Abstract
The regulation of neutrophil lifespan by induction of apoptosis is critical for maintaining an effective host response and preventing excessive inflammation. The hypoxia-inducible factor (HIF) oxygen-sensing pathway has a major effect on the susceptibility of neutrophils to apoptosis, with a marked delay in cell death observed under hypoxic conditions. HIF expression and transcriptional activity are regulated by the oxygen-sensitive prolyl hydroxylases (PHD1–3), but the role of PHDs in neutrophil survival is unclear. We examined PHD expression in human neutrophils and found that PHD3 was strongly induced in response to hypoxia and inflammatory stimuli in vitro and in vivo. Using neutrophils from mice deficient in Phd3, we demonstrated a unique role for Phd3 in prolonging neutrophil survival during hypoxia, distinct from other hypoxia-associated changes in neutrophil function and metabolic activity. Moreover, this selective defect in neutrophil survival occurred in the presence of preserved HIF transcriptional activity but was associated with upregulation of the proapoptotic mediator Siva1 and loss of its binding target Bcl-xL. In vivo, using an acute lung injury model, we observed increased levels of neutrophil apoptosis and clearance in Phd3-deficient mice compared with WT controls. We also observed reduced neutrophilic inflammation in an acute mouse model of colitis. These data support what we believe to be a novel function for PHD3 in regulating neutrophil survival in hypoxia and may enable the development of new therapeutics for inflammatory disease.
Introduction
Therapeutic agents that selectively limit neutrophil lifespan during inflammation are a major unmet medical need, since persistent and inappropriate activation of neutrophils is central to the pathogenesis of a number of inflammatory diseases. In contrast, neutropenia and defective neutrophil function are associated with catastrophic impairment of host defense. A fine balance exists therefore between the requirement for fully competent neutrophils at the onset of the inflammatory response and the need for their prompt deactivation and/or removal to limit tissue damage. Neutrophil apoptosis is recognized as a major mechanism for the clearance of effete neutrophils from inflammatory foci, and, in vivo, acceleration of neutrophil apoptosis enhances resolution of neutrophilic inflammation (1). Since many proinflammatory mediators inhibit neutrophil apoptosis, the selective removal of these cells, after they have discharged their phagocytic and bactericidal functions, represents an attractive antiinflammatory strategy, with the potential to limit the side effects associated with existing antiinflammatory drugs while preserving basal host defense functions.
A key regulator of neutrophil apoptosis is the HIF-1α subunit of the oxygen-sensitive transcription factor hypoxia-inducible factor (HIF) (2). HIF effector genes increase tissue oxygen delivery and adaptation to anaerobic metabolism in all cell types (3). In myeloid cells, HIF-1α deficiency reduces cell aggregation, motility, invasiveness, and bacterial killing (4). HIF therefore regulates many aspects of the adaptive response of neutrophils to hypoxia during inflammation and, uniquely among nontransformed cells, extends neutrophil lifespan by delay of apoptosis (2). HIF also regulates myeloid responses to proinflammatory stimuli (5, 6) with a critical role in the neutrophil’s ability to phagocytose and kill bacteria even under normoxic conditions. Therefore, direct targeting of HIF may lack the precision required of novel treatments for inflammatory disease.
The HIF-α subunit is regulated by hydroxylation, both by a family of proyl hydroxylase domain–containing enzymes (PHDs, also known as EGL-N1-3; refs. 7, 8), leading to ubiquitination and proteasomal degradation, and by transcriptional inactivation following asparaginyl hydroxylation by factor inhibiting HIF (FIH) (9). The enzymatic function of PHDs and FIH is believed to be critical for inhibition of HIF transcriptional activity in vivo (9–12). To date, 4 PHD proteins with HIF hydroxylation activity have been described (PHD1, PHD2, PHD3, and P4H-TM) with wide but varied tissue expression (13, 14). While PHD enzymes are major regulators of HIF activity, there is also increasing evidence of roles for individual PHDs in regulating cellular processes independently of HIF-1 interactions (11, 12, 15–17).
Since HIF has such an important role in neutrophil survival, we proposed that PHD enzymes are fundamentally involved in this process. More specifically, given our original description of hypoxia-mediated neutrophil survival (18, 19), the previously described hypoxic induction of PHD2 and PHD3 by HIF, and our initial observation of selective regulation of PHD3 by neutrophil hypoxia, aging, TLR agonists, and chronic inflammatory disease, we hypothesized that PHD3 may be an important regulator of neutrophil function and fate.
Results
PHD3 is differentially regulated in human neutrophils by pro-survival stimuli.
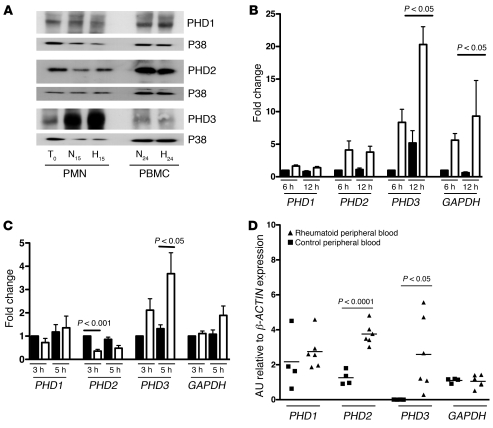
Human peripheral blood neutrophils constitutively express PHD1, PHD2, and PHD3 proteins (Figure 1A). Neutrophil PHD3 protein expression was markedly increased by time in culture and further increased by hypoxic conditions, whereas expression of PHD1 and PHD2 proteins was unaltered (Figure 1A). Sustained culture in hypoxic conditions, at an oxygen tension that induced the HIF target gene GAPDH in neutrophils, induced significantly greater increases in PHD3 transcript abundance than PHD1 or PHD2 (Figure 1B). PHD3 transcript was also the most responsive to stimulation of neutrophils with the NOD agonist peptidoglycan (ref. 20 and Figure 1C). PHD3 (and PHD2) was also significantly upregulated in peripheral blood neutrophils isolated from individuals with active rheumatoid arthritis (Figure 1D). PHD3 was thus inducible both by hypoxia and inflammatory stimulation, suggesting a role for PHD3 in regulating neutrophil function and lifespan.
Figure 1. PHD3 is upregulated in human neutrophils in response to hypoxia and proinflammatory stimuli.
(A) Expression of PHD1, -2, and -3. Human peripheral blood PMNs and PBMCs were lysed at time 0 or following 15 hours (PMNs) or 24 hours (PBMCs) culture in normoxia (N) or hypoxia (H) and then protein separated by SDS-PAGE. Blots shown are representative of n = 9 experiments, with p38-MAPK loading controls. (B) Induction of PHD3 by hypoxia. Human PMNs were aged for 6 or 12 hours in normoxia (black bars) or hypoxia (white bars). TaqMan analysis of cDNA was performed with data normalized to β-ACTIN expression. Data show fold change with respect to normoxic samples at 6 hours (n = 6). (C) Peptidoglycan induces PHD3. Human PMNs were cultured in the presence (white bars) or absence (black bars) of peptidoglycan (10 μg/ml) for 3 or 5 hours. TaqMan analysis of cDNA was performed with data normalized to β-ACTIN. Data shown represent fold change with respect to normoxic samples at 3 hours (n = 5). (D) PMNs from patients with rheumatoid arthritis show increased PHD2 and PHD3 transcript levels. TaqMan analysis of cDNA from freshly isolated peripheral blood PMNs of 6 healthy controls (squares) and 6 individuals with active rheumatoid arthritis (triangles) normalized to β-ACTIN. Significant P values only are shown for healthy compared with rheumatoid neutrophil expression.
Phd3-null mice have a normal peripheral blood phenotype.
To determine whether Phd3 influences basal neutrophil numbers or function, we studied peripheral blood neutrophils from mice deficient in Phd3 (21) and compared them with those from WT mice. Phd3-deficient animals were viable and healthy and had normal differential white cell counts, and, specifically, normal numbers of neutrophils. Furthermore, they mounted a normal peripheral blood neutrophil response to zymosan-mediated peritonitis (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI43273DS1).
Phd3–/– neutrophils have preserved function in normoxia and hypoxia.
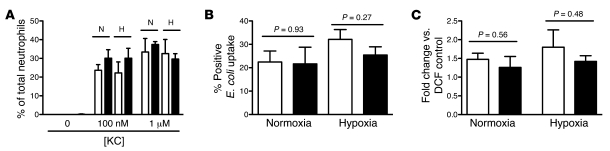
Peripheral blood neutrophils isolated from Phd3-null and WT mice were used in a panel of assays to investigate the effects of Phd3 loss on neutrophil function. Phd3–/– neutrophils migrated normally toward the chemokine KC during both normoxia and hypoxia (Figure 2A). Likewise, coculture of Phd3–/– or WT neutrophils with heat-inactivated E. coli resulted in equivalent uptake of bacteria in both normoxia and hypoxia (Figure 2B). The ability of Phd3–/– neutrophils to undergo respiratory burst following stimulation with heat-inactivated E. coli was also preserved (Figure 2C). Unlike the previously reported effect of HIF-1α deletion in lowering neutrophil intracellular ATP levels (4), we found no significant differences in either intracellular glucose or ATP concentration in Phd3–/– versus WT neutrophils (Supplemental Figure 1, B and C).
Figure 2. Phd3–/– neutrophils function normally in vitro.
Murine peripheral blood PMNs from WT (white bars) and Phd3–/– (black bars) animals were studied. (A) Chemotaxis toward 0–1 μM KC was equivalent in normoxic and hypoxic conditions (n = 4). (B) Phagocytosis. The percentage of PMNs ingesting heat-inactivated Alexa Fluor 488–labeled E. coli (MOI of 1:1) was determined by flow cytometry (n = 3). (C) Respiratory burst. PMNs were preincubated in normoxia or hypoxia; DCF was added, then heat inactivated E. coli (MOI of 10:1 E. coli/neutrophil). Change in DCF emission was determined by flow cytometry. Data represent fold change in geometric mean fluorescence from DCF-only controls (n = 5).
Loss of Phd3 results in a selective loss of hypoxic neutrophil survival.
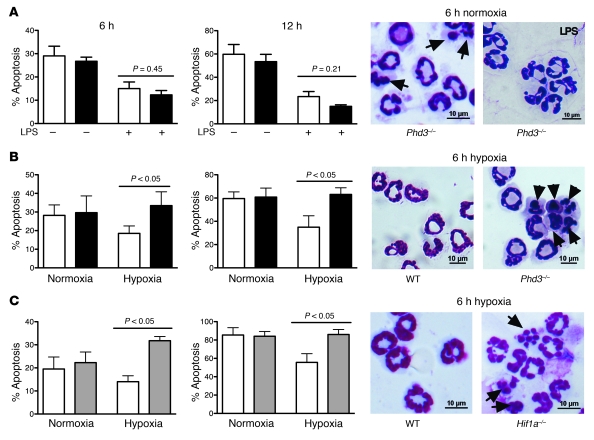
We next determined the role of Phd3 in neutrophil pro-survival pathways. Neutrophils were cultured in the presence or absence of known survival stimuli and rates of apoptosis determined. In keeping with multiple studies of human neutrophils, murine neutrophils underwent constitutive apoptosis, but at a slightly faster rate, with 20%–30% of WT neutrophils apoptotic at 6 hours and 60%–80% at 12 hours (Figure 3, A–C). WT and Phd3–/– neutrophils showed a similar survival response to the TLR4 agonist LPS, equivalent to that of human neutrophils (ref. 22 and Figure 3A). Hypoxia also prolonged WT neutrophil survival, again as in human neutrophils (18, 19), but this survival response was completely absent in Phd3–/– neutrophils (Figure 3B). The observed loss of survival mirrors the loss of hypoxic survival we previously reported in bone marrow–derived Hif1a–/– neutrophils (2) and now confirm in peripheral blood neutrophils from these animals (Figure 3C). The morphological phenotype of loss of hypoxic neutrophil survival in Phd3–/– neutrophils was verified by two biochemical assays, which demonstrated increases in both annexin V/TO-PRO-3 positivity and caspase activity, as determined by increased cleavage of the synthetic caspase substrate FITC-VAD (Supplemental Figure 2).
Figure 3. Selective loss of hypoxic survival in Phd3–/– neutrophils.
Murine peripheral blood PMNs were isolated from WT (white bars), Phd3–/– (black bars), and Hif1a–/– (gray bars) animals. Representative cytospins are shown, with apoptotic cells highlighted by arrowheads and apoptosis assessed by morphology (A–C) (original magnification, ×1,000). (A) LPS-mediated survival. PMNs were cultured in the presence/absence of LPS (100 ng/ml) for 6 or 12 hours (n = 4). (B and C) PMNs from WT, Phd3–/–, and Hif1a–/– animals were cultured in normoxia or hypoxia for 6 or 12 hours (n = 4).
Since Phd3-mediated effects on cell survival may be Hif-2α or Hif-1α dependent (21), we sought but found no evidence of altered Hif1a or Hif2a transcriptional upregulation of target genes in Phd3–/– neutrophils. Glut1 (Supplemental Figure 3A) and Gapdh (Supplemental Figure 3B) were upregulated to a similar extent in WT and Phd3–/– neutrophils following hypoxic stimulation. Of note, we saw no compensatory change in expression of other PHD isoforms in neutrophils isolated from the Phd3–/– mice (Supplemental Figure 3C). Hif1a–/– neutrophils showed significantly less Phd3 upregulation in hypoxia (Supplemental Figure 3D), raising the possibility that loss of PHD3 specifically impairs hypoxic survival in these cells.
PHD3-dependent hypoxic neutrophil survival alters expression of Siva1 and Bcl-xL.
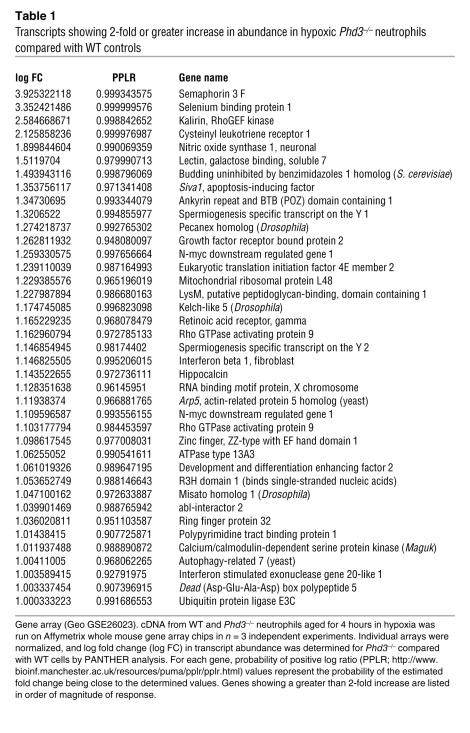
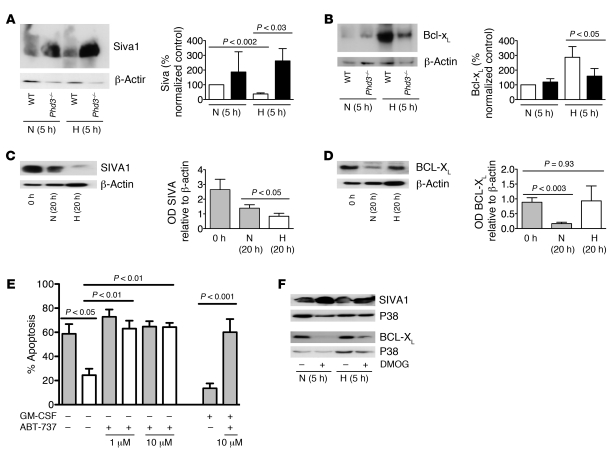
Given the preserved HIF transcriptional responses to hypoxia in Phd3–/– neutrophils, a gene array approach was undertaken to identify other regulators of neutrophil lifespan that might be altered in Phd3–/– cells. Using a whole mouse genome Affymetrix array, we compared transcript abundance in WT and Phd3–/– neutrophils exposed to hypoxia for 4 hours (data available via GEO accession number GSE26023). In keeping with our previous observations in human neutrophils, murine neutrophils expressed a relatively select set of genes (2). We identified 39 genes that were upregulated (Table 1) and 52 genes (excluding replicates) downregulated (Supplemental Table 1) in Phd3–/– compared with WT neutrophils in hypoxia, the gene downregulated to the greatest extent in Phd3–/– neutrophils being Phd3 itself. We also identified downregulation of Kif-1bβ a recently described PHD3 target modulating neuronal cell apoptosis (11), but KIF-1Bβ protein expression in murine neutrophils was not altered by aging or hypoxia (data not shown). A proapoptotic factor, Siva1 (23), showed 2.3-fold greater expression in Phd3–/– compared with WT neutrophils (verified by quantitative PCR). WT neutrophils cultured in vitro showed reduced SIVA1 protein in hypoxia compared with normoxia, whereas levels were significantly higher in Phd3–/– neutrophils (Figure 4A). Intriguingly, we had previously shown SIVA1 is downregulated by hypoxia in human peripheral blood neutrophils (2). SIVA1 is a proapoptotic protein that binds BCL-XL and inhibits BCL-XL–mediated enhancement of cell survival (24). BCL-XL is expressed in human neutrophils following inflammatory mediator stimulation in vitro (25) and in exudative neutrophils in vivo (26), but the role of BCL-XL in regulating neutrophil apoptosis has not been studied. Bcl-xL is upregulated in hypoxia-stimulated WT neutrophils, but this is significantly reduced in Phd3–/– cells (Figure 4B). Further confirming the importance of SIVA1 and BCL-XL expression in determining neutrophil lifespan, we found downregulation of SIVA1 in human neutrophils in hypoxia compared with normoxia (Figure 4C), while the loss of BCL-XL observed with time in culture in normoxic cultures did not occur in hypoxia (Figure 4D). The dependence of neutrophils on BCL-XL expression for hypoxic survival was confirmed using ABT-737, a small molecule BH3 mimetic with specificity for BCL-XL, BCL-2, and BCL-W proteins (27), of which only BCL-XL is expressed in neutrophils (25, 26). ABT-737 completely abrogated the enhanced survival of neutrophils under hypoxia at a concentration that also reversed the survival effect of GM-CSF, a pro-survival stimulus known to induce BCL-XL (ref. 28 and Figure 4E), but was without effect upon constitutive apoptosis. In addition a pan-hydroxylase inhibitor, DMOG, previously shown to inhibit PHD3 and other dioxygenases (29), mimicked in human neutrophils the changes in SIVA1/BCL-XL protein levels seen in Phd3–/– compared with WT murine neutrophils (Figure 4F). Collectively, these data establish what we believe to be novel roles for SIVA1 and BCL-XL in regulating survival of both murine and human neutrophils in hypoxia.
Table 1 .
Transcripts showing 2-fold or greater increase in abundance in hypoxic Phd3–/– neutrophils compared with WT controls
Figure 4. Phd3 modifies Siva1 and Bcl-xL expression.
(A and B) Murine peripheral blood neutrophil protein expression. PMNs from WT and Phd3–/– animals were aged for 5 hours in normoxia or hypoxia. Total protein was separated by SDS-PAGE and densitometry performed relative to β-actin for Phd3–/– (black bars) compared with WT (white bars) lysates in normoxia and hypoxia (n = 6). (C and D) Human peripheral blood neutrophil protein expression. PMNs were aged for 20 hours in normoxia or hypoxia. Total protein was separated by SDS-PAGE and densitometry performed relative to β-actin for normoxia (gray bars) and hypoxia (white bars) (n = 7). (E) BCL-XL mediates human neutrophil survival. PMNs were preincubated with the BH3 mimetic ABT-737 (1–10 μM), then cultured in normoxia (gray bars) with or without GM-CSF (500 U/ml) or hypoxia (white bars) for 20 hours, and apoptosis was assessed by morphology (n = 4). (F) The hydroxylase inhibitor DMOG modifies SIVA1 and BCL-XL expression. PMNs were aged for 5 hours in normoxia or hypoxia with or without DMOG (100 μM), and total protein was separated by SDS-PAGE.
Phd3 mediates hypoxic neutrophil survival in acute lung injury and colitis.
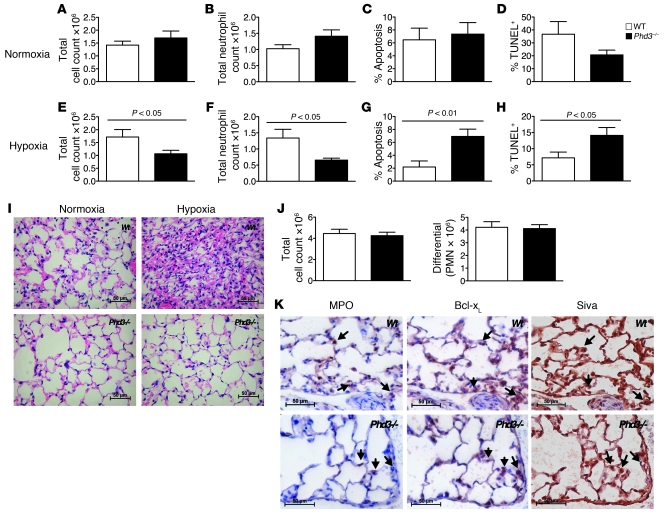
To investigate the importance of Phd3 in regulating neutrophilic inflammation in vivo, we used a well-characterized model of LPS-induced acute lung injury, which induces rapid neutrophil influx to the lungs followed by spontaneous resolution (30). Given the selectivity of Phd3 for hypoxic neutrophil survival, this model was studied in both hypoxia and normoxia. There were no significant differences in bronchoalveolar lavage (BAL) total cell counts, total neutrophil counts, morphologically scored neutrophil apoptosis, or TUNEL-positive events between WT and Phd3-null mice recovered in normoxia (Figure 5, A–D). However, in ambient hypoxia (0.1 FiO2), there was a significant reduction in total cell counts and neutrophil counts in Phd3-null mice, with a concomitant increase in both morphologic neutrophil apoptosis and TUNEL-positive events (Figure 5, E–H). This correlated with a reduction in the characteristic neutrophil-dominant pulmonary inflammation as detailed by histology (Figure 5I). There were no significant differences in BAL macrophage or lymphocyte counts in WT compared with Phd3–/– mice in normoxia or hypoxia. The reduction in total neutrophil count was independent of neutrophil recruitment in the first 6 hours (Figure 5J), a time point at which phenotypic differences in neutrophil recruitment are revealed (31, 32). Immunohistochemical staining of serial lung sections colocalized myeloperoxidase-stained (MPO-stained) neutrophils with both Bcl-xL and Siva1 staining (Figure 5K), although other cell types do also express these proteins, in keeping with previous data (33, 34).
Figure 5. Enhanced resolution of neutrophilic inflammation in Phd3–/– animals in hypoxia.
(A–I) Acute lung injury was induced by intratracheal LPS installation. Six hours after LPS, animals were recovered in environmental normoxia (21%) or hypoxia (10%) for 18 hours. BAL samples were assessed for total cell counts (A and E), total neutrophil counts (B and F), percent morphological neutrophil apoptosis (C and G), and percent TUNEL-positive PMNs (D and H) in WT (white bars) versus Phd3-null (black bars) mice for both normoxic recovery (A–D) and hypoxic recovery (E–H) (n = 9). (I) Animals were sacrificed at 18 hours, lungs fixed with 10% buffered formalin and paraffin-embedded, and sections stained with hematoxylin and eosin; original magnification, ×400. (J) Acute lung injury was induced by nebulized LPS and BAL samples assessed at 6 hours for total cell and neutrophil differential counts in WT (white bars) versus Phd3-null (black bars) mice (n = 4). (K) Bcl-xL and Siva1 expression by inflammatory cells. Serial paraffin-embedded sections from hypoxia-recovered mice were stained for expression of the neutrophil marker MPO, Bcl-xL, and Siva1, and individual MPO-positive matched cells are indicated by arrows; original magnification, ×400.
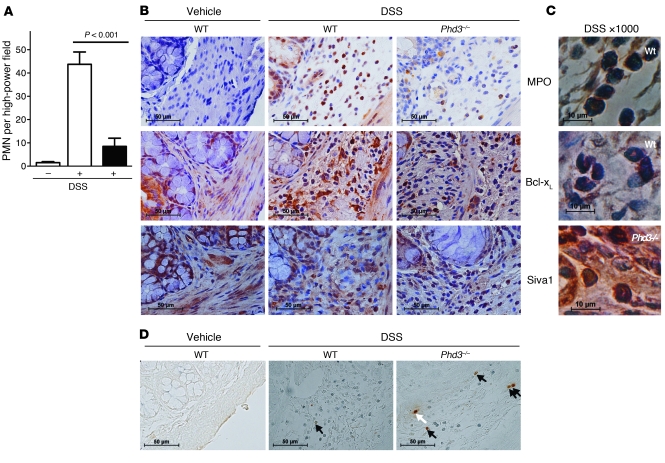
Dextran sulfate sodium–induced (DSS-induced) colitis is an in vivo model of inflammation where neutrophils are prominent and that is associated with a marked degree of tissue hypoxia (35). Using the acute 6-day time point, we determined the number of neutrophils present in the intestinal mucosa using immunohistochemical staining. We observed significantly fewer MPO-positive cells, i.e., neutrophils, in the intestinal mucosa of Phd3-null compared with WT mice (Figure 6, A and B). In keeping with our observations in the lung injury model, the submucosal regions of the bowel, where the inflammatory cell infiltrate is mostly seen, displayed fewer MPO-positive cells and less intense Bcl-xL staining in Phd3–/– than WT sections. Siva1 was expressed at high levels in all sections (Figure 6B), in keeping with its known tissue expression (34). Examination of cells at higher power clearly showed that Bcl-xL and Siva1 staining was associated with cells with the morphologic appearance of neutrophils (Figure 6C). Apoptosis detection by TUNEL staining revealed more frequent apoptotic cells in the inflammatory regions of the Phd3–/– compared with the WT sections (Figure 6D). These experiments again support an important role for Phd3 in regulating neutrophil persistence in inflammation.
Figure 6. Reduced neutrophilic inflammation in Phd3–/– animals in colitis.
Six days following DSS diet-induced colitis, colonic sections from WT (white bars), and Phd3–/– (black bars) mice were harvested and fixed in paraffin, and sections were stained with anti-MPO, anti–Bcl-xL, or anti-Siva1 antibodies. (A) Neutrophil numbers per high-power field were determined by MPO positivity (n = 4). (B) Representative MPO, Bcl-xL, and Siva1 sections from untreated WT and treated WT/Phd3–/– animals are shown; original magnification, ×400. (C) At higher magnification, cells with morphologic features of neutrophils are shown to stain for MPO, Bcl-xL, and Siva1; original magnification, ×1,000. (D) In situ apoptosis was shown with TUNEL staining, with MPO-positive TUNEL-positive neutrophils highlighted by black arrows and MPO-negative TUNEL-positive cells by the white arrow; original magnification, ×400.
Discussion
Neutrophilic inflammation typically occurs in extremely hypoxic environments, with oxygen tensions close to zero in neutrophilic exudates. Hypoxia is a profoundly antiapoptotic stimulus to neutrophils (18, 19), an effect that is principally mediated via HIF-1α (2). HIF-1 inactivation, however, also results in wider impairment of neutrophil host defense functions (4, 5, 6). Since HIF expression is regulated by prolyl hydroxylase enzymes, and since PHD2 and -3 are induced in hypoxia, we proposed PHDs might also regulate neutrophil survival at sites of inflammation and hypoxia.
We found human neutrophils express all 3 PHD isoforms constitutively as both mRNA and protein, but importantly only PHD3 protein was increased by culture in hypoxia. PHD3 transcript was induced by both hypoxia and peptidoglycan treatment and elevated in patient samples, whereas PHD2 transcript showed a relatively modest fold change with hypoxic stimulation, and was downregulated by the NOD agonist peptidoglycan. This differential regulation of PHD2 and PHD3 suggests that individual PHDs may have distinct roles in disease pathogenesis and innate immune responses. In Phd3-deficient neutrophils, we identified a direct and highly specific role for Phd3 in maintaining neutrophil survival in hypoxia. Importantly, this hypoxic regulation of survival can be dissociated from the delay of apoptosis induced by the prototypic proinflammatory mediator LPS. This is particularly interesting given the role of HIF-1α in the development of LPS-induced sepsis syndrome (6) and the enhanced production of immune-defense molecules in an HIF-1α–dependent fashion following macrophage coculture with both Gram-positive and -negative pathogens (5), suggesting that the mechanisms invoked in PHD3-dependent regulation of hypoxic neutrophil survival are more selective and complex than a simple feedback into regulation of HIF signaling pathways. This is further supported by the phenotypic divergence between Phd3-null and Hif1a-null neutrophils with respect to their functional and metabolic responses. In marked contrast to Hif-1α deficiency, in which myeloid cells display a profound impairment in aggregation, motility, invasiveness, and bacterial killing (4), Phd3-null neutrophils maintain normal in vitro chemotactic, phagocytic, and respiratory burst activity to both chemokine and bacterial stimuli. This is seen on the background of preserved intracellular ATP and glucose concentrations and again contrasts with the dramatic loss of intracellular ATP pools in Hif-1α–deficient cells (4). Indeed, the independence of the PHD3-selective phenotype from HIF regulation is further indicated by the maintenance of WT Hif target gene transcriptional responses in the Phd3-null neutrophils. Conversely, it is possible the Hif1a–/– apoptosis phenotype, which we previously described (2), may in part be explained by loss of Phd3, since Hif1a–/– neutrophils showed reduced Phd3 expression in hypoxia. However, given the profound nature of the metabolic dysregulation in myeloid cells lacking Hif-1α, it is likely that ATP deficiency itself may also impact on the ability of these cells to remain viable, a response we would predict to occur independently of Phd3 regulation.
It is currently unclear whether the role of PHD3 in regulating hypoxic neutrophil survival is dependent upon its catalytic activity, since noncatalytic effects of PHDs have been described (9). It is also unclear whether the effect requires ongoing PHD3 activity in hypoxia or reflects the persistence of a biological effect of PHD3 in normoxia that “programs” the neutrophil for subsequent survival in hypoxia. The ability of PHD enzymes to retain activity at low oxygen levels has certainly been described, with loss of PHD-mediated HIF transcriptional activity in glioblastoma cell lines reported at pO2 levels of 1% by Henze et al. (36) and persistent hydroxylation of recombinant HIF proteins and HIF ODDD constructs also shown under physiological oxygen tensions (9).
The downstream mechanism(s) by which PHD3 regulates hypoxic survival are clearly of great interest. In neuronal progenitor cells, Schlisio et al. identified PHD3-dependent regulation of KIF-1Bβ to modulate neuronal cell apoptosis following growth factor withdrawal, with KIF-1Bβ both necessary and sufficient for neuronal apoptosis (11). We, however, have found no change in Kif-1bβ protein expression in murine bone marrow–derived neutrophils following aging or hypoxia. Moreover, neutrophils express a converse apoptotic phenotype in the absence of Phd3, with hypoxia being a profound survival stimulus in contrast to its effects on other primary cells. In superior cervical ganglion cells isolated from Phd3–/– mice, Bishop et al. originally described a reduction in apoptosis, again in contrast to our observed restoration of constitutive rates of apoptosis in the absence of Phd3, which was associated with an increase in cell number within the ganglion as well as in the adrenal medulla and carotid body (21). The subsequent heterozygous inactivation of Hif1a and Hif2a in explanted Phd3–/– neurons demonstrated an HIF-2 dependence of this survival response. More recently, Henze et al. described the hypoxic induction of PHD2 and PHD3 to protect glioblastoma cell lines from hypoxia-induced cell death, with PHD inhibition enhancing staurosporine- and TNF-mediated apoptosis (36). They conclude the biological function of PHDs within this system is exerted through the negative feedback on both HIF-1 and HIF2 protein stability. Of note, we found no evidence for modulation of Hif transcriptional activity in Phd3-deficient neutrophils, compared with WT controls, either by whole mouse genome Affymetrix array or subsequently by TaqMan time courses of expression for recognized Hif-1 and Hif-2 target genes. It would seem highly likely, therefore, that individual cell types have adapted unique hypoxic survival mechanisms and that the PHD3 target for hypoxic regulation of neutrophil survival will be specific to the neutrophil.
In neutrophils we showed that PHD3-dependent hypoxic survival is associated with altered expression of SIVA1, a known proapoptotic protein originally identified as a molecule interacting with the cytoplasmic portion of a TNF receptor family member CD27 (34). There is a previous report of SIVA1 expression in neutrophils (37), but no modulation of SIVA1 by hypoxia has been described in any cell type. SIVA1 interacts with BCL-XL, and this binding interaction is likely unique for BCL-XL among BCL-2 proteins, although other non–BCL-2 family binding partners of this protein have been described (24). SIVA1 has been shown in vitro to sensitize cancer cell lines to UV-induced apoptosis through this ability to bind to and sequester BCL-XL (24), and we therefore postulate the interaction between SIVA1 and BCL-XL represents an important step in the regulation of PHD3-dependent hypoxic neutrophil survival. While a role for BCL-XL in regulating hypoxic survival has not previously been studied, we show that BCL-XL is induced by hypoxia and that inhibition of BCL-XL by a BH3 mimetic abrogates hypoxic survival of neutrophils. Interestingly, Bcl-xL was not identified as being significantly downregulated in Phd3–/– neutrophils in our Affymetrix array. However, Bcl-xL mRNA is stably expressed in murine neutrophils and unaltered by a range of inflammatory mediators (25). Moreover, Bcl-xL protein expression in other cell types is predominantly mediated by posttranslational mechanisms (38), although the direct hydroxylation of Bcl-xL is not currently reported. Thus, alterations in proteins known or predicted to influence rates of neutrophil apoptosis are observed in Phd3–/– neutrophils, supporting the observed morphologic phenotype of apoptosis. Our experiments support a role for SIVA1/BCL-XL in PHD3-mediated neutrophil survival, but the mechanism by which PHD3 modifies these proteins will require further study. Identification of novel targets of 2-oxoglutarate–dependent dioxygenases with functional biological consequences is currently a significant challenge in the field. A summary of the potential interactions between BCL-XL/SIVA1- and HIF-1α–associated pro-survival and antimicrobial pathways in PHD3-dependent hypoxic neutrophil survival is provided in Figure 7. Not only does this highlight the complexity and degree of potential crosstalk between these proinflammatory responses, it emphasizes the selectivity of PHD3 loss for neutrophil survival, in marked contrast to the HIF-1α/NF-κB pathways, which have broad links to many key antimicrobial responses.
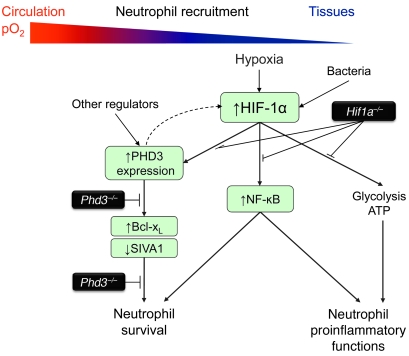
Figure 7. The roles of HIF-1 and PHD3 in regulating neutrophilic inflammation.
HIF-1α expression is induced in neutrophils in hypoxia and positively regulates neutrophil metabolic function (glycolysis, ATP generation), cytokine and inflammatory mediator generation, and survival. HIF-1α extends neutrophil survival in hypoxia, with NF-κB, a known regulator of neutrophil proinflammatory function, implicated in this response. However, hypoxic neutrophil survival requires PHD3, which itself regulates neutrophil survival downstream of HIF-1α, in association with upregulation of BCL-XL and downregulation of SIVA1. PHD3 does not directly modify neutrophil metabolic or proinflammatory functions. PHD3 therefore demonstrates an increased selectivity in the regulation of neutrophil lifespan compared with HIF-1α.
While our in vitro data provide important mechanistic insights into the PHD3-dependent regulation of hypoxic neutrophil survival, it is clearly important to establish whether this translates to a discernible antiinflammatory phenotype in vivo. To this end we investigated two independent models of neutrophilic inflammation where hypoxia has either been experimentally induced or is a recognized feature of the inflammatory process. Acute lung injury is widely recognized to be associated with a profound reduction in circulating oxygen tensions and poor clinical outcomes in patients within the intensive care setting. In a murine model of acute lung injury, we demonstrated a significant reduction in neutrophilic inflammation in hypoxic Phd3–/– animals, which correlated with an increase in neutrophil apoptosis, both confirming our in vitro phenotype and supporting the importance of a selective loss of hypoxic neutrophil survival in a disease process in vivo. This reduced inflammation occurred in the absence of a difference in rates of neutrophil recruitment between WT and Phd3–/– animals, at a time point when such differences are typically apparent if there is a recruitment phenotype (31, 32). This is important both to rule out altered neutrophil migration (which was not observed in vitro) but also because, in these whole animal knockouts, PHD3 deficiency of resident cells such as epithelial and endothelial cells could have modified neutrophil influx into the lungs. Murine DSS-induced colitis is also recognized to be profoundly hypoxic (35) and can be characterized histologically by neutrophil-predominant inflammation within the intestinal mucosa, although the specific role neutrophils play in the disease pathogenesis is unclear. In keeping with a reduction in neutrophil-dominant pulmonary inflammation and increased neutrophil apoptosis, we again saw a reduction in neutrophil numbers in the intestinal mucosa of Phd3–/– animals compared with WT controls. Moreover, disease development and susceptibility in this model again do not differ between WT and Phd3–/– animals, as evidenced by daily weight and symptom score monitoring in this model up to day 6 (39). Thus, in two discrete models of acute neutrophilic inflammation, loss of PHD3 in a hypoxic setting resulted in the modulation of the end disease phenotype with enhanced inflammation resolution.
In summary, we identify PHD3 as a selective regulator of neutrophil hypoxic survival, and identify the transcriptional regulation of PHD3 in circulating neutrophils from individuals with rheumatoid arthritis. We describe the preservation of key antimicrobial functions in Phd3–/– cells and thus dissociate the Phd3–/– neutrophil phenotype from the previously described broader Hif1a–/– phenotype with its critical defects in effective host defense, and further postulate that PHD3 itself may mediate neutrophil survival downstream of HIF-1α. Importantly, we confirm the importance of PHD3 regulation of hypoxic neutrophil survival in vivo in both an acute lung injury model and a DSS model of colitis. Consequently, we propose the selective inhibition of PHD3 may provide a more potent and less toxic antiinflammatory strategy for targeting neutrophilic inflammation, an unmet clinical need with respect to a broad range of both acute and chronic inflammatory disease states.
Methods
For cell count determination, murine neutrophil glucose and ATP assays, and apoptosis assays, see Supplemental Methods.
Isolation and culture of neutrophils from healthy human volunteers and knockout mice.
Human peripheral blood neutrophils were isolated from individuals with active rheumatoid arthritis, in accordance with 1987 American Rheumatism Association criteria (40), and with one or more swollen joints and from healthy volunteers using dextran sedimentation and discontinuous plasma-Percoll gradients (41). Ethical approval was obtained from the South Sheffield Research Ethics Committee, and all participants gave written informed consent in accordance with the Declaration of Helsinki principles. Murine bone marrow–derived neutrophils were isolated using modified discontinuous HBSS-Percoll gradients (82%, 62%, and 51%) and peripheral blood neutrophils by negative magnetic selection following a terminal inferior vena cava bleed into a 23 gauge pre-heparinized needle (42). Cell purity, assessed by cytospin, was routinely greater than 97% human peripheral blood, greater than 70% murine bone marrow, and greater than 80% murine peripheral blood.
Purified cells were resuspended at 5 × 106/ml (human peripheral blood and murine bone marrow–derived neutrophils) or 1 × 106/ml (murine peripheral blood neutrophils) in RPMI with 10% fetal calf serum and 50 U/ml streptomycin and penicillin. Cells were cultured in the presence or absence of peptidoglycan (10 μg/ml), E. coli LPS (100 ng/ml), GM-CSF (500 U/ml), or ABT-737 (10 μM) (Selleck Chemicals) in normoxia (19 kPa) or hypoxia (3 kPa) and apoptosis determined by cytospin morphology. Hypoxia was achieved using an in vivo 400 hypoxic work station (Ruskinn) with a 5% CO2/balance N2 gas mix preset to deliver an oxygen tension of 0.75 kPa into the chamber, which correlated with a culture media oxygen tension of 3 kPa as determined by automated blood gas analysis (NPT7, Radiometer). All media were allowed to equilibrate overnight prior to use.
Animals.
Phd3–/–, myeloid-targeted Hif1a–/–, and WT mice were used in these experiments and have previously been described (4, 21). Phd3–/– mice were initially on a mixed Swiss/129SvEv genetic background, generated through heterozygous cross, with mice from the same litter used for comparison. For functional assays Phd3–/– mice backcrossed onto a C57BL/6 background were subsequently used. All key results presented were confirmed in this background (data not shown). Lysozyme M–driven Cre (lysMcre) was used to target Hif1a deletions to myeloid lineage cells (4) with animals also backcrossed to a C57BL/6 background. All animal experiments were conducted in accordance with the Home Office Animals (Scientific Procedures) Act of 1986, with approval of the Sheffield Ethical Review Committee, Sheffield, United Kingdom.
Immunoblot detection of human and murine neutrophil protein.
Whole cell lysates were prepared by resuspending 0.5 × 106 murine neutrophils in 30 μl SDS lysis buffer or 10 × 106 human neutrophils in 100 μl hypotonic lysis buffer (10 mM Tris-HCl, pH 7.8, 1.5 mM EDTA, 10 mM KCl, 0.5 mM DTT, 1 mM sodium orthovanadate, 2 mM levamisole, 0.5 mM benzamidine, and 0.05% NP40 and complete protease inhibitor cocktail [Roche Applied Science]), sonication lysing (30 seconds on, 30 seconds off) (Bioruptor, Diagenode), and boiling in 100 μl 2× SDS buffer with 1 × 106 to 5 × 106 neutrophils loaded per lane. Protein recovery was assessed by immunoblotting with primary antibodies against PHD1–3 (generated by our research team), SIVA1 (clone M-175, Santa Cruz Biotechnology Inc.), and Bcl-xL (clone aa 18–233, Santa Cruz Biotechnology Inc.). Sample loading was confirmed by β-actin or P38 expression. All bands shown were at the predicted molecular weight for the protein of interest.
Human RNA isolation and relative quantification.
Neutrophils (20 × 106/condition) were lysed with 2 ml TRI Reagent (Sigma-Aldrich), and RNA was extracted using chloroform phase partitioning and isopropanol. Samples were DNase digested and random hexamer cDNA synthesized by reverse transcription, with cDNA run in TaqMan assays at a final concentration of 100 ng/μl. Assays-On-Demand gene expression TaqMan MGB 6FAM dye–labeled products (Applied Biosystems) were used for GAPDH and β-actin and assays performed according to the manufacturer’s instructions. 6FAM dye–labeled probes and primers were designed for PHD1 (sense 5′-AGCGGGCAGCAGCCAAAGACAAG-3′, antisense 5′-TGCCATGCGGCTCTGGGACTG-3′, probe 5′-ATCAGCTAGCATCAGGACAGAAAGGTGTCC-3′), PHD2 (sense 5′-AGCCCGGCTGCGAAACCATTG-3′, antisense 5′-TTCGTCCGGCCATTGATTTTGT-3′, probe 5′-GCTGCTCATGAGCAGCATGGACGACC-3′), PHD3 (sense 5′-AGTCCGGAAACGGGTCGTGGAG-3′, antisense 5′-AGCGTCGGGGACAAGGGAAAGTT-3′, probe 5′-TCCGCACCACTCCCGCTGGTTCCCGAAG-3′). Relative quantification for each gene was determined against β-actin expression.
Murine neutrophil functional assays.
For chemotaxis assays, neutrophil chemotaxis to KC (0–1 mM) over 3 hours was measured using normoxia/hypoxia pre-equilibrated Neuro Probe ChemoTx microplates with a 5-μm filter. The number of cells in each well was expressed as a percentage of the positive control minus the percent migration of the chemokinesis control. For phagocytosis assays, following a 1 hour pre-equilibration of neutrophils (1 × 106/ml) in either normoxia or hypoxia, neutrophil uptake of Alexa Fluor 488–labeled heat-inactivated E. coli after 30 minutes coculture (MOI of 1:1) was determined by flow cytometry (FACSCalibur, BD). For respiratory burst assays, following a 1 hour pre-equilibration of neutrophils (1 × 106/ml) in either normoxia or hypoxia, cells were cultured with 6 mM DCF for 30 minutes and then stimulated for a further 30 minutes with heat-inactivated E. coli at an MOI of 10:1 (E. coli/PMN) before FL1 geometric mean fluorescence was determined by flow cytometry.
Murine RNA isolation and relative quantification.
Neutrophils (1 × 106/condition) were lysed and RNA extracted using the mirVana total RNA isolation protocol (Ambion). Samples were DNase digested, and biotin-labeled antisense RNA was prepared for array analysis using the Affymetrix one cycle target labeling method as per the manufacturer’s instructions. Fragmented antisense RNA (15 μg) was applied to each array chip following determination of integrity by Agilent Bioanalysis (Agilent Technologies). Affymetrix data were normalized and log fold change was determined using PANTHER software ( http://www.pantherdb.org/). Microarray data were deposited in the MIAME-compliant GEO database (accession number GSE26023). For TaqMan quantitative PCR, random hexamer cDNA was synthesized by reverse transcription and run at a final concentration of 100 ng/μl. Assays-On-Demand gene expression TaqMan MGB 6FAM dye–labeled products (Applied Biosystems) were used for Phd1, Phd2, Phd3, Vegf, Glut1, Pai1, Bcl-xL, Kc, Gapdh, Siva1, and β-actin and assays performed according to the manufacturer’s instructions. 6FAM dye–labeled probes and primers were designed for Nf-κb (sense 5′-GGCGGCACGTTTTACTCTTT-3′, antisense 5′-CCGTCTCCAGGAGGTTAATGC-3′, probe 5′-CGCTTTCGGAGGTGCTTTCGCAG-3′), Ikk (sense 5′-TGCACACCGTGCAGAGTCA-3′, antisense 5′-TGCTTGCAGCCCAACAACT-3′, probe 5′-CGTGTTCTCAAGGAGCTGTTTGGTCACC-3′), and I-κb (sense 5′-CGGAGGACGGAGACTCGTT-3′, antisense 5′-CTTCCATGGTCAGCGGCTT-3′, probe 5′-TGCACTTGGCAATCATCCACGAAGA-3′). Relative quantification for each gene was determined against β-actin expression. Murine LPS acute lung injury model. Direct tracheal instillation of bacterial LPS (0.3 mg) was performed on anesthetized mice (28), and the animals recovered for 6 hours in a warmed cage in room air. At this point, after placement in a sealed work station (Wolf Laboratories) set to 21% O2, mice were maintained either at 21% for the duration of the experiment or, following reduction of O2 to 10% over 1 hour, at 10% O2 for the rest of the experiment. For 6-hour recruitment data, 3 mg nebulized LPS was instilled without a requirement for anesthesia, and animals were maintained at 21% for 6 hours. At the end time point, mice were sacrificed (30), the trachea re-cannulated, and the lungs instilled with 3.5 ml ice-cold PBS in 0.5- to 1.0-ml aliquots. The recovered lavage fluid was maintained on ice. Hemocytometer counts were determined, and then samples were divided into 2 aliquots, pelleted (1,000 g, 5 minutes, 4°C), and resuspended in either fetal calf serum or 1% paraformaldehyde prior to cytocentrifugation. Cytospins of serum-suspended cells were stained by Diff-Quick for differential cell counts and morphologic scoring of apoptosis, and paraformaldehyde-suspended cytospins stored at 4°C for subsequent analysis of apoptosis by deoxynucleotidyltransferase-mediated TUNEL. TUNEL was performed using an ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon International) as per the manufacturer’s instructions.
For histological sections, unlavaged lungs were fixed via the trachea with 10% buffered formalin at 20 cm H2O and paraffin-embedded blocks prepared. Serial sections were subsequently stained with hematoxylin and eosin or anti-MPO antibody (clone ab15484, Abcam), anti–Bcl-xL antibody (BD Biosciences — Transduction Laboratories), or anti-SIVA1 antibody (clone M-175, Santa Cruz Biotechnology Inc.) following deparaffinization. Neutrophil count per high-power field was calculated from the individual counts of 5 high-power fields per section.
Murine DSS-induced acute colitis model.
Colitis was induced using a previously described method (35), and mice were sacrificed at 6 days. Sections of intestine were stained with hematoxylin and eosin or anti-MPO, anti–Bcl-xL, or anti-Siva1 antibodies (as above) following deparaffinization. Total neutrophils per high-power field were calculated from the individual counts of 5 high power-fields per section. TUNEL was performed using an ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon International) as per the manufacturer’s instructions.
Statistics.
Gene array data were analyzed for statistical significance after normalization using PANTHER software. For all other experiments, where data allowed, significance was determined by paired 2-tailed t tests unless otherwise specified, with P values less than 0.05 considered significant. Data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Yvonne Stephenson for help with immunohistochemistry, Endre Kiss-Toth for help with TaqMan primer/probe design, Claudia Knight for help with Western blotting, Paul Heath and Hazel Holden for help with array experiments, and Ian Cree and Sharon Glaysher for ATP measurements. This work was supported by the Wellcome Trust via an Intermediate Clinical Fellowship (ref. 078244) to S.R. Walmsley and also by a Senior Clinical Fellowship (ref. 076945) to D.H. Dockrell and a Science Foundation Ireland grant to C.T. Taylor; by the NIHR Sheffield Biomedical Research Unit in Cardiovascular Disease; and by the NIHR Cambridge Biomedical Research Centre. The work of P. Carmeliet is supported by long term-structural funding–Methusalem funding from the Flemish Government.
Footnotes
Conflict of interest: Christopher W. Pugh, Peter J. Ratcliffe, and Patrick H. Maxwell are founding scientists of ReOx Ltd.
Citation for this article: J Clin Invest. 2011;121(3):1053–1063. doi:10.1172/JCI43273.
References
- 1. Rossi AG, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12(9):1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 2. Walmsley SR, et al. The role of HIF-1α and NF-κB in hypoxia-induced survival in human and murine neutrophils. J Exp Med. 2005;201(1):105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2 regulated gene expression. . FASEB J. 2002;16(10):1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 4. Cramer T, et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peyssonnaux C, et al. HIF-1α expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115(7):1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178(12):7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 7. Epstein ACR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 8. Bruick R, McKnight S. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 9. Kaelin WG, Ratcliffe P. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 10. Appelhoff RJ, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279(37):38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 11. Schlisio S, et al. The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008;22(7):884–893. doi: 10.1101/gad.1648608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Q, et al. Control of cyclin D1 and breast tumorigenesis by the EglN2 prolyl hydroxylase. Cancer Cell. 2009;16(5):413–424. doi: 10.1016/j.ccr.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metzen E, et al. Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J Cell Sci. 2003;116(pt 7):1319–1326. doi: 10.1242/jcs.00318. [DOI] [PubMed] [Google Scholar]
- 14. Koivunen P, et al. An endoplasmic reticulum transmembrane prolyl 4-hydroxylase is induced by hypoxia and acts on hypoxia-inducible factor alpha. J Biol Chem. 2007;282(42):30544–30552. doi: 10.1074/jbc.M704988200. [DOI] [PubMed] [Google Scholar]
- 15. Cummins EP, et al. Prolyl hydroxylase-1 negatively regulates IκBkinase-β, giving insight into hypoxia-induced NFκB activity. Proc Natl Acad Sci U S A. 2006;103(48):18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee S, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial phaeochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8(2):155–167. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 17. Koditz J, et al. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood. 2007;110(10):3610–3617. doi: 10.1182/blood-2007-06-094441. [DOI] [PubMed] [Google Scholar]
- 18. Hannah S, et al. Hypoxia prolongs neutrophil survival in vitro. FEBS Lett. 1995;372(2-3):233–237. doi: 10.1016/0014-5793(95)00986-J. [DOI] [PubMed] [Google Scholar]
- 19. Mecklenburgh KI, et al. Involvement of a ferroprotein sensor in hypoxia-mediated inhibition of neutrophil apoptosis. Blood. 2002;100(8):3008–3016. doi: 10.1182/blood-2002-02-0454. [DOI] [PubMed] [Google Scholar]
- 20. Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005;73(12):7967–7976. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bishop T, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3–/– mice. . Mol Cell Biol. 2008;28(10):3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168(9):4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 23. Yoon Y, Ao Z, Cheng Y, Schlossman SF, Prasad KV. Murine Siva-1 and Siva-2, alternate splice forms of the mouse Siva gene, both bind to CD27 but differentially transduce apoptosis. Oncogene. 1999;18(50):7174–7179. doi: 10.1038/sj.onc.1203144. [DOI] [PubMed] [Google Scholar]
- 24. Xue L, et al. Siva-1 binds to and inhibits BCL-X(L)-mediated protection against UV radiation-induced apoptosis. Proc Natl Acad Sci U S A. 2002;99(10):6925–6930. doi: 10.1073/pnas.102182299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J Leukoc Biol. 2001;70(5):783–792. [PubMed] [Google Scholar]
- 26. Sawatzky DA, Willoughby DA, Colville-Nash PR, Rossi AG. The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo. Am J Pathol. 2006;168(1):33–41. doi: 10.2353/ajpath.2006.050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuroda J, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A. 2006;103(40):14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Estrov Z. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces antiapoptotic and proapoptotic signals in acute myeloid leukemia. Blood. 2003;102(2):630–637. doi: 10.1182/blood-2002-06-1890. [DOI] [PubMed] [Google Scholar]
- 29. Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathway s J Biol Chem. 2006. 281 22 15215 15226 10.1074/jbc.M511408200 [DOI] [PubMed] [Google Scholar]
- 30. Rowe SJ, Allen L, Ridger VC, Hellewell PG, Whyte MK. Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J Immunol. 2002;169(11):6401–6407. doi: 10.4049/jimmunol.169.11.6401. [DOI] [PubMed] [Google Scholar]
- 31. Brass DM, Hollingsworth JW, McElvania-Tekippe E, Garantziotis S, Hossain I, Schwartz DA. CD14 is an essential mediator of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L77–L83. doi: 10.1152/ajplung.00282.2006. [DOI] [PubMed] [Google Scholar]
- 32. Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol. 2008;180(10):6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krajewski S, et al. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994;54(21):5501–5507. [PubMed] [Google Scholar]
- 34. Prasad KV, et al. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci U S A. 1997;94(12):6346–6351. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hindryckx P, et al. Absence of placental growth factor blocks dextran sodium sulfate–induced colonic mucosal angiogenesis, increases mucosal hypoxia and aggravates acute colonic injury. Lab Invest. 2010;90(4):566–576. doi: 10.1038/labinvest.2010.37. [DOI] [PubMed] [Google Scholar]
- 36. Henze AT, et al. Prolyl hydroxylases 2 and 3 act in gliomas as protective negative feedback regulators of hypoxia-inducible factors. Cancer Res. 2010;70(1):357–366. doi: 10.1158/0008-5472.CAN-09-1876. [DOI] [PubMed] [Google Scholar]
- 37. Balci-Peynircioglu B, et al. Pyrin, product of the MEFV locus, interacts with the proapoptotic protein SIVA. J Cell Physiol. 2008;216(3):595–602. doi: 10.1002/jcp.21435. [DOI] [PubMed] [Google Scholar]
- 38. Pardo OE, Arcaro A, Salerno G, Raguz S, Downward J, Seckl MJ. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway: correlation with resistance to etoposide-induced apoptosis. J Biol Chem. 2002;277(14):12040–12046. doi: 10.1074/jbc.M109006200. [DOI] [PubMed] [Google Scholar]
- 39. Tambuwala MM, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139(6):2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 40. Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 41. Haslett C, Guthrie LA, Kopamiak MM, Johnson RB, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- 42. Cotter MJ, Norman KE, Hellewell PG, Ridger VC. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am J Pathol. 2001;159(2):473–481. doi: 10.1016/S0002-9440(10)61719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.