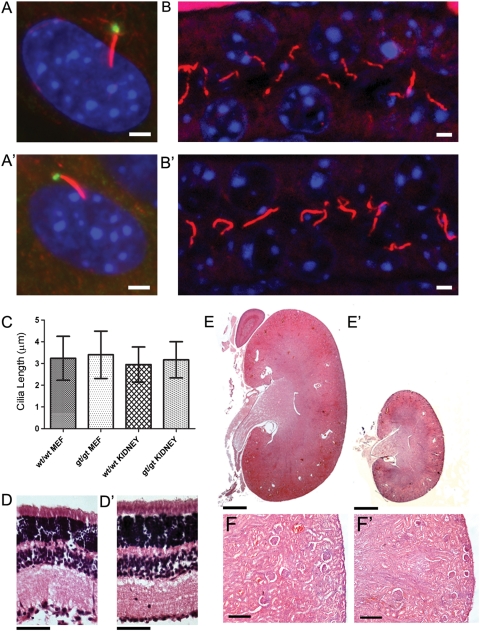
All IFT mutants previously described show a loss of cilia or an impairment in cilial growth or structure. This led us to investigate whether the phenotypes observed in the Ift80gt/gt line were also caused by ciliary dysfunction. Ift80gt/gt and wild-type MEFs were labelled to visualize the ciliary axoneme and basal body. This revealed that cilia were present on Ift80gt/gt MEFs (Fig. 5A and A′). To investigate whether ciliogenesis occurs in vivo, sections from P21 kidneys were labelled for cilia, which were found to be evident in the tubules of Ift80gt/gt kidneys (Fig. 5B and B′). MEF and kidney cilia were counted and their lengths measured; neither showed any significant difference compared with wild-type littermate controls (Fig. 5C). Although cilia are present and structurally normal, it is possible that they are non-functional. However, we do not observe classic ciliopathy phenotypes in this line. No isomerism was observed in any of the Ift80gt/gt animals. Recent work in Danio rerio has shown that morpholino knockdown ift80 led to retinal defects including defects in photoreceptor outer segment formation and photoreceptor death (18). Analysis of the retina in Ift80gt/gt mice at P21 showed that all retinal layers were present and appeared normal (Fig. 5D and D′). Kidney sections from P21 null mice showed that while small, the kidneys appeared to be properly developed with the formation of mature glomeruli and no evidence of cysts (Fig. 5E and E′). These data suggest that in Ift80gt/gt animals cilia are present, structurally normal and apparently functional aside from the observed hedgehog signalling defects, which indicates these defects and resulting skeletal phenotype do not result from the same ciliary dysfunction that has been shown in other IFT mutants.
Figure 5.
No loss of ciliation is seen Ift80gt/gt animals. (A and A′) Immunofluorescence of MEF cells. Cells are stained with anti gamma tubulin (green) and anti-acetylated alpha-tubulin (red) and nuclei were marked with DAPI (blue). Cilia are present on both wild-type (A) and Ift80gt/gt (A′) cells. Scale bar = 2 µm. (B and B′) Immunofluorescence of P21 kidney sections. Sections are stained with anti-acetylated alpha-tubulin (red) and nuclei were marked with DAPI (blue). Cilia are present in both wild-type (B) and Ift80gt/gt (B′) tubules. Scale bar = 2 µm. (C) Length analysis of both MEF and tubule cilia. Five hundred wild-type and Ift80gt/gt MEF cilia were measured as were 200 wild-type and Ift80gt/gt kidney cilia. The data show no significant difference between the lengths of cilia in either genotype. Error bars represent one standard deviation from the mean. (D and D′) Haematoxylin and eosin staining of wild-type (D) and Ift80gt/gt (D′) retina at P21. All retinal layers are present in the Ift80gt/gt. Scale bar = 100 µm. (E and F′) Haematoxylin and eosin staining of wild-type (E and enlargement F) and Ift80gt/gt (E′ and enlargement F′) kidneys at P21. Ift80gt/gt kidneys are smaller in line with the size of the mouse but appear normally developed with mature glomeruli and no evidence of cysts. Scale bars = 500 µm (E and E′) and 125 µm (F and F′).
DISCUSSION
In this study, a novel mouse mutant expressing hypomorphic levels of Ift80 was investigated and shown to recapitulate the skeletal defects of SRP spectrum diseases. These phenotypes were shown to be accompanied by defects in hedgehog signalling seen in similar models of skeletal dysplasia. However, this is the first demonstration of an IFT gene causing hedgehog signalling defects without a concurrent loss or malformation of cilia.
All homozygous animals produced from this line were shown to be hypomorphs expressing low levels of wild-type Ift80. While the hypomorphic levels of Ift80 expression observed do not permit an exhaustive assessment of the contribution played by Ift80 during development, they more accurately model the result of JATD and SRPIII patient mutations than a full knockout line. All four published mutations in IFT80 leading to JATD and SRPIII have been single missense mutations or deletions of a single amino acid (2,3). The minimal protein changes resulting from these mutations suggest that, like the Ift80 model described here, the patients’ phenotypes also represent a hypomorphism. The key phenotypes observed in the two SRP spectrum diseases caused by these ‘hypomorphic' IFT80 mutations are perinatal lethality and skeletal dysplasia, both of which are phenocopied in this line. This emulation of the human disease phenotype further validates Ift80 as a causative gene in the SRP spectrum of diseases and highlights the absolute requirement for Ift80 during development.
All SRP spectrum disorders feature skeletal malformation. Work in this study complements previously published investigations of the SRP spectrum disease Ellis van Creveld (EVC) syndrome, where mutations in EVC1 were associated with skeletal malformations and defects in hedgehog signalling (19). The limb outgrowth abnormality observed here is markedly similar to phenotypes seen in a variety of mouse lines, including Evc1 and Ihh mutants, but also to tissue-specific knockouts of IFT components, including Ift172, Ift88 and the molecular motor Kif3a (10,11,19–21). Ihh has been shown to be a key signalling molecule in endochondral ossification, the process by which most skeletal elements including the ribs and long bones are formed (20). In this process, a cartilage model of the skeleton is laid down which is then gradually ossified as chondrocytes mature through resting, proliferative and hypertrophic states. Ihh regulates the timing of this maturation and therefore the loss or disruption of Ihh signalling leads to shortening of the long bones due to aberrant progression of this process (22).
While Ihh is needed for the outgrowth skeletal elements, sonic hedgehog and its downstream effectors are needed at earlier stages for patterning of the forming skeleton. Defects in Shh signalling have also been shown to underlie both polydactyly and skull malformations similar to those seen in the Ift80gt/gt gene-trap line. A gradient of hedgehog pathway component Gli3 is formed across the developing limb bud to allow correct digit patterning and specification (23–25). Most polydactyly phenotypes described are believed to be caused by aberrant Gli3 expression at this time. Shh signalling is also required for craniofacial development with signalling driving cranial neural crest migration that establishes the skeletal elements of the skull (26,27). An anterior posterior shortening of the skull similar to that observed in Ift80gt/gt mutants is present in mouse models of Bardet–Biedl syndrome, and has been shown to be caused by aberrant crest migration and defective hedgehog signalling (28).
Thus, the skeletal phenotypes observed in the Ift80gt/gt hypomorphic line described here and in a variety of IFT and ciliary component knockout lines can be ascribed to abnormal hedgehog signalling. In turn, this can be explained by the recent findings that cilia and therefore IFT are absolutely required for efficient hedgehog signalling. In the presence of a hedgehog ligand, smoothened is translocated to the primary cilium. Other components of the pathway including the Gli family of transcription factors are then trafficked through the cilium as a key step in their processing from inactive to active forms (14–16). Without a cilium, hedgehog signalling is abrogated leading to a variety of phenotypes, including embryonic lethality and skeletal malformations. All murine models of IFT components described to date which have skeletal malformations accompanied by aberrant hedgehog signalling also have structural defects in the cilium. In addition, full knockout models of IFT complex B components including Ift88 and Ift172 usually show a total loss of ciliation and early embryonic lethality (29,30). We cannot rule out that this would also be the case for a full knockout of Ift80. Evidence from Tetrahymena and Danio rerio has shown that Ift80 is required for normal ciliation in these organisms (2,18). It is possible that in higher organisms, the requirement for Ift80 for ciliation has been lost. However, it is more likely that ciliation is simply less sensitive to reduction in Ift80 levels than hedgehog signalling so the role of Ift80 in ciliation is masked by the hypomorphic levels of expression seen in this line.
We propose a threshold model of Ift80 requirement during development. Total loss of expression would lead to the loss of ciliation and associated defects including early embryonic lethality as seen in the other complex B knockouts. Low to mid level expression as seen in hypomorphs of this line is sufficient for normal ciliation and thus the establishment of normal left–right asymmetry and correct early development, but leads to disruption of the hedgehog signalling pathway and thus to perinatal lethality and skeletal malformations. Wild-type levels of Ift80 are required for efficient hedgehog signalling and therefore for development to proceed normally.
As IFT is required for both the formation and function of the cilium, it is difficult to ascertain whether certain phenotypes are due to a defect in ciliation or to perturbation of IFT that also results in secondary ciliogenesis defects. With this model, we have shown for the first time that both hedgehog signalling defects and the resulting skeletal dysplasias can be caused by a defect in IFT without disruption to ciliary structure. This suggests a more direct role for Ift80 in hedgehog signalling when compared with other IFT mutants. Further analysis of this role should permit us new insights into the reasons why hedgehog signalling has become dependent on IFT and the cilium in higher organisms, and into the precise roles played by IFT in this fundamental developmental pathway. It will also shed new light on the pathomechanism of SRP spectrum diseases, the overlapping phenotypes of which suggest they are all likely to be caused by similar ciliary-related abrogation of hedgehog signalling.
MATERIALS AND METHODS
Itf80gt/gt mouse generation and genotyping and breeding
Embryonic stem cell line AN0245 containing gene-trap vector pGT0lxr in intron 9 of Ift80 was obtained from Baygenomics (San Francisco, USA). Microinjection and founder breeding was carried out using standard procedures (31). Genotyping primers were as follows:
IFT80_GTGT_5′-CCCTCTGCCTTTGACTCTTG,
IFT80_GTGT_3′-AATTATCTGTTGGAACGCGG,
IFT80_GTGT_int ATAATACCGCGCCACATAGC.
For timed matings; noon on the day a plug was found was designated as embryonic day 0.5 (E0.5).
RNA extraction, sequencing and qRT–PCR
Ift80gt/gt and wild-type littermate control tissue was dissected and homogenized in a dounce homogenizer. RNA was extracted using Trizol (Invitrogen) and reverse transcribed using Omniscript (Qiagen). Primers were designed to amplify both the wild-type and gene-trap fusion transcripts and PCRs were sequenced (Geneservice) and analysed using Sequencher (Genecodes). Primer sequences are available on request. Quantitative real-time PCR was carried out using Sybr Green (Qiagen) and run on a 7900HT Fast Real-Time PCR system (Applied Biosystems). Data were normalized to GAPDH. Primer sets used are as follows:
ift80_14_15_f TACATCACCTCTGTGAAACGATTTGGG,
ift80_14_15_r TGCATGTATCACACCATGCCAAAGT,
GAPDH_rt_F, TGCACCACCAACTGCTTAGC,
GAPDH_rt_R GGCATGGACTGTGGTCATGAG.
Skeletal preparations
Alcian blue and alizarin red staining was done using standard protocols. Briefly mice were eviscerated, fixed in 95% ethanol for 2 days, kept in acetone for 2 days and rinsed with water. Staining cocktail (1 volume 0.3% alcian blue in 70% EtOH, 1 volume 0.1% alizarin red in 95% EtOH, 1 volume 100% acetic acid and 17 volume 100% EtOH) was added and bones incubated at 37°C for 5–10 days until visible through surrounding tissue and fully stained. Surrounding tissue was cleared by immersion in 1% KOH for 24 h followed by a graded 1% KOH/glycerol series. Stained skeletal preparations were stored and photographed in 100% glycerol. Measurements were made using ImageJ (http://rsbweb.nih.gov/ij).
Tissue sectioning, H&E and immunostaining
Tissue was fixed in 4% (w/v) paraformaldehyde (PFA) in PBS at 4°C (kidneys and retina) or neutral-buffered formalin (bone). Bones were decalcified for 3 days prior to dehydration using a 1:1 solution of 8% HCl to 8% formic acid. All tissues were then dehydrated through an ethanol series, cleared using Histoclear (Flowgen), rehydrated in paraffin and embedded. Sections were taken at 10 µm. Haematoxylin and eosin stains followed standard protocols. For immunostaining, tissues were deparaffinized and hydrated through a graded ethanol series. Antigen retrieval was carried out in heated pressurized citrate buffer (10 mm citric acid, 0.05% Tween 20, pH 6.0). Primary antibodies used were as follows: polyclonal anti-Ift80, mouse monoclonal anti-γ-tubulin (1:100, GTU-88, Sigma) and anti-acetylated tubulin (1:800, 6-11B-1, Sigma). Secondary antibodies were: donkey anti-mouse Alexa Fluor 594 (Molecular Probes) and goat anti-rabbit Alexa Fluor 488 (Molecular Probes). Samples were mounted in Vectashield with DAPI (Vector) and images captured on an Imager.Z1 fluorescence microscope (Zeiss). Cilia length measurements were made using Image J.
Purmorphamine treatment of cells and quantification of target genes
MEFs were established from Ift80gt/gt and control E14.5 embryos using standard protocols. Briefly, embryos were dissected and heads and visceral organs were removed and kept for genotyping. The remaining tissue was homogenized by drawing through a 25-gauge needle 10 times. The resulting cell suspension was incubated in Trypsin-EDTA (Gibco) at 37°C for 10min to create a single-cell suspension. This was plated in 0.1% gelatin-coated flasks and maintained in DMEM + glutamax (Gibco) supplemented with 10% FBS (Gibco) and pen/strep (Gibco). For Hh pathway induction experiments, 1 × 105 cells were grown overnight in 0.1% gelatin-coated 12-well plates or on 0.1% gelatin-coated glass cover slips and treated for 72 h with either 2 μm purmorphamine (10 mm stock in DMSO, Calbiochem) or DMSO alone. Quantitative real-time PCR was carried as described above with the following primer sets:
IFT80 and GAPDH as above,
ptch1_rt_f CTGGCAGCCGAGACAAGCCC,
ptch1_rt_r TGGCCTGGGAGGCAGCGTAA,
gli1_rt_f GCCAGCTGGAGGTCTGCGTG,
gli1_rt_r TGGCTGTGGAGGGGTCGGAG.
Assays were carried out twice on cultures derived from four Ift80gt/gt and two wild-type littermate controls. Statistical analysis of results was carried out using t-tests. MEF cultures for immunostaining were fixed in ice-cold MeOH and stained as described above.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported by the Wellcome Trust (Grant No. 082311/Z/07/Z) and the British Heart Foundation.
ACKNOWLEDGEMENTS
We thank Juan Pedro Martinez-Barbera for help with microinjection and founder breeding.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Ho N.C., Francomano C.A., van Allen M. Jeune asphyxiating thoracic dystrophy and short-rib polydactyly type III (Verma-Naumoff) are variants of the same disorder. Am. J. Med. Genet. 2000;14:310–314. doi: 10.1002/(sici)1096-8628(20000214)90:4<310::aid-ajmg9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Beales P.L., Bland E., Tobin J.L., Bacchelli C., Tuysuz B., Hill J., Rix S., Pearson C.G., Kai M., Hartley J., et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat. Genet. 2007;39:727–729. doi: 10.1038/ng2038. doi:10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 3.Cavalcanti D.P., Huber C., Le Quan Sang K.H., Baujat G., Collins F., Delezoide A.L., Dagoneau N., Le Merrer M., Martinovic J., Mello M.F., et al. Mutation in IFT80 gene in a foetus with a phenotype of Verma-Naumoff provides molecular evidence for the Jeune-Verma-Naumoff dysplasia spectrum. J. Med. Genet. 2009 doi: 10.1136/jmg.2009.069468. Doi:10.1136/jmg.2009.06946. [DOI] [PubMed] [Google Scholar]
- 4.Dagoneau N., Goulet M., Geneviève D., Sznajer Y., Martinovic J., Smithson S., Huber C., Baujat G., Flori E., Tecco L., et al. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am. J. Hum. Genet. 2009;84:706–711. doi: 10.1016/j.ajhg.2009.04.016. doi:10.1016/j.ajhg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrill A.E., Merriman B., Farrington-Rock C., Camacho N., Sebald E.T., Funari V.A., Schibler M.J., Firestein M.H., Cohn Z.A., Priore M.A., et al. Ciliary abnormalities due to defects in the retrograde transport protein DYNC2H1 in short-rib polydactyly syndrome. Am. J. Hum. Genet. 2009;84:542–549. doi: 10.1016/j.ajhg.2009.03.015. doi:10.1016/j.ajhg.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozminski K.G., Johnson K.A., Forscher P., Rosenbaum J.L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl Acad. Sci. 1993;15:5519–5523. doi: 10.1073/pnas.90.12.5519. doi:10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucker B.F., Behal R.H., Qin H., Siron L.C., Taggart W.D., Rosenbaum J.L., Cole D.G. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J. Biol. Chem. 2005;280:27688–27696. doi: 10.1074/jbc.M505062200. doi:10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- 8.Pazour G.J., Dickert B.L., Witman G.B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. doi:10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song B., Haycraft C.J., Seo H.S., Yoder B.K., Serra R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 2007;305:202–216. doi: 10.1016/j.ydbio.2007.02.003. doi:10.1016/j.ydbio.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haycraft C.J., Zhang Q., Song B., Jackson W.S., Detloff P.J., Serra R., Yoder B.K. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. doi:10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 11.Song B., Serra R., Pacifici M. Conditional Kif3a ablation causes abnormal hedgehog signalling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. doi:10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huangfu D., Liu A., Rakeman A.S., Murcia N.S., Niswander L., Anderson K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. doi:10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 13.Liu A., Wang B., Niswander L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. doi:10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 14.Corbit K.C., Aanstad P., Singla V., Norman A.R., Stainier D.Y., Reiter J.F. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. doi:10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 15.May S.R., Ashique A.M., Karlen M., Wang B., Shen Y., Zarbalis K., Reiter J., Ericson J., Peterson A.S. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. doi:10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 16.Caspary T., Larkins C.E., Anderson K.V. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. doi:10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Ocbina P.J., Anderson K.V. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev. Dyn. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. doi:10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudak L.M., Lunt S., Chang C.H., Winkler E., Flammer H., Lindsey M., Perkins B.D. The intraflagellar transport protein ift80 is essential for photoreceptor survival in a zebrafish model of jeune asphyxiating thoracic dystrophy. Invest. Ophthalmol. Vis. Sci. 2010;51:3792–3799. doi: 10.1167/iovs.09-4312. doi:10.1167/iovs.09-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Perez V.L., Blair H.J., Rodriguez-Andres M.E., Blanco M.J., Wilson A., Liu Y.N., Miles C., Peters H., Goodship J.A. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. doi:10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- 20.St-Jacques B., Hammerschmidt M., McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard P.W., Howard T.L., Maurer R.A. Generation of mice with a conditional allele for Ift172. Transgenic Res. 2010;19:121–126. doi: 10.1007/s11248-009-9292-x. doi:10.1007/s11248-009-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp S.J., Schipani E., St-Jacques B., Hunzelman J., Kronenberg H., McMahon A.P. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- 23.Marigo V., Johnson R.L., Vortkamp A., Tabin C.J. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev. Biol. 1996;180:273–283. doi: 10.1006/dbio.1996.0300. doi:10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- 24.Wang B., Fallon J.F., Beachy P.A. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. doi:10.1016/S0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 25.Litingtung Y., Dahn R.D., Li Y., Fallon J.F., Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. doi:10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- 26.Helms J.A., Kim C.H., Hu D., Minkoff R., Thaller C., Eichele G. Sonic hedgehog participates in craniofacial morphogenesis and is down-regulated by teratogenic doses of retinoic acid. Dev. Biol. 1997;187:25–35. doi: 10.1006/dbio.1997.8589. doi:10.1006/dbio.1997.8589. [DOI] [PubMed] [Google Scholar]
- 27.Jeong J., Mao J., Tenzen T., Kottmann A.H., McMahon A.P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. doi:10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobin J.L., Di Franco M., Eichers E., May-Simera H., Garcia M., Yan J., Quinlan R., Justice M.J., Hennekam R.C., Briscoe J., et al. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung's disease in Bardet-Biedl syndrome. Proc. Natl Acad. Sci. 2008;105:6714–6719. doi: 10.1073/pnas.0707057105. doi:10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murcia N.S., Richards W.G., Yoder B.K., Mucenski M.L., Dunlap J.R., Woychik R.P. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- 30.Gorivodsky M., Mukhopadhyay M., Wilsch-Braeuninger M., Phillips M., Teufel A., Kim C., Malik N., Huttner W., Westphal H. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev. Biol. 2008;325:24–32. doi: 10.1016/j.ydbio.2008.09.019. doi:10.1016/j.ydbio.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogan B., Beddington R., Costantini F., Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbour, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.