Abstract
Plasmacytoid DCs (pDCs) are innate immune cells that are specialized to produce IFN-α and to activate adaptive immune responses. Although IFN-α inhibits HIV-1 replication in vitro, the production of IFN-α by HIV-activated pDCs in vivo may contribute more to HIV pathogenesis than to protection. We have now shown that HIV-stimulated human pDCs allow for persistent IFN-α production upon repeated stimulation, express low levels of maturation molecules, and stimulate weak T cell responses. Persistent IFN-α production by HIV-stimulated pDCs correlated with increased levels of IRF7 and was dependent upon the autocrine IFN-α/β receptor feedback loop. Because it has been shown that early endosomal trafficking of TLR9 agonists causes strong activation of the IFN-α pathway but weak activation of the NF-κB pathway, we sought to investigate whether early endosomal trafficking of HIV, a TLR7 agonist, leads to the IFN-α–producing phenotype we observed. We demonstrated that HIV preferentially traffics to the early endosome in human pDCs and therefore skews pDCs toward a partially matured, persistently IFN-α–secreting phenotype.
Introduction
Plasmacytoid DCs (pDCs) are innate immune cells that circulate in the blood and lymphoid tissues and are specialized to produce copious amounts of type I IFNs (IFN-α/β) in response to stimulation by virus-associated single-stranded RNA and unmethylated CpG DNA through the engagement of TLR7 and TLR9 within the endosomal compartment (1–3). HIV recognition by pDCs is mediated by the innate receptor TLR7 and requires its endocytosis followed by endosomal acidification (1). IFN-α inhibits HIV-1 (HIV) replication in vitro, but pDCs are now implicated in vivo in promoting both mucosal infection and chronic immune activation (4–7).
pDCs are the earliest cells to arrive to the mucosal site of SIV inoculation, and their production of cytokines recruits and activates CD4+ T cells for infection (4). HIV infection is marked by aberrant immune activation, which correlates more with disease progression than with viremia (8–13). The cause of immune activation in AIDS is unknown, but stimulation of innate immune cells directly by HIV and indirectly by products of bacterial translocation may be major contributors. Both human and animal studies support a role for IFN-α in HIV pathogenesis. High plasma titers of IFN-α during acute and late-stage disease have been shown to correlate with disease progression (5). Women progress to AIDS more rapidly than men, express higher markers of immune activation, and produce more IFN-α per pDC when challenged with HIV ex vivo (14). Lymphoid tissue and circulating PBMCs derived from HIV-infected subjects with progressive disease express much higher levels of IFN-α and related inducible genes compared with uninfected controls (6, 7). Transcriptional profiling in pathogenic and nonpathogenic SIV-infected primates reveal differences in IFN-α responses: both hosts have strong IFN-α response signatures during acute infection, but only the pathogenically infected animals that go on to develop AIDS maintain elevated IFN-α response signatures over the course of chronic infection (15–18). Classically, pDCs are described as being refractory to IFN-α production upon repeated stimulation with synthetic TLR7 or TLR9 agonists, which is thought to be a protective mechanism against excessive immune activation (19, 20). To our knowledge, pDC tolerance to repeated stimulation with HIV, a TLR7 agonist, has never been evaluated, but we and others have observed that pDCs from HIV-infected subjects can be stimulated ex vivo with HIV to produce IFN-α (21, 22). Because pDCs derived from HIV-infected subjects have been exposed to HIV in vivo, we reasoned that HIV may uniquely allow for repeated stimulation of pDCs.
The studies presented here demonstrate that HIV-activated human pDCs were incompletely matured upon activation and were not refractory to repeated stimulation to produce IFN-α. In comparison, R848 (Resiquimod), a synthetic TLR7/8 ligand; type B CPG oligodeoxyribonucleotide (CpGB), a synthetic TLR 9 ligand; and heat-inactivated influenza virus and Sendai virus, 2 other viruses thought to activate pDCs through TLR7; matured pDCs more fully and rendered the cells either totally or partially refractory to further stimulation to produce IFN-α. We provide evidence for a mechanism whereby HIV traffics predominantly to early endosomes rather than late endosomes, favoring persistent IFN-α production over maturation. Persistent bioactive IFN-α production by HIV-stimulated pDCs correlated with increased levels of the transcription factor IRF7 and was dependent upon the autocrine IFN-α/β receptor feedback loop. By skewing pDCs toward a partially matured and persistently IFN-α–secreting phenotype, HIV may promote its survival by blunting adaptive immune responses and by inciting inflammatory responses to amplify activated target cells for infection.
Results
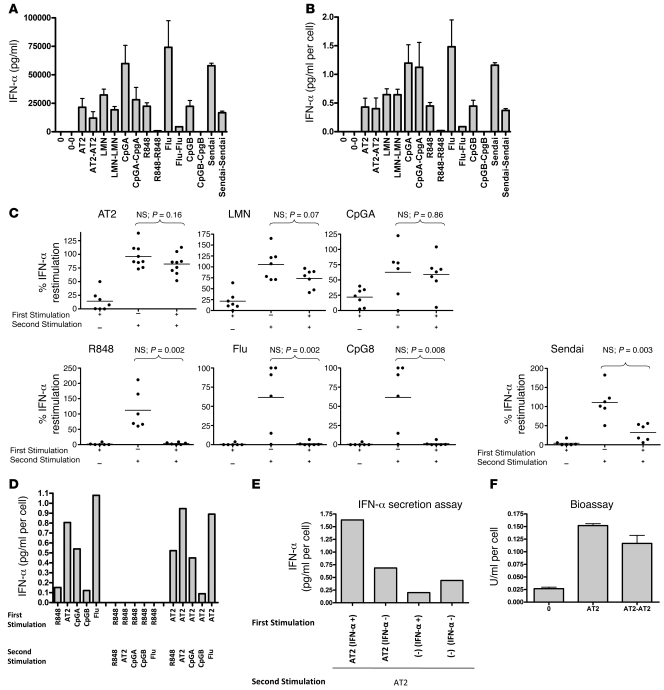
HIV-activated pDCs are not refractory to restimulation to produce IFN-α.
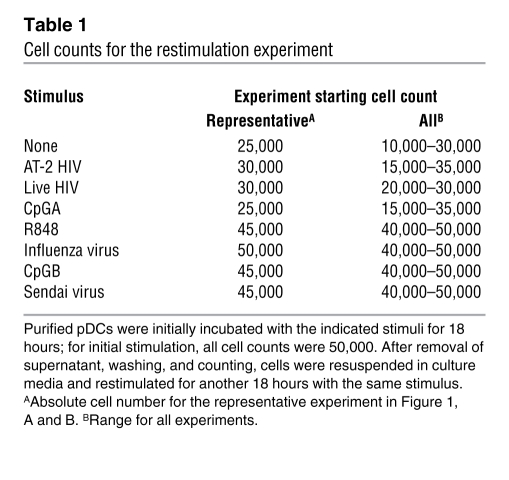
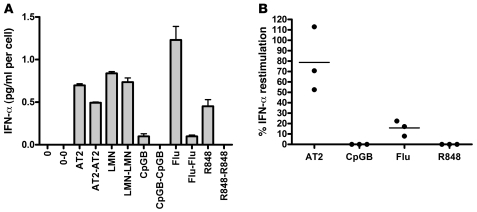
We first evaluated whether pDCs can be restimulated by HIV to produce IFN-α after incubation with HIV compared with other TLR7 and TLR9 agonists. Purified pDCs were incubated with live HIV, 2,2′ dithiodipyridine–inactivated HIV-1 (AT-2 HIV), R848, heat-inactivated influenza virus, Sendai virus, CpGA, or CpGB for 18 hours. Culture supernatants were removed after centrifugation, and cells were washed, counted, resuspended in culture media, and restimulated for another 18 hours. IFN-α was measured in these culture supernatants by ELISA and corrected for cell number (Figure 1, A and B). Cell numbers for the representative experiment shown in Figure 1, A and B, and ranges for all experiments are shown in Table 1. Purified pDCs stimulated with live HIV, AT-2 HIV, or CpGA were not refractory to restimulation. All experiments were performed with 300 ng/ml of CXCR4 tropic MN HIV, as this amount and type of HIV lab strain causes maximal IFN-α production by pDCs based on prior studies (1, 23), but pDCs stimulated with CXCR4 MN HIV and CCR5 tropic ADA HIV at doses ranging from 3 ng/ml to 3 μg/ml were also not refractory to restimulation (data not shown). In comparison, pDCs stimulated with R848, CpGB, and inactivated influenza were completely refractory to restimulation, whereas those stimulated with Sendai virus were partially refractory (Figure 1C). Furthermore, pDCs first stimulated by R848 were refractory to restimulation with both TLR7 and TLR9 ligands and pDCs first stimulated by AT-2 HIV were not refractory to restimulation with either TLR7 or TLR9 ligands (Figure 1D). When the length of initial stimulation of pDCs was extended to 36 hours, HIV-activated pDCs still produced IFN-α upon 18-hour restimulation, but R848- and CpGB-stimulated pDCs could not be restimulated (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI44960DS1). Of note, R848-stimulated pDCs produced a range of IFN-α (0–1 pg/cell) depending on the donor tested; however, regardless of the amount of IFN-α produced or the amount of R848 used (100 nM to 10 μM), the cells were always refractory to further stimulation to produce IFN-α. As demonstrated in our previous studies, RNAs obtained from control microvesicles prepared from HIV-uninfected cells matched to those used to produce HIV virions are ineffective at activating pDCs (1, 24). To confirm that we were restimulating HIV-activated IFN-α–producing pDCs and not merely activating pDCs that had not been activated during the first overnight incubation, we used the Miltenyi IFN-α secretion assay kit to select IFN-α–producing pDCs. pDCs were first stimulated with AT-2 HIV for 12 hours, sorted and separated into IFN α–secreting and non–IFN-α–secreting cells, and then stimulated again with AT-2 HIV for 24 hours. Instead of becoming refractory to further stimulation, AT-2 HIV–activated IFN-α–secreting pDCs produced even more cytokine per cell than did pDCs that did not produce IFN-α upon initial stimulation (Figure 1E). To evaluate whether type I IFNs produced by pDCs upon repeated stimulation with HIV are bioactive, we used a cell-based bioassay using the COS1 pRLpISRE cell line that produces luciferase upon exposure to bioactive type I IFNs, including all IFN-α subtypes and IFN-β. We found that pDCs were able to produce bioactive type I IFNs upon repeated stimulation with HIV (Figure 1F). We next investigated whether pDCs from HIV-infected subjects retain the capacity to produce IFN-α upon repeated stimulation with HIV. We isolated fresh pDCs from HIV-infected subjects and found that pDCs from HIV-infected subjects, similar to those of uninfected donors, produced IFN-α upon restimulation with HIV but not with CpGB, influenza, or R848 (Figure 2). All donors were antiretroviral naive; 2 were early-infected subjects obtained from the NIAID Center for HIV/AIDS Vaccine Immunology (CHAVI) cohort with HIV EIA positive, Western blot indeterminate with CD4 346, VL 191,008, and CD4 428, VL 434,625, respectively, and the third was a chronically infected patient (>5 years) recruited through Bellevue Hospital/NYU with CD4 681, VL 59,800.
Figure 1. HIV-activated pDCs are not refractory to restimulation to produce IFN-α.
(A and B) pDCs were incubated with media alone (0), AT-2 HIV (AT2), live HIV (LMN), R848, influenza virus (Flu), Sendai virus, CpGA, or CpGB for 18 hours. Supernatants were removed, and cells were washed, counted, and restimulated for 18 hours (restimulation denoted as 0-0, AT2-AT2, LMN-LMN, etc.). IFN-α was (A) measured in the supernatants by ELISA and (B) corrected for cell number. (C) Experiments were repeated for 6–10 donors. Percent IFN-α restimulation denotes IFN-α produced after the second stimulation relative to that after the first. Dots represent mean of 2 replicates per donor. (D) HIV-stimulated pDCs could be restimulated with different TLR ligands, whereas R848-stimulated pDCs could not be restimulated. Data are means (n = 2 replicates) and representative of 2 independent experiments. (E) pDCs were stimulated with media or AT-2 HIV for 12 hours; sorted; separated into IFN-α–secreting and non–IFN-α–secreting cells; and then stimulated with AT-2 HIV for 24 hours. AT-2 HIV–activated IFN-α–secreting pDCs produced more cytokine per cell than did HIV-activated pDCs that did not produce IFN-α upon initial stimulation. Data are means (n = 2 replicates) and representative of 2 independent experiments. (F) Culture supernatants were applied to a COS1 pRLpISRE cell line that produces luciferase upon exposure to bioactive type I IFNs. pDCs produced bioactive type I IFN upon repeated stimulation with HIV. Data are mean ± SEM (n = 2 replicates) and representative of 3 independent experiments.
Table 1 .
Cell counts for the restimulation experiment
Figure 2. HIV-infected subjects, similar to uninfected donors, produce IFN-α upon restimulation with HIV, but not with CpGB, influenza, or R848.
(A) pDCs from a chronically HIV-infected subject, incubated with AT-2 HIV, live HIV, CpGB, influenza virus, or R848 for 18 hours. Culture supernatants were removed after centrifugation, and cells were washed, counted, and restimulated for 18 hours. IFN-α was measured in the culture supernatants by ELISA and corrected for cell number. (B) Restimulation experiments were repeated with AT-2 HIV, CpGB, influenza virus, or R848 for 3 donors, 1 chronically infected (as in A) and 2 with early infection. Percent IFN-α restimulation denotes IFN-α produced after second stimulation relative to that after the first. Dots represent mean of 2 replicates per donor.
HIV-activated pDCs express lower levels of costimulatory molecules but are refractory to inflammatory cytokine production upon restimulation.
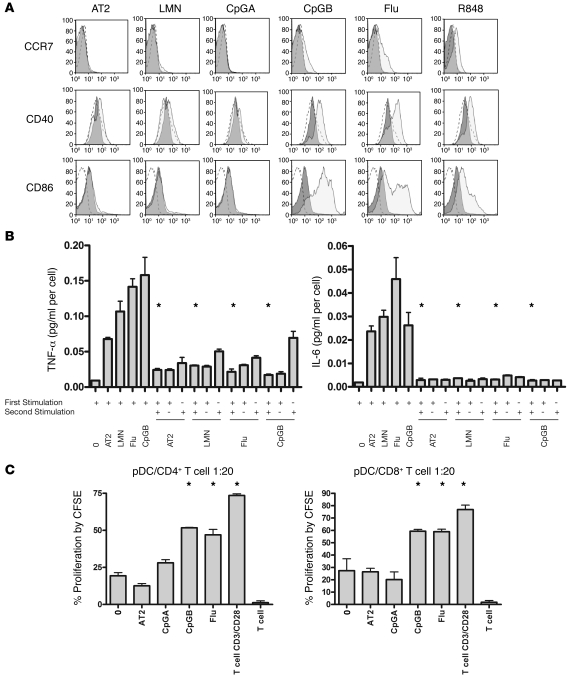
It is known that pDCs are activated by TLR agonists through the engagement between endosomal TLR7 or TLR9 and the adaptor molecule MyD88, leading to the assembly of a multiprotein signal-transducing complex in the cytoplasm that activates and phosphorylates IRF7 for initiation of type I IFN gene transcription and activates NF-κB and MAPKs to induce the transcription of proinflammatory cytokines, chemokines, and costimulatory molecules (25). Because the TLR signaling pathways diverge after MyD88 engagement, we were interested to study whether HIV-activated pDCs are unique only in their persistence of IFN-α production, or whether there are also differences between HIV and other TLR agonists in the NF-κB pathway. pDCs were placed in culture with live HIV, AT-2 HIV, R848, CpGA, CpGB, Sendai virus, or heat-inactivated influenza virus. After incubation, cells were washed and stained with anti-CD123–PE, anti-CD86–APC, anti-CD40–PerCP, anti-CCR7–FITC, or isotype control and analyzed by flow cytometry. HIV-activated and CpGA-activated pDCs upregulated the migration molecule CCR7 and costimulatory molecule CD86 to a much lesser degree than did pDCs activated with comparator agonists (including Sendai virus; data not shown). CD40 upregulation was not different between groups (Figure 3A). To study differences between HIV-activated pDCs and pDCs stimulated with the other agonists in terms of tolerance to inflammatory cytokine production upon restimulation, culture supernatants were tested using cytometric bead array analysis. None of the agonists, including HIV, allowed for restimulation of inflammatory cytokines (i.e., TNF-α and IL-6; Figure 3B). To study whether decreased expression of maturation molecules correlates with defective T cell stimulation, pDCs were incubated overnight with the various agonists, washed, and then cocultured with CFSE-labeled allogenic naive CD4+ or CD8+ T cells for 6 days. In concordance with the lower expression of costimulatory molecules, pDCs stimulated with HIV and CpGA elicited less allogeneic T cell proliferation than did those stimulated with CpGB or heat-inactivated influenza (Figure 3C).
Figure 3. HIV-activated pDCs, like CpGA-activated pDCs, express lower levels of costimulatory molecules; however, all cells are refractory to inflammatory cytokine production upon restimulation, regardless of TLR agonist used.
(A) Maturation of pDCs after 18 hours induced by HIV AT-2, live HIV, CpGA, CpGB, influenza virus, or R848, as measured by CD86, CD40, and CCR7. Data are from 1 experiment, representative of 5 independent experiments. Dashed line is isotype control, dark gray histogram is unstimulated 18 hours, light gray histogram is stimulated with agonist. (B) Inflammatory cytokine production by HIV-activated pDCs versus comparator agonists, tested using cytometric bead array analysis. None of the agonists, including HIV, allowed for restimulation of inflammatory cytokines compared with initial stimuli (*P < 0.05, Student’s t test). Data are mean ± SEM (n = 3 replicates) and representative of 3 independent experiments. (C) Allogeneic T cell proliferation at day 6 after coculture with differentially matured pDCs, expressed as percent proliferation by CFSE. pDCs matured with HIV and CpGA stimulated T cell proliferation similar to that by unstimulated pDCs, whereas pDCs matured with CpGB, influenza virus, or anti-CD3/anti-CD28 stimulated significantly more T cell proliferation than did unstimulated pDCs (*P < 0.05, 2-tailed Student’s t test). Data are represented as mean ± SEM (n = 3 replicates) and representative of 4 independent experiments.
HIV-activated pDCs increase mRNA expression of IRF7, which correlates with ability to produce secondary IFN-α responses.
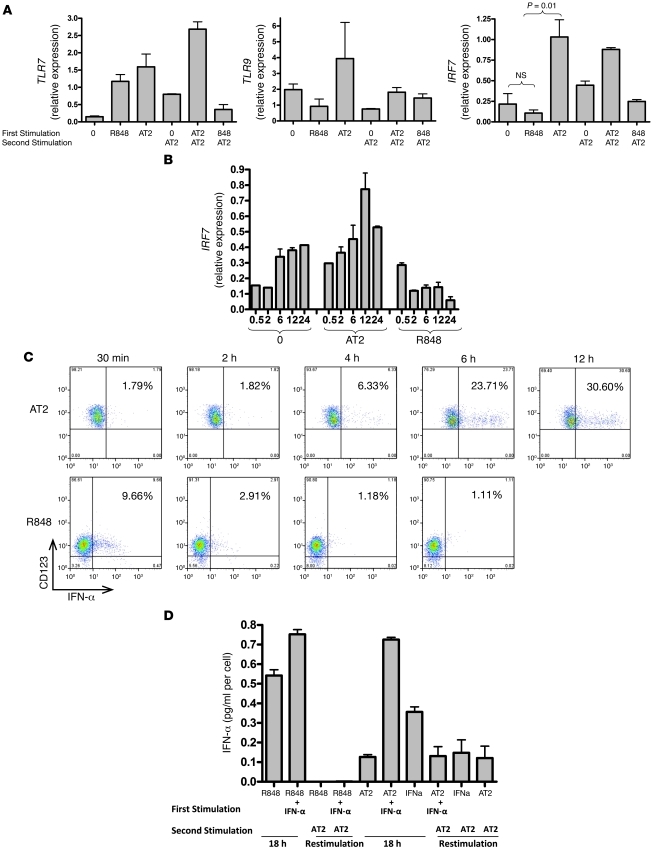
To understand the mechanism by which HIV-activated pDCs do not become tolerant to persistent IFN-α production, we first sought to compare mRNA expression of TLR7, TLR9, and IRF7 in pDCs exposed to either AT-2 HIV or R848. We hypothesized that the maintenance or upregulation of these molecules, which are necessary for pDC IFN-α signaling, may be responsible for the persistence of IFN-α production upon restimulation. As AT-2 HIV is equivalent to live HIV in terms of pDC activation through TLR7 (1, 24), we completed subsequent experiments using AT-2 HIV. Because pDCs are rare and cells available for experiments are limited, we focused on comparing AT-2 HIV with R848, as R848 is also a TLR7 agonist but fully matures pDCs and makes them completely refractory to secondary IFN-α responses. pDCs were incubated with media alone, AT-2 HIV, or R848 for 18 hours and were either washed for immediate preparation of mRNA or washed and placed back in culture with AT-2 HIV for 18 hours. TLR7, TLR9, and IRF7 mRNA levels were quantified by quantitative real-time RT-PCR (qRT-PCR). We found differences in IRF7 expression, but not TLR7 or TLR9 expression, of pDCs incubated with AT-2 HIV compared with R848. Whereas TLR7 expression increased after exposure to either AT-2 HIV or R848 compared with unstimulated pDCs, IRF7 expression increased only in AT-2 HIV–exposed pDCs, decreasing in R848-exposed pDCs. TLR9 expression was variable in pDCs exposed to either AT-2 HIV or R848 (Figure 4A). When IRF7 mRNA expression was measured over 24 hours, unstimulated cells spontaneously increased IRF7 expression, and AT-2 stimulation augmented IRF7 expression further, but R848 decreased IRF7 expression over the time course (Figure 4B). The kinetics of IFN-α production were measured using intracellular staining of AT-2 HIV– or R848-exposed pDCs. R848-stimulated pDCs produced IFN-α within 30 minutes but were no longer producing by 2–4 hours. In contrast, AT-2 HIV–stimulated pDCs did not begin to produce IFN-α for 6 hours, but continued to produce at 12 hours (Figure 4C). To test whether exogenous IFN-α could rescue the block to restimulation with R848, purified pDCs were incubated with IFN-α and R848, but again, pDCs could not be restimulated to produce IFN-α (Figure 4D). Nuclear translocation of IRF7 after stimulation of HIV versus R848 was also tested. pDCs were exposed to R848 or live HIV for 2–4, 6, or 12 hours. IRF7 nuclear translocation was apparent at 2–4 hours after R848 stimulation (Supplemental Figure 1C) and was unchanged at 6 and 12 hours (data not shown). HIV-activated pDCs did not show evidence of IRF7 nuclear translocation at 2–4, 6, or 12 hours (data not shown). This is likely because a minority of pDCs are infected with HIV by 12 hours; therefore, nuclear translocation of IRF7 in these few cells is not readily apparent. To select the minority of activated pDCs for microscopic analysis, we sorted HIV-activated IFN-α–producing pDCs from those that were not producing IFN-α after 12 hours, using the Miltenyi IFN-α secretion assay kit. Using this strategy, we clearly saw IRF7 nuclear translocation in HIV-activated IFN-α–producing pDCs compared with those that were not producing IFN-α after 12 hours (Supplemental Figure 2D). There was no discernible pattern (i.e., increased cytoplasmic as well as nuclear IRF7) in HIV-activated compared with R848-activated pDCs.
Figure 4. HIV-activated pDCs increase mRNA expression of IRF7, which correlates with ability to produce secondary IFN-α responses.
(A) pDCs were incubated with media alone, AT-2 HIV, or R848 for 18 hours, then washed for immediate preparation of mRNA or washed and placed back in culture with AT-2 HIV for 18 hours. cDNA was prepared for qRT-PCR. Relative expression of gene products was normalized to GAPDH. Whereas TLR7 expression increased in pDCs exposed to either AT-2 HIV or R848, and TLR9 expression was variable, IRF7 expression increased relative to unstimulated pDCs (*P < 0.05; Student’s t test) in AT-2 HIV–exposed pDCs, but decreased in R848-exposed pDCs. Data are mean ± SEM (n = 3 replicates) and representative of 3 independent experiments. (B) IRF7 expression was spontaneously increased by unstimulated cells and further augmented by AT-2 HIV stimulation, but was decreased by R848 over the time course. (C) R848-stimulated pDCs produced IFN-α within 30 minutes, but were no longer producing by 2–4 hours. In contrast, AT-2 HIV–stimulated pDCs did not begin to produce IFN-α for 6 hours, but continued to produce at 12 hours. Data are representative of 5 independent experiments. (D) To test whether exogenous IFN-α could rescue the block to restimulation with R848, purified pDCs were incubated with IFN-α and R848; again, pDCs could not be restimulated to produce IFN-α (*P < 0.05 versus first stimulation with R848; Student’s t test). Data are mean ± SEM (n = 3 replicates) and representative of 2 independent experiments.
HIV-activated IFN-α production is IFN-α inducible, in contrast to R848-activated IFN-α production.
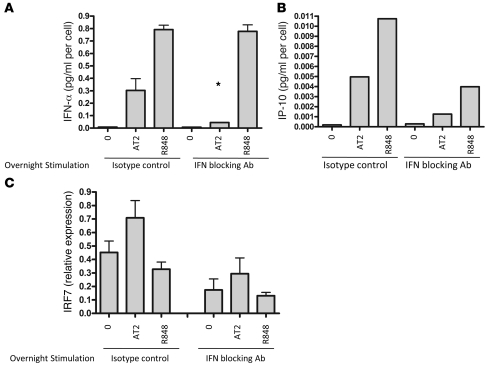
To evaluate whether the autocrine type I IFN feedback loop is necessary for IFN-α production when pDCs are stimulated with AT-2 HIV in comparison to R848 (26), we preincubated the pDCs for 30 minutes with blocking antibodies to IFN-α, IFN-β, and IFN-α/β receptor and then stimulated the cells for 18 hours with AT-2 HIV or R848. IFN blocking antibodies attenuated IFN-α production upon AT-2 HIV stimulation, but not upon R848 stimulation (Figure 5A), which suggests that the IFN-α feedback loop enhances IFN-α secretion upon stimulation with HIV, but not R848. Bioactive IFN-α production was confirmed by testing the culture supernatants by ELISA for IP-10, an IFN-α–inducible protein (Figure 5B). In addition, we noted that IFN blockade reduced IRF7 expression of AT-2 HIV–activated pDCs, unstimulated pDCs, and R848-stimulated pDCs (Figure 5C). Our observation that IFN blocking antibodies reduced IRF7 expression in unstimulated pDCs was surprising and suggested that pDCs use the type I IFN autocrine feedback loop to maintain increased baseline levels of IRF7.
Figure 5. HIV-activated IFN-α production is IFN-α inducible, whereas R848-activated IFN-α production is not.
(A) pDCs were preincubated for 30 minutes with blocking antibodies to IFN-α, IFN-β, and IFN-α/β receptor and then stimulated for 18 hours with AT-2 HIV or R848. IFN blocking antibodies attenuated IFN-α production upon AT-2 HIV stimulation (*P < 0.05 versus AT-2 HIV stimulation without antibodies; 2-tailed Student’s t test), but not R848 stimulation. Data are mean ± SEM (n = 3 replicates) and representative of 4 independent experiments. (B) Bioactive IFN-α production was confirmed by testing the culture supernatants by ELISA for IP-10, an IFN-α–inducible protein. (C) IFN blocking antibodies also reduced IRF7 expression of AT-2 HIV–activated pDCs and reduced expression of IRF7 in unstimulated and R848-stimulated cells.
High baseline mRNA expression of IRF7 in unstimulated pDCs is likely a result of constitutive low-level type I IFN production by pDCs.
To test the hypothesis that high IRF7 expression in unstimulated pDCs is not constitutive, but is induced by spontaneous type I IFN production, we used a reporter COS cell line that is highly sensitive to type I IFNs (lower limit of detection, 1 U/ml) and produces luciferase upon exposure to these cytokines. We purified pDCs from 6 different donors and incubated the cells for 18 hours. Culture supernatants were applied to the COS cell line and assayed for bioactive type I IFN. All 6 donors produced varying amounts of bioactive type I IFNs in the absence of stimulation (Figure 6A). To explore this phenomenon further, we sorted pDCs and myeloid DCs (mDCs) to 99% purity and prepared fresh cells and incubated cells overnight in culture media without additional stimuli. IFNA and IFNB mRNA were expressed at low levels in pDCs, but not mDCs, although expression did not increase significantly after overnight incubation. However, in pDCs, but not mDCs, IRF7 and IFN-stimulated gene 54 (ISG54) increased significantly after overnight incubation (Figure 6, B and C), again suggesting that human pDCs express low levels of bioactive type I IFNs that maintain increased baseline IRF7 and other IFN-stimulated genes.
Figure 6. High baseline mRNA expression of IRF7 in unstimulated pDCs is likely a result of constitutive low-level type I IFN production by pDCs.
(A) pDCs from 6 different donors were incubated in media without stimulation for 18 hours. Culture supernatants were applied to the COS cell line and assayed for bioactive type I IFN. All 6 donors produced varying amounts of bioactive type I IFNs in the absence of stimulation compared with control (CTL) mDCs. Data are mean ± SEM (n = 3 replicates). (B) pDCs and mDCs from 5 different donors were sorted with FACS ARIA to 99% purity. mRNA was isolated from fresh cells (0 h) and after overnight incubation in culture media without stimulation (18 h). qRT-PCR analysis revealed that IFNA and IFNB mRNA transcripts were expressed at low levels in pDCs but not mDCs, and expression was variable after overnight incubation. However, in pDCs but not mDCs, IRF7 and ISG54 increased after overnight incubation. (C) Pooled data for 5 pDC donors. Change in relative expression was calculated as the difference between expression levels of fresh and overnight-stimulated cells divided by the overnight expression level, and expressed as a percentage. *P < 0.05, 2-tailed Student’s t test. Data are mean ± SEM (n = 5 donors).
SOCS1 and SOCS3 are not inhibited by HIV.
The SOCS protein family has been implicated in the negative regulation of many cytokine-stimulated pathways. SOCS1 and SOC3 are strongly induced by IFN-α, and overexpression of these proteins has been shown to suppress IFN-α production by interfering with the JAK/STAT pathway (J.J. Krolewski, unpublished observation). We were interested to investigate whether there are differences in SOCS1 and SOCS3 mRNA expression in pDCs exposed to AT-2 HIV or R848. AT-2 HIV caused gradually increased expression of both SOCS1 and SOCS3 over 24 hours compared with unstimulated pDCs. R848 caused a sharp and immediate increase in SOCS3, but not SOCS1, although the increased expression was not sustained (Figure 7). Early upregulation of SOCS3 might contribute to inhibition of further IFN-α production after R848 stimulation.
Figure 7. SOCS1 and SOCS3 are not inhibited by HIV.
mRNA expression of IRF7, SOCS1, and SOCS3 in pDCs exposed to AT-2 HIV or R848 is shown. Unstimulated pDCs spontaneously increased IRF7 expression; AT-2 HIV stimulation augmented this expression further, but R848 decreased it over the time course. AT-2 HIV caused gradual increased expression of both SOCS1 and SOCS3 over 24 hours compared with unstimulated pDCs; R848 caused a sharp and immediate increase in SOCS3, but not SOCS1, but the increased expression was not sustained. Data are mean ± SEM (n = 3 replicates) and representative of 2 independent experiments.
HIV is retained in early endosomes.
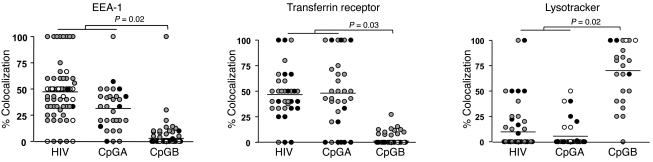
Studies have suggested that TLR9 signaling in early endosomes leads to IFN-α production by pDCs, whereas TLR9 signaling in late endosomes induces pDC maturation and inflammatory cytokine production (27–29). Because HIV and CpGA similarly stimulate pDCs to produce persistent IFN-α responses and express low levels of markers of maturation, we sought to investigate whether HIV, like CpGA, also traffics primarily to early endosomes. pDCs were stimulated with fluorescently labeled GFP-HIV, FAM-CpGA, or FAM-CpGB. GFP-HIV was generated using a Vpr mutant of the X4-tropic HIV pNL4-3 and eGFP-Vpr plasmids; therefore, the viruses are nonreplicative, and the GFP protein visualized by microscopy incorporates with the conical core of the HIV particle, which is thought to be closely associated with the viral RNA (30, 31). This system has been used to tag HIV particles in order to follow intracellular virus behavior during the early steps of infection of target cells (32, 33). After overnight incubation, the fluorescently labeled agonists stimulated pDCs in a manner similar to that of unlabeled agonists (i.e., GFP-HIV), and FAM-CpGA stimulated pDCs to produce strong IFN-α responses but minimal expression of CD86, whereas FAM-CpGB–stimulated pDCs produced minimal IFN-α and induced high expression of CD86 (data not shown). For lysotracker imaging, pDCs were incubated with GFP-HIV or CpGA for 18 hours or CpGB for 1 hour. These time points were chosen because minimal GFP-HIV or FAM-CpGA was taken up by pDCs before 12 hours, and FAM-CpGB was taken up entirely by 1 hour and was subsequently degraded and no longer visualized at 12 hours (data not shown). After incubation, pDCs were imaged live for analysis of late endosomal/lysosomal (lysotracker) trafficking or fixed and stained with specific antibodies for early endosomal and recycling compartments (EEA-1 and transferrin receptor). Live lysotracker trafficking and fixed cells were imaged using the Advanced Precision PersonalDV imaging system at ×60. Images were deconvoluted and analyzed using ImageJ. Colocalization of fluorescently labeled ligands with endosomal markers were analyzed quantitatively using intensity correlation analysis (Figure 8). For each cell that contained both green and red points, image contrast was set to minimize bleed-through (range, 100–2,500), background was subtracted, and intensity correlation analysis was performed, including product of differences from the mean (PDM) — with positive PDM values correlating with Pearson and Mander coefficients consistently greater than 0.7. The distributions of percent colocalization of agonists with endosomal markers were not normal and varied among donors. In order to compare characteristics of these divergent distributions and to maintain the correlated information within each donor, medians and interquartile ranges (IQRs) obtained for each subject were compared among subjects exposed to HIV, CpGA, and CpGB. Nonparametric Kruskal-Wallis tests were used to compare the 3 conditions with respect to medians and IQRs. The Wilcoxon rank-sum test was then used to compare the combined data for HIV and CpGA to CpGB. Table 2 presents the results of analyses of pooled pDC microscopy experiments from 2–3 different donors. For EEA-1 and transferrin receptor, percent colocalization of HIV, CpGA, and CpGB in pDCs differed with respect to intrasubject medians and intrasubject IQRs (borderline P ≤ 0.08 in all instances). HIV- and CpGA-exposed pDCs combined had significantly greater percent colocalization with EEA-1 and transferrin receptor, but significantly less with lysotracker, compared with CpGB. For lysotraker, results were as above with respect to intrasubject medians only, and HIV- and CpGA-stimulated pDCs combined had significantly less percent colocalization with lysotracker than did CpGB-stimulated pDCs. CpGA and HIV trafficked similarly to each other and differently from CpGB for all endosomal markers studied, and this pattern was statistically significant using the Wilcoxon rank-sum test (Figure 9).
Figure 8. HIV and CpGA traffic predominantly to early (EEA-1) and recycling (transferrin receptor) endosomes, whereas CpGB traffics predominantly to lysosomes (lysotracker).
Shown are representative images of pDCs incubated with GFP-HIV, FAM-CpGA, or FAM-CpGB and stained with (A) EEA-1 or (B) transferrin receptor (Trans) or incubated with (C) lysotracker (Lyso). Differential interference contrast (DIC), fluorescent, merged, and PDM images are shown. Yellow in merged and PDM images indicates colocalization; blue/white points in PDM images correspond to negative intensity correlation (no colocalization). Images demonstrating colocalization are shown at higher magnification in the insets. Original magnification, ×60; ×120 (insets).
Table 2 .
Statistical analysis of microscopy data
Figure 9. Statistical analysis of pooled microscopy data.
Shown are pooled data from 2–3 different donors per experiment; each symbol represents a cell, and different colors represent cells from different donors. Percent colocalization was calculated as PDM-positive green/red intensity correlation points relative to green points. Wilcoxon rank-sum tests were used to compare intrasubject medians and IQRs of combined HIV and CpGA subjects with those of CpGB-exposed pDCs from different donors. HIV and CpGA had similar trafficking colocalization; both differed significantly from CpGB.
Discussion
HIV infection induces an inflammatory and immune-activated state characterized by increased levels of plasma inflammatory cytokines and increased markers of cell turnover, activation, and exhaustion (12, 34–39). Potent antiretroviral therapy restores the immune system by suppressing HIV to clinically undetectable levels, but does not completely reverse inflammation and immune activation (40). Recent findings reveal that HIV-infected persons, even with full virologic suppression on antiretroviral therapy, are at increased risk of cardiovascular events and of renal and liver disease (41). An evolving model suggests that these increased comorbidities are linked to heightened inflammation and immune activation. The cause of immune activation is unknown, but pathogenic stimulation of innate immune cells by HIV is thought to be a major contributor (42). For the first time to our knowledge, we have found that pDCs are dysregulated by HIV to persistently produce IFN-α upon restimulation and to incompletely mature the cells, which may contribute more to disease pathogenesis (inflammation and immune activation) than to protection (stimulation of protective adaptive immune responses). Notably, previous studies have reported decreased IFN-α production by PBMCs from HIV-infected subjects in response to activation by TLR agonists ex vivo (22, 43–46), whereas we showed preserved and sustained IFN-α responses in response to HIV activation of pDCs in vitro. Discrepancies between our results and those of others may be based upon differences in TLR agonists used to activate pDCs. For example, Anthony et al. evaluated pDC function upon stimulation of PBMCs with the synthetic TLR9 agonist CpG (43), Chehimi et al. used influenza virus (44), and Kamga et al. used herpes simplex virus–1 (45). In our recent study we found that pDCs purified from acutely HIV-infected subjects produce normal to high IFN-α in response to HIV, but low IFN-α in response to herpes simplex virus–1, a TLR9-activating virus (21). Moreover, it has previously been shown that TLR9- but not TLR7-mediated activation of pDCs is inhibited by HIV gp120, further supporting the idea that the impairment of pDC function in HIV infection is specific to the TLR agonist used (47). Although it is likely that pDCs exposed to HIV in vivo may respond less well to other TLR agonists, especially TLR9 agonists, we have previously shown that they are hyperresponsive to HIV ex vivo (21). We now provide mechanistic in vitro data in support of this ex vivo observation.
There were striking differences between pDC maturation and IFN-α responses to various TLR7 and TLR9 agonists. Whereas R848, CpGB, and heat-inactivated influenza virus fully matured pDCs and made them refractory to repeated stimulation to produce IFN-α, Sendai virus was intermediate, and HIV and CpGA allowed for persistent IFN-α production upon restimulation. There was a delayed onset of IFN-α production by pDCs after HIV stimulation, but the production of IFN-α was prolonged, in contrast to the effect of R848 stimulation. Moreover, HIV-stimulated pDCs maintained high levels of IRF7 mRNA expression, which correlated with their ability to produce IFN-α upon repeated stimulation. In comparison, R848 fully matured pDCs and downregulated expression of IRF7. Microscopy experiments evaluating nuclear translocation of IRF7 after HIV versus R848 activation did not reveal discernible differences, which suggests that IFN-α–producing cells have undergone nuclear translocation of IRF7 regardless of stimuli. Inhibiting IFN-α signaling using IFN-α/β and IFN-α/β receptor blocking antibodies attenuated the ability of HIV to stimulate pDCs to produce IFN-α. In fact, blocking IFN-α signaling also decreased expression of IRF7 in control nonactivated pDCs in culture media overnight. Using qRT-PCR and a reporter cell line–based bioassay, we showed that elevated baseline IRF7 mRNA expression in human pDCs is likely not constitutive, but rather induced by the spontaneous production of IFN-α/β. To our knowledge, this has been demonstrated previously in murine pDCs (48), but not conclusively in humans.
Recent studies reveal that pDCs may be functionally dichotomous; depending upon which TLR agonist is used, either they produce IFN-α or they develop a mature antigen-presenting phenotype capable of stimulating antigen-specific effector memory T helper cells (49). Much of the work exploring differences in pDC functionality based on distinct TLR ligation has been done comparing TLR9 synthetic agonists CpGA and CpGB. It has been shown that CpGA-stimulated pDCs cause higher and prolonged kinetics of type I IFN production compared with those caused by CpGB stimulation. In contrast, CpGB is more active than CpGA in stimulating IL-8 production and increasing costimulatory and antigen-presenting molecules. Additionally, CpGA, but not CpGB, activates the type I IFN receptor–mediated autocrine feedback loop (50). Studies using confocal microscopy have shown that CpGA forms multimeric complexes and is retained for long periods in the endosomal vesicles of pDCs, together with the MyD88-IRF7 complex, whereas monomeric CpGB traffics rapidly to late endosomal vesicles (27–29). A recent study showed that CpGB and influenza stimulate pDCs to form NF-κB–dependent intracellular pools of MHC II molecules that are persistently neosynthesized and accumulate in antigen loading compartments. In contrast, CpGA stimulation of pDCs does not lead to the formation of MHC II intracellular clusters (51). CpGB-stimulated pDCs efficiently process and present CMV antigens and are capable of stimulating CMV-specific effector memory T helper cells. CpGA-stimulated pDCs produce large amounts of type I IFNs, but fail to induce CMV-specific CD4+ effector memory T cells to produce IFN-γ (49). Interestingly, influenza virus induces IFN-α production but also matures pDCs fully, in comparison to CpGB, which stimulates minimal IFN-α production. Agonists such as influenza virus or CpGC can both stimulate IFN-α production and antigen-presenting capacity in pDCs; however, these pDCs that obtain the mature antigen-presenting phenotype cannot be restimulated to produce IFN-α (50). Thus, strong maturation correlates with a refractory state to further cytokine production. Potential mechanisms for inhibited IFN-α production after strong maturation include changes at the transcriptional and posttranscriptional level. At the transcriptional level, we showed downregulation of IRF7 mRNA after a strong maturational stimulus (i.e., R848). At the posttranscriptional level, it has previously been shown that strong activation of the NF-κB signaling pathway causes ubiquitination and proteasomal degradation of IRAK1, a necessary component of the transductional transcriptional processor complex necessary for IRF7 phosphorylation and nuclear translocation (52).
In our experiments, we have shown that HIV, like CpGA, partially matured pDCs and activated the type I IFN receptor–mediated autocrine feedback loop, allowing for persistent IFN-α production upon repeated stimulation. We found that HIV behaved like CpGA, trafficking to the early endosome to stimulate persistent type I IFN responses, instead of to the lysosome, where NF-κB and MAPKs are activated to induce the transcription of proinflammatory cytokines, chemokines, and costimulatory molecules and where MHC complexes colocalize for antigen presentation. We speculate that it is the lack of strong activation of the NF-κB signaling pathway that allows for restimulation of pDCs to produce IFN-α. If CpGA and HIV are being retained in the early endosome and therefore not strongly activating the NF-κB signaling (maturation) pathway, pDCs can be persistently stimulated to produce IFN-α. Additionally, because IFN-α production relies on a positive feedback loop involving IRF7 upregulation, retention of activating ligands in the early endosomes may further facilitate ongoing IFN-α production. In vivo, HIV may act upon pDCs to skew their responses toward an IFN-α–producing phenotype while failing to induce efficient HIV antigen presentation to HIV-specific effector memory T cells. Effective HIV-specific CD4+ and CD8+ T cells are deficient in chronic HIV infection and likely strongly contribute to lack of virologic control (53, 54). Little is known regarding the mechanisms by which HIV-stimulated pDC dysregulation may contribute to immune activation and poor adaptive immune stimulation. Deeper understanding of HIV-pDC interactions and the TLR-induced signaling pathways that regulate type I IFN production versus maturation and antigen presentation could elucidate new therapies targeting immune activation and inflammation in HIV disease.
Methods
Cells.
PBMCs were separated on Ficoll-Hypaque (Amersham Biosciences) from buffy coats (New York Blood Center). pDCs were purified by BDCA-4 magnetic bead separation (Miltenyi Biotec) as described previously (1), with a purity ranging from 80% to 95%. Cells were cultured in RPMI 1640 Glutamax (Invitrogen) with 5% PHS (Innovative Research), gentamicin, and HEPES.
HIV subjects.
Subjects were recruited through NYU and CHAVI 012 clinical sites (Aaron Diamond AIDS Research Center, New York, New York, USA, and University of North Carolina, Chapel Hill, North Carolina, USA) to undergo leukapheresis to allow for collection of large numbers of PBMCs. This study was reviewed and approved by the Institutional Review Board of Bellevue Hospital, New York University School of Medicine, University of North Carolina Chapel Hill, Aaron Diamond AIDS Research Center, and CHAVI. Written informed consent was obtained from all study participants in accordance with the Declaration of Helsinki.
Activating agonists.
Purified pDCs were stimulated at 50,000 cells/100 μl media at 37°C, 5% CO2, with R848 (10 μM; 3M Corp.), CpGB (5′-T*C*G*T*C*G*T*T*T*T*G*T*C*G*T*T*T*T*G*T*C*G*T*T*-3′, with asterisks denoting phosphorothioate bonds; 2 μg; IDT), CpGA (5′-G*G*G*GGACGATCGTC*GG*G*G*G*G-3′; 2 μg; IDT), Sendai virus (100 U/ml), and influenza virus PR8 heat inactivated at 56°C for 30 minutes in a water bath (MOI 5).
HIV virions.
HIV-1MN (X4-tropic) and HIV-1ADA (R5-tropic) were produced at the AIDS Vaccine Program, National Cancer Institute, as previously described (1, 23, 24). For preparation of noninfectious virus (AT-2 HIV) with functionally intact envelope glycoproteins, virus was inactivated by treatment with 1 mM AT-2 (Sigma-Aldrich) and purified and concentrated as previously described (55). To study markers of maturation, after 18 hours of incubation, cells were washed and stained with anti-CD123–PE, anti-CD86–allophycocyanin, anti-CD40–PerCP, anti-CCR7–FITC, or isotype control (BD Biosciences — Pharmingen).
Cytokine/chemokine analysis.
Supernatants were analyzed using cytometric bead array (BD Biosciences — Pharmingen). The presence of IFN-α was detected by the multisubtype IFN-α ELISA kit (PBL Biomedical). IP-10 was detected by ELISA (Invitrogen). For intracellular staining, pDCs were incubated at 100,000 cells/200 μl media with either R848 or AT-2 HIV, and brefeldin A was added after 30 minutes, 2 hours, 6 hours, or 12 hours; at 18 hours, cells were washed and stained with PE-conjugated CD123 and FITC-conjugated IFN-α in 0.05% saponin.
Allogenic T cell proliferation.
pDCs were incubated overnight with media alone, AT-2 HIV, CpGA, CpGB, or heat-inactivated influenza; washed; and incubated at 1:20 (10,000 pDCs/200,000 T cells) with Miltenyi-bead purified CFSE-labeled allogeneic CD4+ or CD8+ T cells, as compared with T cells alone or T cells with anti-CD3 (1 μg/ml) and anti-CD28 (0.5 μg/ml; BD Biosciences — Pharmingen). T cells were isolated from PBMCs using negative selection with Miltenyi anti-RO, CD19, CD25, CD14, CD56, and then positive selection with anti-CD4 or anti-CD8, purity greater than 90%. T cells were incubated with CFSE (1 μM) for 10 minutes and washed and counted prior to coculture with pDCs. After 6 days of coculture, CFSE dilution was analyzed by FACS.
IFN-α/β blocking.
pDCs were incubated (50,000 cells/200 μl) with mouse monoclonal antibody against IFN-α, IFN-β, and IFN-α/β receptor chain (10 μg/ml; PBL Biomedical) or isotype control anti-mouse IgG1κ for 30 minutes. AT-2 or R848 was then added, and cells were placed back in culture for 18 hours. Cells were washed and prepared for qRT-PCR, and culture supernatants were removed and tested for IFN-α and IP-10 by ELISA.
qRT-PCR.
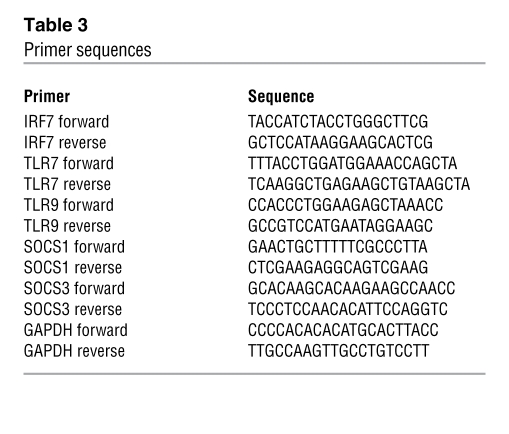
pDC RNA was isolated using the RNAeasy Mini kit (Qiagen) and converted to cDNA using the Qiagen SuperScript III RT kit, and assays were performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) as per protocol. All primers (Table 3) were purchased from Operon except the IRF7 primers, which were purchased from Sigma-Aldrich. Reactions were conducted using the BioRad icycler IQ5 RT-PCR detection system. For experiments evaluating spontaneous production of IFN-α by examining IFN-related genes of mDCs and pDCs, DC subsets were enriched from buffy coats using blood DC isolation kits (Miltenyi Biotec), followed by staining with antibodies recognizing lineage markers (CD3, CD14, CD16, CD19, CD20, and CD56), CD11c (all from BD Biosciences — Pharmingen), and CD4 (Invitrogen) before sorting by flow cytometry using BD FACS Aria. Both DC subsets were sorted to 99% purity based on the absence of lineage markers and the high expression of CD4, with mDCs expressing CD11c and pDCs not expressing CD11c.
Table 3 .
Primer sequences
Quantitative bioassay for type I IFNs.
Supernatants of cell cultures after stimulatory conditions were assayed for IFN activities using a recombinant COS-1 cell line, which carries a luciferase reporter containing multiple repeats of IFN-stimulated response element (ISRE). The reporter cells were exposed to cell culture supernatants for 8 hours to overnight, assayed for luciferase activities, and then translated to IFN activities using a standard curve generated from a serial dilution of human IFN-α2a.
Microscopy.
Nonreplicative GFP-HIV was generated by cloning a Vpr mutant of the X4-tropic HIV pNL4-3 and eGFP-Vpr plasmids in E. coli; purified using a Midi/Maxi kit (Qiagen); and transfected (Mirus TransIT-293) for 48 hours using HEK 293T cells. Supernatants were collected, cellular debris was removed, and virus was concentrated by ultracentrifugation (133,907 g, 1 hour at 4°C), and quantified using p24 ELISA at 0.23 ng/ml (AIDS Vaccine Program). 5′FAM-labeled CpGA and CpGB were purchased from IDT. pDCs were stimulated with GFP-HIV (230 pg/ml), FAM-CpGA (1 μg), or FAM-CpGB (1 μg). After incubation, cells were washed and added to 0.01% poly-l-lysine–coated (Sigma-Aldrich) Lab-Tek chambered no. 1.0 borosilicare system wells (Nunc). 30 minutes prior to imaging, lysotracker was added at 1 μM (Invitrogen). For EEA-1 and transferrin receptor staining, pDCs were stimulated with GFP-HIV, FAM-CpGA, or FAM-CpGB; washed; plated on 0.01% poly-l-lysine–coated culture slides (BD Biosciences — Falcon); fixed with 4% paraformaldehyde; permeabilized with 0.1% Triton X-100; blocked with 0.5% BSA in PBS; and then stained with mouse anti–transferrin receptor (0.5 μg; Invitrogen) or mouse anti-EEA1 (0.5 μg; BD Biosciences) for 1 hour. Cells were washed and then stained with goat anti-mouse 568 (5 μg/ml; Invitrogen) for 1 hour. Cells were washed, dried, and mounted in Vectashield mounting media (Vector Labs). For IRF7 staining, pDCs were stimulated with live HIV or R848 for 2–4, 6, and 12 hours; washed; plated on 0.01% poly-l-lysine–coated culture slides (BD Biosciences — Falcon); fixed with 4% paraformaldehyde; permeabilized with 0.1% Triton X-100; blocked with 0.5% BSA in PBS; and then stained with rabbit anti-IRF7 (5 μg/ml; Santa Cruz) for 1 hour. Cells were washed and then stained with goat anti-rabbit 488 (5 μg/ml; Invitrogen) for 1 hour. Cells were washed, dried, and mounted in Vectashield mounting media (Vector Labs).
Statistics.
Statistical analysis was performed on at least 2–3 independent experiments using 2-tailed Student’s t test (Figures 1–7). Statistical analysis of microscopy data (Figure 9) used nonparametric Kruskal-Wallis tests and the Wilcoxon rank-sum test using SPSS version 16.0 and SAS version 9.2. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We would like to acknowledge David Ott and Jeffrey Lifson (AIDS Vaccine Program, Frederick, Maryland, USA) for providing HIV-1 MN, HIV-1 ADA, and AT-2 HIV and plasmids for generating GFP-HIV; Barton Haynes for guidance; Joel Ernst and Persephone Borrow for critical review of the manuscript; Demetre Daskalakis for study subject recruitment; and Yan Deng for his advice and assistance in using the NYULMC microscopy core. The authors acknowledge the following funding support: NIH grants P01 AI057127-01A1, R37 AI044628, R01-AI28900, and AI047033; CHAVI grant U01 AI 067854; Bill and Melinda Gates Foundation grant RFP-GH-HTR-05-02; the Rockefeller University CCTA; grant UL1 RR024143-01 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research; General Clinical Research Centers Clinical Research Feasibility Funds grant M01 NIH RR00096; Center for AIDS Research pilot project grant P30 AI027742; NIH 1UL1RR029893 CTSI pilot grant; and the NYULMC Grunebaum AIDS research scholarship.
Footnotes
Conflict of interest: N. Bhardwaj has patents related to DCs and has stocks in Bristol-Myers Squibb, Pfizer, and Dendreon.
Citation for this article: J Clin Invest. 2011;121(3):1088–1101. doi:10.1172/JCI44960.
References
- 1. Beignon AS, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115(11):3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 3. Latz E, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5(2):190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 4. Li Q, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Sydow M, Sonnerborg A, Gaines H, Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7(4):375–380. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- 6. Herbeuval JP, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103(18):7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lehmann C, et al. Increased interferon alpha expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2008;48(5):522–530. doi: 10.1097/QAI.0b013e31817f97cf. [DOI] [PubMed] [Google Scholar]
- 8. Hazenberg MD, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 9. Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 10. Papagno L, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2(2):E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benito JM, et al. Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4+ T cells under successful highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;38(4):373–381. doi: 10.1097/01.qai.0000153105.42455.c2. [DOI] [PubMed] [Google Scholar]
- 12. Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 13. Bofill M, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS. 1996;10(8):827–834. doi: 10.1097/00002030-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 14. Meier A, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15(8):955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119(12):3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris LD, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84(15):7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119(12):3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diop OM, et al. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol. 2008;82(11):5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107(6):2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 20. Bjorck P. Dendritic cells exposed to herpes simplex virus in vivo do not produce IFN-alpha after rechallenge with virus in vitro and exhibit decreased T cell alloreactivity. J Immunol. 2004;172(9):5396–5404. doi: 10.4049/jimmunol.172.9.5396. [DOI] [PubMed] [Google Scholar]
- 21. Sabado RL, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116(19):3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101(11):4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 23. Manches O, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118(10):3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fonteneau JF, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78(10):5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 26. Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2(5):378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 27. Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434(7036):1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 28. Harada N, Honda S. Analysis of spatiotemporal regulation of aromatase in the brain using transgenic mice. J Steroid Biochem Mol Biol. 2005;95(1–5):49–55. doi: 10.1016/j.jsbmb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 29. Guiducci C, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203(8):1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welker R, Hohenberg H, Tessmer U, Huckhagel C, Krausslich HG. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J Virol. 2000;74(3):1168–1177. doi: 10.1128/JVI.74.3.1168-1177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang S, Pointer D, Singer G, Feng Y, Park K, Zhao LJ. Direct binding to nucleic acids by Vpr of human immunodeficiency virus type 1. Gene. 1998;212(2):157–166. doi: 10.1016/S0378-1119(98)00178-4. [DOI] [PubMed] [Google Scholar]
- 32. McDonald D, et al. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159(3):441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300(5623):1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 34. Neuhaus J, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neuhaus J, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24(5):697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuller LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med . 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cockerham L, et al. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53(1):102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalayjian RC, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201(12):1796–1805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ehrhard S, Wernli M, Durmuller U, Battegay M, Gudat F, Erb P. Influence of antiretroviral therapy on programmed death-1 (CD279) expression on T cells in lymph nodes of human immunodeficiency virus-infected individuals. Hum Pathol. 2009;40(10):1427–1433. doi: 10.1016/j.humpath.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 40. El-Far M, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5(1):13–19. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 41. El-Sadr WM, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 42. Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:54. doi: 10.1186/1742-4690-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anthony DD, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172(8):4907–4916. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 44. Chehimi J, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168(9):4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 45. Kamga I, et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192(2):303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 46. Siegal FP, Fitzgerald-Bocarsly P, Holland BK, Shodell M. Interferon-alpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS. 2001;15(13):1603–1612. doi: 10.1097/00002030-200109070-00002. [DOI] [PubMed] [Google Scholar]
- 47. Martinelli E, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2007;104(9):3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gresser I, et al. The essential role of endogenous IFN alpha/beta in the anti-metastatic action of sensitized T lymphocytes in mice injected with Friend erythroleukemia cells. Int J Cancer. 1995;63(5):726–731. doi: 10.1002/ijc.2910630520. [DOI] [PubMed] [Google Scholar]
- 49. Jaehn PS, Zaenker KS, Schmitz J, Dzionek A. Functional dichotomy of plasmacytoid dendritic cells: antigen-specific activation of T cells versus production of type I interferon. Eur J Immunol. 2008;38(7):1822–1832. doi: 10.1002/eji.200737552. [DOI] [PubMed] [Google Scholar]
- 50. Kerkmann M, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170(9):4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 51. Sadaka C, Marloie-Provost MA, Soumelis V, Benaroch P. Developmental regulation of MHC II expression and transport in human plasmacytoid-derived dendritic cells. Blood. 2009;113(10):2127–2135. doi: 10.1182/blood-2008-10-178152. [DOI] [PubMed] [Google Scholar]
- 52. Kubo-Murai M, Hazeki K, Nigorikawa K, Omoto T, Inoue N, Hazeki O. IRAK-4-dependent degradation of IRAK-1 is a negative feedback signal for TLR-mediated NF-kappaB activation. J Biochem. 2008;143(3):295–302. doi: 10.1093/jb/mvm234. [DOI] [PubMed] [Google Scholar]
- 53. Rosenberg ES, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 54. Kalams SA, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73(8):6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arthur LO, et al. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res Hum Retroviruses. 1998;14 suppl 3:S311–S319. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.