Abstract
Muscular dystrophies (MDs) comprise a group of degenerative muscle disorders characterized by progressive muscle wasting and often premature death. The primary defect common to most MDs involves disruption of the dystrophin-glycoprotein complex (DGC). This leads to sarcolemmal instability and Ca2+ influx, inducing cellular necrosis. Here we have shown that the dystrophic phenotype observed in δ-sarcoglycan–null (Sgcd–/–) mice and dystrophin mutant mdx mice is dramatically improved by skeletal muscle–specific overexpression of sarcoplasmic reticulum Ca2+ ATPase 1 (SERCA1). Rates of myofiber central nucleation, tissue fibrosis, and serum creatine kinase levels were dramatically reduced in Sgcd–/– and mdx mice with the SERCA1 transgene, which also rescued the loss of exercise capacity in Sgcd–/– mice. Adeno-associated virus–SERCA2a (AAV-SERCA2a) gene therapy in the gastrocnemius muscle of Sgcd–/– mice mitigated dystrophic disease. SERCA1 overexpression reversed a defect in sarcoplasmic reticulum Ca2+ reuptake that characterizes dystrophic myofibers and reduced total cytosolic Ca2+. Further, SERCA1 overexpression almost completely rescued the dystrophic phenotype in a mouse model of MD driven solely by Ca2+ influx. Mitochondria isolated from the muscle of SERCA1-Sgcd–/– mice were no longer swollen and calpain activation was reduced, suggesting protection from Ca2+-driven necrosis. Our results suggest a novel therapeutic approach using SERCA1 to abrogate the altered intracellular Ca2+ levels that underlie most forms of MD.
Introduction
MD broadly encompasses a diverse group of genetic disorders that result in progressive muscle wasting and premature death (1). The majority of genes identified in patients with MD appear to contribute to or affect the multi-protein sarcolemmal-spanning dystrophin-glycoprotein complex (DGC), which is critical for maintaining integrity of the membrane and the proper activity of signaling complexes and channels (2). A disruption in the DGC is hypothesized to promote direct Ca2+ influx and/or abnormal cytosolic Ca2+ homeostasis, leading to increased vulnerability of myofibers to necrosis (3). However, direct measurement of intracellular Ca2+ concentration in dystrophic muscle has revealed conflicting results as to whether or not Ca2+ is elevated at baseline (4–9). Despite the lack of consensus with direct physical measurements, numerous studies have demonstrated that increased cytosolic Ca2+ activity can cause or enhance the dystrophic phenotype in muscle (10–12). For example, overexpression of transient receptor potential canonical (TRPC3) in skeletal muscle, which facilitates Ca2+ and Na+ influx, directly induced the entire dystrophic phenotype without destabilizing the sarcolemma (10). Similarly, blunting of select Ca2+-mediated disease processes can reduce the dystrophic phenotype of muscle in various mouse models of MD (10, 13, 14).
In skeletal muscle, the sarcoplasmic reticulum Ca2+ ATPase 1 (SERCA1) isoform is dominantly responsible for Ca2+ reuptake into the sarcoplasmic reticulum (SR) during excitation contraction (EC) coupling (15). Interestingly, a reduction in SERCA activity has been observed in dystrophic muscle (16–18), which is likely responsible for aspects of defective Ca2+ handling in MD, leading to greater cytoplasmic levels and cellular necrosis through calpain activation and mitochondrial permeability transition pore (MPTP) formation (3, 7, 12, 14). Here we hypothesized that Ca2+ is a final-common pathway for mediating cellular necrosis and end-stage disease across most of the MDs. Indeed, we show that SERCA1 overexpression in skeletal muscle dramatically attenuates manifestations of dystrophic disease in a Duchenne and limb-girdle model of MD in the mouse.
Results
Generation of SERCA1 Tg mice.
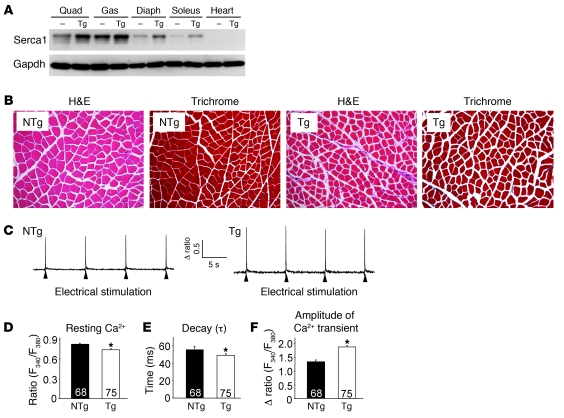
Increased ryanodine receptor (RyR) Ca2+ leak and reduced SR Ca2+ cycling are thought to contribute to MD pathogenesis (16, 18–20). Moreover, diminished SERCA1 activity characteristic of dystrophic muscle should exacerbate a Ca2+ overload problem associated with an unstable sarcolemma (3, 21, 22). In an attempt to restore and enhance SERCA1 activity and SR Ca2+ reuptake, we generated a series of Tg mice using the skeletal muscle–specific skeletal α-actin promoter to drive SERCA1. While 12 lines were generated and partially analyzed for protein expression and phenotypic effects, 1 line was selected for in-depth analysis. Western blotting from 1 line showed 2- to 4-fold overexpression of SERCA1 protein across the quadriceps, gastrocnemius, diaphragm, and soleus, while no expression was observed in the heart (Figure 1A). H&E- and Masson’s trichrome–stained histological sections showed no pathological features in the muscles of SERCA1 Tg mice compared with non-Tg littermates, although fiber cross-sectional areas were slightly decreased (Figure 1B). Next, electrically evoked Ca2+ transients were assessed in acutely isolated flexor digitorum brevis (FDB) fibers to determine whether SERCA1 overexpression promotes SR loading and enhanced Ca2+ clearance. As predicted, peak Ca2+ transient amplitudes were significantly increased and the time to decay of the Ca2+ transient was reduced (Figure 1, C, E, and F). Interestingly, the resting Ca2+ ratio was also significantly decreased in FDB fibers isolated from SERCA1 Tg mice compared with non-Tg littermates (Figure 1D). Taken together, these results demonstrate that overexpression of SERCA1 in skeletal muscle enhances Ca2+ clearance and cycling. However, expression levels of critical SR Ca2+ handling proteins were not significantly changed by the SERCA1 transgene, as assessed by quantitative Western blotting (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI43844DS1).
Figure 1. Overexpression of SERCA1 in skeletal muscle enhances Ca2+ cycling during EC coupling.
(A) Western blot analysis for SERCA1 expression in different muscle groups isolated from non-Tg (NTg) and SERCA1 Tg (Tg) mice at 3 months of age. Quad, quadriceps; Gas, gastrocnemius; Diaph, diaphragm. (B) H&E and Masson’s trichrome sections of quadriceps. Original magnification, ×200. (C) Representative traces of F340/F380 fluorescence ratio recordings from single FDB myofibers isolated from NTg and SERCA1 Tg mice in response to electrical stimulation. (D) Resting Ca2+ ratio, (E) time constant of decay (τ), and (F) peak Ca2+ transient amplitude in isolated myofibers from the indicated genotypes. *P < 0.05 compared with NTg mice; n = total number of fibers recorded from 4 animals in each genotype shown in the graphs, D–F.
SERCA1 overexpression ameliorates MD in δ-sarcoglycan null mice.
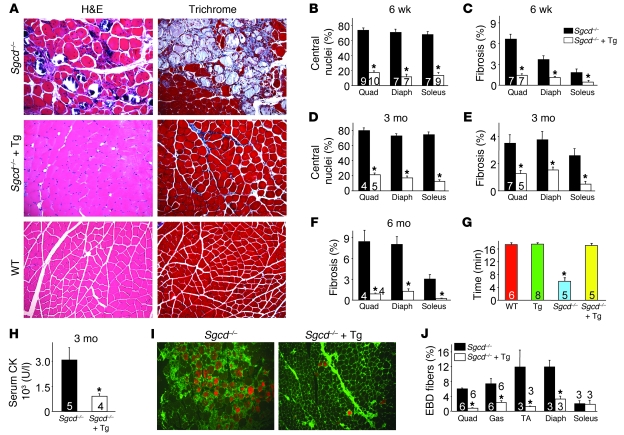
To assess the potential beneficial effect of SERCA1 overexpression in the context of MD, we crossed the SERCA1 transgene into the δ-sarcoglycan null (Sgcd–/–) genetic background, the latter of which is a mouse model with fulminant dystrophic disease (23, 24). The presence of the SERCA1 transgene resulted in a remarkable reduction in histopathology in Sgcd–/– mice at 6 weeks, 3 months, and 6 months of age, showing severe reductions in myofibers with centrally located nuclei and overall rates of fibrosis compared with Sgcd–/– only controls (Figure 2, A–F). In addition, serum creatine kinase (CK) levels were significantly reduced in Sgcd–/– mice with the SERCA1 transgene compared with Sgcd–/– littermates (Figure 2H). Associated with this improvement in histopathology, Sgcd–/– mice with the SERCA1 transgene showed a restoration in their ability to run on a treadmill comparable to WT control mice, while Sgcd–/– mice alone were severely compromised (Figure 2G). We also investigated whether sarcolemmal rupture rates and subsequent Evans blue dye (EBD) uptake were affected by the SERCA1 transgene in Sgcd–/– mice. Normally, muscle fibers from Sgcd–/– mice show uptake of EBD after systemic injection due to membrane breaks, which was observed in the quadriceps, gastrocnemius, tibialis anterior (TA), and diaphragm from control null mice only (Figure 2, I and J). However, the presence of the SERCA1 transgene uniformly reduced total EBD uptake in Sgcd–/– mice by 3- to 5-fold (Figure 2, I and J). We interpret this reduction in EBD uptake with the SERCA1 transgene to result from enhanced Ca2+ reuptake, which likely reduces membrane and mitochondrial reactive oxygen species generation that could secondarily diminish membrane stress and a presumed feed-forward mechanism of disease (see discussion). The soleus muscle was not differentially affected, presumably because it is a postural muscle that is always under tension/use (Figure 2J).
Figure 2. SERCA1 mitigates biochemical and histological features of MD in Sgcd–/– mice.
(A) H&E- and Masson’s trichrome–stained sections of quadriceps from WT, Sgcd–/–, and Sgcd–/–-SERCA1 Tg mice at 3 months of age. Original magnification, ×200. (B and D) Percentage of myofibers with centrally located nuclei in Sgcd–/– and Sgcd–/–-SERCA1 Tg mice at 6 weeks and 3 months of age. (C, E, and F) Interstitial fibrosis in muscle histological sections analyzed using metamorph analysis software in Sgcd–/– and Sgcd–/–-SERCA1 Tg mice at 6 weeks, 3 months, and 6 months of age. Number of mice used for quantitation is shown in the graphs. (G) Time to fatigue in minutes with forced treadmill running in the indicated groups of mice. (H) Quantitation of serum CK levels in Sgcd–/– and Sgcd–/–-SERCA1 Tg mice at 3 months of age. (I) Representative immunofluorescence images of EBD uptake in histological sections from quadriceps of 3-month-old mice subjected to running for 2 days in the presence of EBD. Membranes are stained green while EBD-positive fibers are in red. Original magnification, ×100. (J) Quantitation of total EBD fibers in quadriceps, gastrocnemius, TA, diaphragm, and soleus from at least 3 mice in each genotype. *P < 0.05 versus Sgcd–/–. Number of mice used is shown in each of the graphs.
SERCA1 overexpression ameliorates MD in mdx mice.
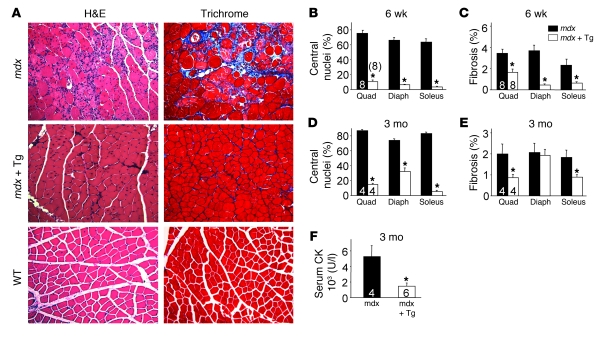
The mouse model of Duchenne MD with defective dystrophin expression results from the mdx mutation. This model, which corresponds to the most prevalent form of human MD, is typically less severe, and mice can live a near normal lifespan compared with Sgcd–/– mice, which manifest more severe disease with premature lethality (23, 24). Here we crossed the SERCA1 transgene into the mdx genetic background, and similarly to what we observed in the Sgcd–/– background, both histological and biochemical markers of MD were dramatically diminished (Figure 3A). At both 6 weeks and 3 months of age, myofiber central nucleation was profoundly reduced in the quadriceps, diaphragm, and soleus (Figure 3, B and D). In fact, at 6 weeks of age, myofiber central nucleation was nearly extinguished in all 3 muscles analyzed (Figure 3B). While fibrosis typically only becomes prominent in older mdx mice, even the mild degree of tissue fibrosis that begins to develop at 6 weeks and 3 months of age was significantly reduced in skeletal muscle of SERCA1/mdx mice (Figure 3, C and E). Finally, serum CK levels at 3 months of age were also reduced by approximately 4-fold in SERCA1/mdx mice when compared with mdx mice (Figure 3F). These results further support the conclusion that enhanced SERCA1 expression can attenuate histopathology and biochemical features of MD in mouse models of disease.
Figure 3. SERCA1 mitigates biochemical and histological features of MD in mdx mice.
(A) H&E- and Masson’s trichrome–stained histological sections from quadriceps of WT, mdx, and mdx-SERCA1 Tg mice at 3 months of age. Original magnification, ×200. (B and D) Percentage of myofibers with centrally located nuclei in mdx and mdx-SERCA1 Tg mice at 6 weeks and 3 months of age. (C and E) Interstitial fibrosis in muscle histological sections analyzed using metamorph analysis software in mdx and mdx-SERCA1 Tg mice at 6 weeks and 3 months of age. Number of mice used for quantitation is shown in the graphs. (F) Quantitation of serum CK levels in mdx and mdx-SERCA1 Tg mice at 3 months of age. *P < 0.05 versus mdx. Number of mice used is shown in the graphs.
SERCA1 overexpression ameliorates MD in TRPC3-overexpressing Tg mice.
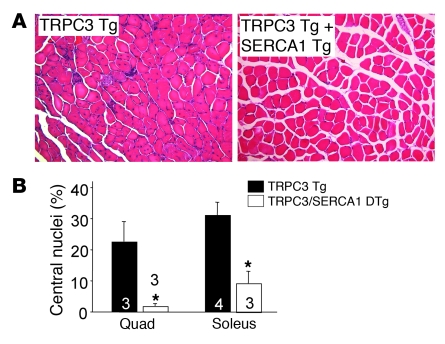
We demonstrated that skeletal muscle–specific overexpression of the ion channel TRPC3 was sufficient to induce skeletal muscle disease that was entirely consistent with MD (10). TRPC channels are thought to contribute to Ca2+ influx in MD associated with destabilization of the sarcolemma. Indeed, overexpression of a dominant negative TRPC6 mutant protein in skeletal muscle blocked Ca2+ leak activity of the sarcolemma and significantly reduced MD disease manifestations in mdx and Sgcd–/– mice (10). Here we intercrossed TRPC3- and SERCA1-overexpressing Tg mice to more directly examine the “Ca2+ hypothesis,” since the only disease-inducing effect associated with the TRPC3 transgene is Ca2+ influx. As predicted, H&E staining of quadriceps and soleus muscle showed dramatically less tissue histopathology at 3 months of age in SERCA1/TRPC3 Tg mice compared with TRPC3 Tg mice (Figure 4A). For example, quantitation of centrally located myofibers showed significant reductions in SERCA1/TRPC3 Tg mice compared with TRPC3 Tg mice (Figure 4B). These results further support the hypothesis that SERCA1 overexpression is protective by augmenting SR Ca2+ handling and counteracting the effect of increased membrane Ca2+ leak in MD.
Figure 4. SERCA1 mitigates histological features of MD in TRPC3 Tg mice.
(A) Representative histological H&E stain of quadriceps from TRPC3 Tg and TRPC3/SERCA1 double-Tg at 3 months of age. Original magnification, ×200. (B) Percentage of myofibers with centrally located nuclei in quadriceps and soleus from TRPC3 Tg and TRPC3/SERCA1 double-Tg mice. At least 3 mice from each genotype were used. *P < 0.05 versus TRPC3 TG. Number of mice used is shown in the graph.
Enhanced SR Ca2+ uptake in SERCA1-overexpressing mice.
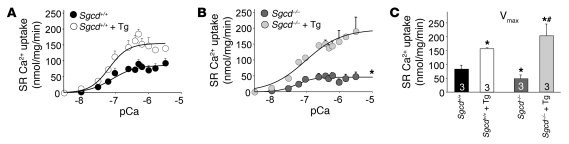
To further investigate the mechanism whereby SERCA1 overexpression can protect against MD, we directly measured SR Ca2+ uptake in lysates from muscle of the relevant genotypes. As predicted, SR Ca2+ uptake was approximately 2-fold higher in WT (Sgcd+/+) mice containing the SERCA1 transgene compared with mice without the SERCA1 transgene (Figure 5, A and C). Consistent with past observations in dystrophic myofibers/myotubes, maximal SR Ca2+ uptake was significantly reduced in the Sgcd–/– mice compared with WT controls (Sgcd+/+) (Figure 5C). More importantly, SR Ca2+ uptake in Sgcd–/– Tg mice was 3-fold greater than in Sgcd–/– mice and activity was restored to levels higher than even that in WT controls (Figure 5, B and C). The calculated EC50 value (pCa) was not significantly different in any of the groups, suggesting that increased expression of SERCA1 did not change its affinity for Ca2+ (data not shown). Collectively, these results indicate that SERCA1 overexpression dramatically enhances SR Ca2+ reuptake capacity and corrects a deficit observed in Sgcd–/– myofibers.
Figure 5. SERCA1 enhances SR Ca2+ uptake in Sgcd–/– mice.
(A) Average mean values of SR Ca2+ uptake in Sgcd+/+ (WT) and Sgcd+/+-SERCA1 Tg mice as a function of pCa2+. (B) Average mean values of SR Ca2+ uptake in Sgcd–/– and Sgcd–/–-SERCA1 Tg mice as a function of pCa2+. (C) Mean maximal velocity determined for each genotype in the SR Ca2+ uptake measurements. *P < 0.05 compared with Sgcd+/+; #P < 0.05 compared with Sgcd–/–. Number of mice used is shown in the graph.
SERCA1 overexpression restores EC-coupling defects observed in dystrophic myofibers.
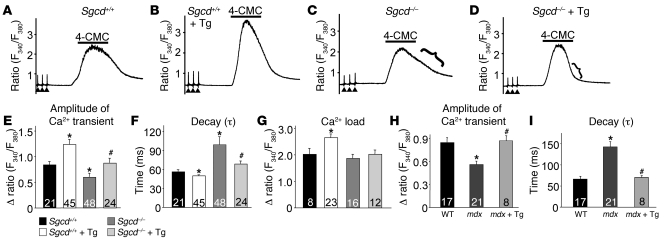
Multiple studies have shown deficits in global EC-coupling in dystrophic muscle fibers (25–27). These include a significant reduction in the peak amplitude of the Ca2+ transient and diminished Ca2+ reuptake into the SR. Here we investigated whether enhancing SR Ca2+ uptake with SERCA1 overexpression could ameliorate the defects in global EC coupling observed in dystrophic myofibers. We assessed electrically evoked Ca2+ transients in acutely isolated individual FDB fibers in Sgcd+/+ and Sgcd–/– mice with and without the SERCA1 transgene. Interestingly, the peak amplitude of the Ca2+ transient was increased in WT (Sgcd+/+) myofibers with the SERCA1 transgene compared with WT alone (Figure 6, A, B, and E). Consistent with results in the literature, the peak amplitude of the Ca2+ transient was reduced in Sgcd–/– myofibers compared with WT fibers, but this decrease was prevented with the SERCA1 transgene (Figure 6, C–E). SR Ca2+ load assessed with 4-chloro-m-cresol (4-CMC, to release SR Ca2+ stores) was not significantly different between Sgcd–/– and WT myofibers (Figure 6G). However, the SERCA1 transgene did significantly elevate SR Ca2+ load in WT myofibers above WT levels, but not in Sgcd–/– myofibers (Figure 6G). More importantly, the reuptake phase of the 4-CMC–induced Ca2+ profile and the decay time of the Ca2+ transient were each significantly prolonged in myofibers from Sgcd–/– mice, and this defect was corrected with the SERCA1 transgene (Figure 6, C, D, and F). Similarly, FDB myofibers from mdx mice also showed a reduction in the amplitude of the Ca2+ transient and an increase in relaxation time, parameters that were again corrected by the SERCA1 transgene (Figure 6, H and I). Taken together, these results demonstrate that dystrophic myofibers have significant defects in SR Ca2+ handling, which can be corrected by SERCA1 expression.
Figure 6. SERCA1 overexpression enhances EC coupling and Ca2+ clearance in Sgcd–/– and mdx myofibers.
(A–D) Representative traces of changes in ratiometric fluorescence ratios in acutely isolated FDB fibers from Sgcd–/– mice in response to electrical and chemical stimulation over time. The brackets in C and D show the Ca2+ reuptake characteristics. (E) Amplitude of the Ca2+ transient after electrical twitch stimulation in the indicated genotypes shown in the legend next to F (applies to E–G). (F) Time constant of decay (τ) and (G) maximal response to 4-CMC in the indicated genotypes as assessed with Fura-2 ratio analysis. *P < 0.05 versus Sgcd+/+; #P < 0.05 versus Sgcd–/–. Number of fibers used in E–G is shown in the graphs. (H) Amplitude of the Ca2+ transient after electrical twitch stimulation in FDB myofibers from mdx and control mice as indicated. *P < 0.05 versus WT; #P < 0.05 versus mdx. (I) Time constant of decay (τ) of the Ca2+ transient in FDB myofibers in the indicated mdx and control groups. *P < 0.05 versus WT; #P < 0.05 versus mdx.
SERCA1 overexpression reverses mitochondrial swelling in dystrophic muscle.
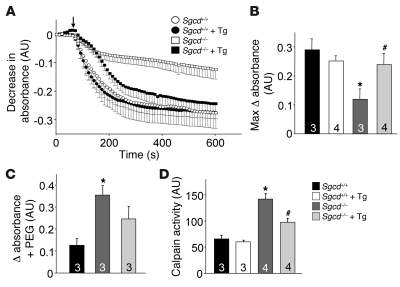
We recently demonstrated that mitochondria isolated from dystrophic skeletal muscle of Sgcd–/– mice are swollen at baseline and that this swelling can lead to myofiber necrosis (14). Indeed, prevention of Ca2+-mediated mitochondrial swelling and myofiber necrosis by deletion of cyclophilin D or use of Debio-025 in mice (both of which inhibit MPTP) significantly reduced MD in Sgcd–/– and mdx mice (14). Here we hypothesized that the SERCA1 transgene would reduce Ca2+ overload and spare mitochondria from swelling and induction of myofiber necrosis. Mitochondrial swelling was measured in purified preparations in vitro after isolation from muscle; thereafter swelling/shrinking was assessed with exogenous Ca2+ or PEG3350, respectively. As we have previously observed, mitochondria from Sgcd–/– muscle showed a significantly reduced capacity to swell upon Ca2+ addition compared with Sgcd+/+ mitochondria given their already swollen state, although shrinkage was enhanced (Figure 7, A–C). As predicted, this swollen state of mitochondria from Sgcd–/– muscle was rescued by the SERCA1 Tg (Figure 7, A–C). Ca2+ overload in dystrophic skeletal muscle is associated with calpain activation, which can lead to myofiber degeneration and cellular necrosis (12). We observed an increase in calpain enzymatic activity in muscle from Sgcd–/– mice, and importantly, this increase was significantly reduced by the presence of the SERCA1 transgene (Figure 7D). Collectively, these results again demonstrate that the SERCA1 transgene corrects Ca2+ overload, which likely protects the myofibers from cell death by diminishing mitochondrial swelling and calpain activation, reducing myofiber necrosis.
Figure 7. SERCA1 overexpression protects against Ca2+-induced disease indexes in skeletal muscle of Sgcd–/– mice.
(A) Mitochondrial swelling after Ca2+ addition (arrow) as a function of increased light scattering (decreased absorbance) from muscle purified mitochondria from Sgcd+/+, Sgcd+/+-SERCA1 Tg, Sgcd–/–, and Sgcd–/–-SERCA1 Tg mice. (B) Average maximal change in absorbance in response to external Ca2+ in purified mitochondria from the indicated groups. *P < 0.05 compared with Sgcd+/+; #P < 0.05 versus Sgcd–/–. (C) Change in absorbance in response to PEG to show degree of mitochondrial shrinkage in purified mitochondria. *P < 0.05 compared with Sgcd+/+. (D) Calpain enzymatic activity in skeletal muscle of the indicated groups *P < 0.05 compared with Sgcd+/+; #P < 0.05 versus Sgcd–/–. Number of mice used is shown in the bars of each panel.
SERCA2a gene therapy in Sgcd–/– mice mitigates dystrophic disease.
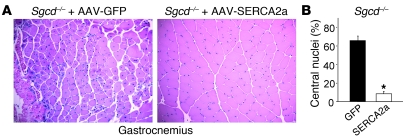
The results presented to this point suggest that SERCA overexpression could serve as a “universal” therapy for many diverse forms of MD that are associated with a Ca2+ imbalance or a leaky plasma membrane. Hence, a gene therapeutic strategy to overexpress SERCA protein in dystrophic skeletal muscle could be an exciting new approach to consider to treat patients. Here we injected 3-day-old Sgcd–/– pups with adeno-associated virus 9–SERCA2a (AAV9-SERCA2a) in the left gastrocnemius or AAV9-GFP (control) in the right gastrocnemius with 1010 viral particles each. The AAV9 serotype yields robust expression in muscle, and the SERCA2a protein functions very similarly to SERCA1. Mice were then harvested 6 weeks later for full histological assessment of muscle disease and expression of GFP. GFP expression was observed throughout the gastrocnemius of Sgcd–/– mice (greater than 90% of the fibers; data not shown), and tissue pathology was fulminant in the control injected muscle, as is typically observed (Figure 8A). However, expression of SERCA2a by AAV-mediated gene therapy dramatically attenuated dystrophic disease in the injected gastrocnemius, showing 8-fold less central nucleation and preserved cellular architecture with little signs of necrosis (Figure 8, A and B). Thus, AAV-SERCA gene therapy is an exciting new avenue to potentially pursue for treating human dystrophic or myopathic diseases.
Figure 8. AAV9-SERCA2a gene therapy mitigates disease in Sgcd–/– gastrocnemius.
(A) Representative histological H&E stain of gastrocnemius from injected neonates taken 6 weeks later given control AAV9-GFP or experimental AAV9-SERCA2a. Original magnification, ×200. (B) Quantitation of myofiber central nucleation in the 2 groups taken from 4 mice analyzed. *P < 0.05 versus GFP.
Discussion
Loss of sarcolemmal stability associated with mutations in genes comprising or affecting components of the DGC has been established as a key insult leading to the development of MD (22, 28, 29). Dysregulated and enhanced Ca2+ influx across an unstable sarcolemma has been proposed to directly program myofiber degeneration and produce disease (3, 21). For example, elevated cytosolic Ca2+ can enhance Ca2+-dependent protease activity (calpain) and mitochondrial Ca2+ overload–induced cell death as well as increase reactive oxygen and nitrogen species to mediate myofiber death (3, 7, 11, 21, 30). In support of this hypothesis, a number of groups have reported abnormally high levels of intracellular Ca2+ in dystrophic myotubes and adult myofibers isolated from dystrophic muscle cells (4–6). However, other groups failed to observe elevated free cytosolic Ca2+ in similar muscle preparations (8, 9). By comparison, studies focusing on cardiomyopathy in sarcoglycan-deficient animals are also consistent with the Ca2+ hypothesis of disease, as the L-type Ca2+ inhibitor verapamil reduced some aspects of heart disease in this mouse model, as did gene therapy with a dominant negative phospholamban protein (enhances SERCA2a function and Ca2+ clearance) in a hamster model (31, 32).
More recent evidence in genetically modified mouse models has further buttressed the Ca2+ hypothesis of disease in MD. For example, overexpression of the Ca2+/Na+ permeable TRPC3 channel in skeletal muscle of Tg mice produced all the histological and biochemical features of MD without membrane instability (10). These mice exhibited enhanced store-operated calcium entry (SOCE) that is also observed in other models of MD due to mutations in DGC components (10). Moreover, this same study also demonstrated that inhibition of endogenous TRPC channels with a dominant negative encoding TRPC6 transgene could significantly attenuate the dystrophic phenotype in 2 separate mouse models of MD (10). Iwata et al. also reported that inhibition of Ca2+ influx via TRPV channels can mitigate the dystrophic phenotype (33). These data support the hypothesis that enhanced Ca2+ influx alone is sufficient to induce MD, either through membrane rupture events or activated channels. That the SERCA1 transgene dramatically attenuated dystrophic muscle disease in TRPC3 Tg mice not only basically proves that the effect is entirely Ca2+ dependent, but also strongly suggests that enhanced SR Ca2+ cycling and reuptake can directly counteract a membrane-leak effect in promoting myofiber degeneration.
Previous work performed in this laboratory demonstrated that increased cytosolic Ca2+ can promote mitochondrial matrix Ca2+ overload and necrotic cell death via MPTP opening events (14). For example, inhibition of MPTP opening by genetic deletion of cyclophilin D or use of Debio-025 prevented cell death and reduced the muscular disease phenotype in Sgcd–/–, Lama2–/–, and mdx mice (14). Consistent with a Ca2+-driven pathway to MPTP opening and cell death, we observed that mitochondria isolated from SERCA1/Sgcd–/– mice were protected from swelling that otherwise occurs in mitochondria isolated from Sgcd–/– muscle (Figure 7, A–C). In addition to activation of MPTP opening and calpain (12, 13), dysregulated Ca2+ entry can result in the generation of reactive oxygen and nitrogen species to further contribute to membrane instability and myofiber degeneration (34–36). Enhanced removal of Ca2+ with the SERCA1 transgene not only protected the mitochondria from swelling and reduced calpain activation, but also provided some degree of membrane protection as assayed by EBD uptake, suggesting that increased Ca2+ influx generates a “feed-forward” disease process that further weakens the sarcolemma.
Overexpression of SERCA1-enhanced skeletal muscle EC coupling, such as a significant decrease in resting Ca2+ levels, enhanced amplitude of the Ca2+ transient and faster Ca2+ clearance from the cytoplasm (Figure 1, D–F). Interestingly, cardiac overexpression of SERCA1 also resulted in similar enhancement in cardiac function at baseline (37). In dystrophic muscle, the kinetics of SR Ca2+ handling are likely depressed, potentially due to reduced SERCA activity (16–18, 38). Although, the mechanism of altered SERCA1 activity in MD is not currently known, we observed a 50% increase in sarcolipin mRNA in the quadriceps of Sgcd–/– mice (data not shown). Sarcolipin is a small SR membrane protein that inhibits SERCA1 activity. Indeed, Campanaro et al. also reported a similar increase in sarcolipin mRNA expression in muscle obtained from dysferlinopathy patients (39).
It is also very interesting to note that muscles spared of disease in mdx mice, the laryngeal muscles, show significant overexpression of SERCA1 with disease (40). More recently, in direct support of our study, AAV-SERCA1a gene transfer in mdx mice was shown to decrease centrally located nuclei in the diaphragm and to reduce eccentric contraction-induced damage (41). This study was particularly interesting, as the beneficial effect of the viral therapy lasted out to 6 months of age, suggesting that such an approach could provide sustained protection in humans (41). In our hands, direct injection of AAV9-SERCA2a in the gastrocnemius produced an astonishing protection from dystrophic disease in Sgcd–/– mice (Figure 8), again suggesting an entirely novel approach for gene therapy in humans with MD.
The experimental approaches discussed above suggested the hypothesis that enhanced Ca2+ removal directly protects against MD. If Ca2+ really is the “final common pathway” for myofiber necrosis downstream of most genetic mutations that induce MD, a universal therapy might easily involve approaches that augment cytosolic Ca2+ clearance. Here we utilized SERCA1 overexpression to increase the rates of Ca2+ reuptake into the SR to possibly reduce the effects associated with chronic Ca2+ leak from activated channels and membrane ruptures. In heart failure, a similar defect in membrane stability and Ca2+ handling has been observed and shown to underlie progressive cell death and worsening of disease (42, 43). In this context, SERCA2 overexpression by transgenesis or gene therapeutic strategies, or disinhibition of endogenous SERCA2 by deletion of phospholamban, dramatically protected mice from heart failure. Indeed, results of a recent gene therapy trial in heart failure patients with AAV-SERCA2a was reported to be effective (44). Thus, a gene therapeutic strategy with SERCA1/2 AAV might represent a “universal” approach to mitigate MD due to many types of gene mutations by correcting a final common defect in Ca2+ handling that appears to underlie most forms of MD.
Methods
Animals.
A modified human skeletal α-actinin promoter (a gift from Edna C. Hardeman, University of Sydney, Sydney, Australia) was used to create Tg mice overexpressing the rat skeletal muscle isoform of SERCA1 (provided by Muthu Periasamy, The Ohio State University, Columbus, Ohio, USA). Twelve Tg lines were generated, out of which 2 lines were analyzed for experiments. Sgcd–/– and TRPC3 Tg mice were described previously (10, 24). Use of animals in this study was approved by the IACUC at the Cincinnati Children’s Hospital.
Mitochondrial isolation and swelling assay.
Mitochondria from adult skeletal muscle hind limbs were isolated as described previously (45). Briefly, mice were sacrificed and hind limb skeletal muscle was removed, minced, and placed in homogenization buffer supplemented with 1 mg/ml trypsin, stirred for 30 minutes at 4°C, and washed and homogenized in a glass-teflon homogenizer. The muscle extract was then subjected to differential centrifugation to isolate mitochondria. Light scattering was measured using 250 μg of mitochondria suspended in 1 ml of buffer, and 200 μM CaCl2 or 5% PEG (w/v) was used to induce mitochondrial swelling and shrinking, respectively. Change in absorbance at 540 nm was recorded every 10 seconds for up to 10 minutes.
Western blotting.
Muscles were isolated, and extracts were prepared by homogenization in lysis buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl, and 1 mM EDTA. Extracts were centrifuged at 13,000 g for 10 minutes, and 5–25 μg of protein was separated on SDS–8% polyacrylamide gels for Western blotting and chemiluminescent detection (Amersham Bioscience). SERCA1 was detected using mouse monoclonal antibody (1:2500) from Affinity Bioreagents.
Histological analysis.
Muscles were paraffin embedded, and 7-μM histological sections were cut at the center of the muscle and stained with either H&E or Masson’s trichrome. Interstitial fibrotic regions were quantified using metamorph analysis of the percentage of blue area in Masson’s trichrome sections.
Calpain activity assay.
Calpain activity from quadriceps was assessed using a manufacturer-recommended protocol (Abcam). In brief, muscles were lysed in extraction buffer and spun at 20,817 g to generate supernatant for analysis. Then 250 μg of protein was incubated in calpain substrate for 1 hour at 37°C. Samples were read in triplicates in a plate reader equipped with a 400-nm excitation filter and 505-nm emission filter. Both positive and negative controls were performed as suggested by the manufacturer.
EBD uptake.
Animals were placed in cages fitted with running wheels for 5 days of voluntary running (all mice ran equivalent rotations per day). On the fifth day, mice were injected with EBD (10 mg/ml, 0.1 ml/10 g body weight) and allowed to run for 48 additional hours. Mice were then sacrificed and quadriceps, TA, diaphragm, soleus, and gastrocnemius were embedded in OCT and snap frozen in liquid nitrogen for viewing as previously described (14).
SR Ca2+ uptake.
Three-month-old mice were sacrificed, and the quadriceps were surgically removed and immediately homogenized in 50 mM KH2PO4 (pH 7.0), 10 mM NaF, 1 mM EDTA, 0.3 M sucrose, 0.3 mM phenylmethylsulfonylfluoride, and 0.5 mM dithiothreitol. Ca45 uptake in muscle homogenates (0.1 mg/ml) was measured by a modification of the Millipore filtration technique as previously described (46). The rates of Ca2+ uptake were calculated by least-squares linear regression analysis at 30-, 60-, and 90-second values of Ca2+ uptake. The data were analyzed by nonlinear regression using ORIGIN (version 6.0) software.
Measurement of Ca2+ transients.
FDB fibers were isolated as previously described (47) and equilibrated in normal Ringer solution (140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, pH to 7.4) for approximately 15 minutes. Fibers were loaded with 5 μM Fura-2 AM by incubating at room temperature for 45 minutes. Ca2+ transients were evoked via field stimulation using platinum electrodes at a stimulation frequency of 0.2 Hz. The peak of the electrically evoked Ca2+ transient was recorded. The decay phase of the Ca2+ transient was analyzed as a parameter of Ca2+ reuptake from the cytosol. Fura 2-AM excitation ratio (340/380 nm) was recorded on a Nikon Ti-U inverted microscope equipped with a photomultiplier tube from Photon Technology Inc. Locally applied 500 μM 4-CMC bolus was used to assess RyR-sensitive SR Ca2+ load at the end of a train of electrically evoked Ca2+ transients. The peak 4-CMC response was analyzed to determine SR Ca2+ load.
Forced treadmill running.
Three-month-old mice were placed in individual lanes of an electrically driven 4-lane treadmill (Omni-Pacer LC4/M; Columbus Instruments International) at the speed of 6 m/min for 3 minutes. The treadmill measured 19 inches wide by 20 inches in length, with a conveyor-type belt for running lanes measuring 3 inches wide by 12 inches long. A training regimen was first instituted for 10 minutes to familiarize them with the environment and shock grids adjustable from 0–2.0 mA. The speed was increased in increments of 2 m/min every 3 minutes to a maximum speed of 18 m/min. Exhaustion was assessed as greater than 5 consecutive seconds on the shock grid without attempting to reengage the treadmill. Time spent on the treadmill before exhaustion or time to complete the protocol was recorded as average maximum time spent in exercise. The treadmill regimen was performed from the flat position (no incline or decline).
AAV9 production and injection.
AAV9-CMV-GFP and AAV9-CMV-SERCA2a were produced using the 2-plasmid protocol and were grown in triple flasks for 24 hours (DMEM, 10% FBS) prior to adding the calcium phosphate precipitate (48). After 72 hours, the virus was purified from benzonase-treated cell crude lysates over an iodixanol density gradient (OptiPrep; Greiner Bio-One Inc.), followed by heparin-agarose type I affinity chromatography (Sigma-Aldrich). Finally, viruses were concentrated and formulated into lactated Ringer’s solution (Baxter Healthcare Corp.) using Vivaspin 20 Centrifugal concentrators 50K MWCO (Vivascience Inc.) and stored at –80°C. AAV9-SERCA2a and AAV9-GFP were injected into the left and right gastrocnemius, respectively, of 3-day-old Sgcd–/– mice, amounting to approximately 1010 viral particles. Mice were sacrificed at 6 weeks and the gastrocnemius from both sides was processed, embedded, sectioned, and stained with H&E, or cryosections were generated for GFP fluorescence assessment.
Statistics.
All results are presented as mean ± SEM. Statistical analysis was performed with unpaired 2-tailed t test (for 2 groups) and 1-way ANOVA with Bonferroni correction (for groups of 3 or more). P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (to J.D. Molkentin, R.J. Hajjar, and E.G. Kranias). J.D. Molkentin was also supported by grants from the Jain Foundation and the Howard Hughes Medical Institute. S.A. Goonasekera was supported by a local affiliate American Heart Association grant.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(3):1044–1052. doi:10.1172/JCI43844.
References
- 1. Muir LA, Chamberlain JS. Emerging strategies for cell and gene therapy of the muscular dystrophies. Expert Rev Mol Med. 2009;11:e18. doi: 10.1017/S1462399409001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan JC. Gene table of monogenic neuromuscular disorders (nuclear genome only). Neuromuscul Disord. 2009;19(1):77–98. doi: 10.1016/j.nmd.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3. Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88(2):83–91. doi: 10.1139/Y09-058. [DOI] [PubMed] [Google Scholar]
- 4. Fong PY, Turner PR, Denetclaw WF, Steinhardt RA. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990;250(4981):673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- 5. Fraysse B, et al. The alteration of calcium homeostasis in adult dystrophic mdx muscle fibers is worsened by a chronic exercise in vivo. Neurobiol Dis. 2004;17(2):144–154. doi: 10.1016/j.nbd.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 6. Turner PR, Fong PY, Denetclaw WF, Steinhardt RA. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991;115(6):1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner PR, Westwood T, Regen CM, Steinhardt RA. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- 8. De Backer F, Vandebrouck C, Gailly P, Gillis JM. Long-term study of Ca(2+) homeostasis and of survival in collagenase-isolated muscle fibres from normal and mdx mice. J Physiol. 2002;542(pt 3):855–865. doi: 10.1113/jphysiol.2002.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gailly P, Boland B, Himpens B, Casteels R, Gillis JM. Critical evaluation of cytosolic calcium determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium. 1993;14(6):473–483. doi: 10.1016/0143-4160(93)90006-r. [DOI] [PubMed] [Google Scholar]
- 10. Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, Molkentin JD. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106(45):19023–19028. doi: 10.1073/pnas.0906591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006;33(7):657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 12. Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270(18):10909–10914. doi: 10.1074/jbc.270.18.10909. [DOI] [PubMed] [Google Scholar]
- 13. Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet. 2002;11(21):2645–2655. doi: 10.1093/hmg/11.21.2645. [DOI] [PubMed] [Google Scholar]
- 14. Millay DP, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14(4):442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossi AE, Dirksen RT. Sarcoplasmic reticulum: the dynamic calcium governor of muscle. Muscle Nerve. 2006;33(6):715–731. doi: 10.1002/mus.20512. [DOI] [PubMed] [Google Scholar]
- 16. Divet A, Huchet-Cadiou C. Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflugers Arch. 2002;444(5):634–643. doi: 10.1007/s00424-002-0854-5. [DOI] [PubMed] [Google Scholar]
- 17. Divet A, Lompre AM, Huchet-Cadiou C. Effect of cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca-ATPase, on skeletal muscles from normal and mdx mice. Acta Physiol Scand. 2005;184(3):173–186. doi: 10.1111/j.1365-201X.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- 18. Kargacin ME, Kargacin GJ. The sarcoplasmic reticulum calcium pump is functionally altered in dystrophic muscle. Biochim Biophys Acta. 1996;1290(1):4–8. doi: 10.1016/0304-4165(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 19. Bellinger AM, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15(3):325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fauconnier J, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107(4):1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88(2):83–91. doi: 10.1139/Y09-058. [DOI] [PubMed] [Google Scholar]
- 22. Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol. 2007;36(1):1–7. doi: 10.1016/j.pediatrneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 23. Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12(3):349–361. doi: 10.1016/S0959-437X(02)00309-X. [DOI] [PubMed] [Google Scholar]
- 24. Hack AA, et al. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J Cell Sci. 2000;113(pt 14):2535–2544. doi: 10.1242/jcs.113.14.2535. [DOI] [PubMed] [Google Scholar]
- 25. Hollingworth S, Zeiger U, Baylor SM. Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J Physiol. 2008;586(pt 21):5063–5075. doi: 10.1113/jphysiol.2008.160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DiFranco M, Woods CE, Capote J, Vergara JL. Dystrophic skeletal muscle fibers display alterations at the level of calcium microdomains. Proc Natl Acad Sci U S A. 2008;105(38):14698–14703. doi: 10.1073/pnas.0802217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woods CE, Novo D, DiFranco M, Vergara JL. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol. 2004;557(pt 1):59–75. doi: 10.1113/jphysiol.2004.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ervasti JM, Sonnemann KJ. Biology of the striated muscle dystrophin-glycoprotein complex. Int Rev Cytol. 2008;265:191–225. doi: 10.1016/S0074-7696(07)65005-0. [DOI] [PubMed] [Google Scholar]
- 29. Turk R, et al. Common pathological mechanisms in mouse models for muscular dystrophies. FASEB J. 2006;20(1):127–129. doi: 10.1096/fj.05-4678fje. [DOI] [PubMed] [Google Scholar]
- 30. Gissel H. The role of Ca2+ in muscle cell damage. Ann N Y Acad Sci. 2005;1066:166–180. doi: 10.1196/annals.1363.013. [DOI] [PubMed] [Google Scholar]
- 31. Cohn RD, Durbeej M, Moore SA, Coral-Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J Clin Invest. 2001;107(2):R1–R7. doi: 10.1172/JCI11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoshijima M, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8(8):864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 33. Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet. 2009;18(5):824–834. doi: 10.1093/hmg/ddn408. [DOI] [PubMed] [Google Scholar]
- 34. Durham WJ, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133(1):53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase - role in Duchenne muscular dystrophy. J Cell Sci. 2008;121(pt 13):2246–2255. doi: 10.1242/jcs.032003. [DOI] [PubMed] [Google Scholar]
- 36. Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586(7):2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Talukder MA, et al. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293(4):H2418–H2428. doi: 10.1152/ajpheart.00663.2007. [DOI] [PubMed] [Google Scholar]
- 38. Tutdibi O, Brinkmeier H, Rudel R, Fohr KJ. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J Physiol. 1999;515(pt 3):859–868. doi: 10.1111/j.1469-7793.1999.859ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campanaro S, et al. Gene expression profiling in dysferlinopathies using a dedicated muscle microarray. Hum Mol Genet. 2002;11(26):3283–3298. doi: 10.1093/hmg/11.26.3283. [DOI] [PubMed] [Google Scholar]
- 40. Ferretti R, Marques MJ, Pertille A, Santo Neto H. Sarcoplasmic-endoplasmic-reticulum Ca2+-ATPase and calsequestrin are overexpressed in spared intrinsic laryngeal muscles of dystrophin-deficient mdx mice. Muscle Nerve. 2009;39(5):609–615. doi: 10.1002/mus.21154. [DOI] [PubMed] [Google Scholar]
- 41. Morine K, Sleeper MM, Barton ER, Sweeney L. Overexpression of SERCA1a in the mdx diaphragm reduces susceptibility to contraction induced damage [published online ahead of print June 11, 2010]. Hum Gene Ther. doi: 10.1089/hum.2010.077. doi: 10.1089/hum.2010.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell Biochem. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- 43. Townsend D, et al. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. J Clin Invest. 2010;120(4):1140–1150. doi: 10.1172/JCI41329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Celladon Corp. announces that MYDICAR meets primary endpoint in phase 2 trial for treatment of advanced heart failure [press release]. La Jolla, California, USA: Celladon Corp.; April 28, 2010. http://www.celladon.net/index.php?option=com_content&view=article&id=74&Itemid=88 . [Google Scholar]
- 45. Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2(2):287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 46. Slack JP, Grupp IL, Ferguson DG, Rosenthal N, Kranias EG. Ectopic expression of phospholamban in fast-twitch skeletal muscle alters sarcoplasmic reticulum Ca2+ transport and muscle relaxation. J Biol Chem. 1997;272(30):18862–18868. doi: 10.1074/jbc.272.30.18862. [DOI] [PubMed] [Google Scholar]
- 47. Lueck JD, et al. Chloride channelopathy in myotonic dystrophy resulting from loss of posttranscriptional regulation for CLCN1. Am J Physiol Cell Physiol. 2007;292(4):C1291–C1297. doi: 10.1152/ajpcell.00336.2006. [DOI] [PubMed] [Google Scholar]
- 48. Suckau L, et al. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119(9):1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.