Abstract
Solid tumors contain hypoxic regions in which cancer cells are often resistant to chemotherapy-induced apoptotic cell death. Therapeutic strategies that specifically target hypoxic cells and promote apoptosis are particularly appealing, as few normal tissues experience hypoxia. We have found that the compound ABT-737, a Bcl-2 homology domain 3 (BH-3) mimetic, promotes apoptotic cell death in human colorectal carcinoma and small cell lung cancer cell lines exposed to hypoxia. This hypoxic induction of apoptosis was mediated through downregulation of myeloid cell leukemia sequence 1 (Mcl-1), a Bcl-2 family protein that serves as a biomarker for ABT-737 resistance. Downregulation of Mcl-1 in hypoxia was independent of hypoxia-inducible factor 1 (HIF-1) activity and was consistent with decreased global protein translation. In addition, ABT-737 induced apoptosis deep within tumor spheroids, consistent with an optimal hypoxic oxygen tension being necessary to promote ABT-737–induced cell death. Tumor xenografts in ABT-737–treated mice also displayed significantly more apoptotic cells within hypoxic regions relative to normoxic regions. Synergies between ABT-737 and other cytotoxic drugs were maintained in hypoxia, suggesting that this drug may be useful in combination with chemotherapeutic agents. Taken together, these findings suggest that Mcl-1–sparing BH-3 mimetics may induce apoptosis in hypoxic tumor cells that are resistant to other chemotherapeutic agents and may have a role in combinatorial chemotherapeutic regimens for treatment of solid tumors.
Introduction
Hypoxia is present in most, if not all, solid tumors and is known to suppress drug-induced cell death and compromise the efficacy of chemotherapy (1). The degree of tumor hypoxia has prognostic significance, and tumors with high levels of hypoxia are most refractory to treatment (2). Thus, novel agents with maintained or enhanced cytotoxicity in hypoxia could potentially improve therapeutic outcome (3). Since tissue hypoxia is rarely observed in healthy adults, hypoxia-targeted therapeutic strategies also offer potential tumor selectivity.
Bcl-2 family proteins are master regulators of apoptotic cell death and have been identified as drug targets for cancer treatment (4, 5). This family is divided into pro- and antiapoptotic members whose interactions via their BH-3 domains determine the threshold for drug-induced apoptosis. Overexpression of antiapoptotic Bcl-2 family proteins is frequent in human cancer (6), and avoidance of apoptosis (a hallmark of neoplasia; ref. 7) facilitates tumorigenesis and underpins pleiotropic drug resistance (8). As the molecular regulation of apoptosis by the Bcl-2 family of proteins was revealed, drug discovery efforts were set in train, and several novel agents that target antiapoptotic Bcl-2 family proteins have been developed, including the BH-3 mimetic agent ABT-737 (Abbott Laboratories; refs. 9, 10).
ABT-737 mimics the BH-3 domain of proapoptotic Bcl-2 family member Bad and binds with nanomolar affinity to the antiapoptotic Bcl-2 family members Bcl-2, Bcl-xL, and Bcl-w, disrupting their interactions with death-promoting Bcl-2 family members to engage apoptosis (9). ABT-737 sensitizes many types of cancer cells to conventional cytotoxic drugs in vitro and in vivo (11) and has single-agent activity in preclinical in vivo models of acute myeloid leukemia and of small cell lung cancer (SCLC) (9, 12, 13). Following encouraging preclinical studies with ABT-737, ABT-263, a structurally related, orally bioavailable analog with comparable Bcl-2 family member specificity, has entered early phases of clinical testing (10). However, ABT-737 and ABT-263 have poor affinity for the antiapoptotic Bcl-2 family member Mcl-1, an established resistance biomarker for these compounds (14, 15).
The efficacy in hypoxia of novel agents that target members of the Bcl-2 family is not known and was investigated here for ABT-737. Decreased expression of several proapoptotic Bcl-2 family members, including Bax, Bad, and Bid, can occur in hypoxia (16). Conversely, other Bcl-2 family members, BNIP3 and Nix, are upregulated in hypoxia (17). Upregulation of the ABT-737 resistance biomarker Mcl-1 in hypoxic hepatoma and tracheobronchial cells (18, 19) was shown to be dependent on hypoxia-inducible factor 1 (HIF-1, a key mediator of the adaptive cellular response to hypoxia; ref. 3). HIF-1–independent loss of Mcl-1 occurred in oxygen-deprived mouse embryonic fibroblasts (MEFs) (20). Noxa, another Bcl-2 family member that regulates Mcl-1 turnover (21), is also a HIF-1 target (22).
With these data in mind, we investigated in this study the comparative efficacy of ABT-737 in hypoxia and normoxia against SCLC cell lines where ABT-737 sensitivity has been shown in normoxia previously and in colorectal cancer (CRC) cells that are relatively resistant to ABT-737 in normoxia. Given that BH-3 mimetics, including ABT-737, synergize with conventional cytotoxic agents in vitro in normoxia (11) and that combination drug regimens are the most likely clinical utility of this class of therapeutic, interactions between ABT-737 and clinically relevant cytotoxics were determined and compared in normoxia and in hypoxia.
Results
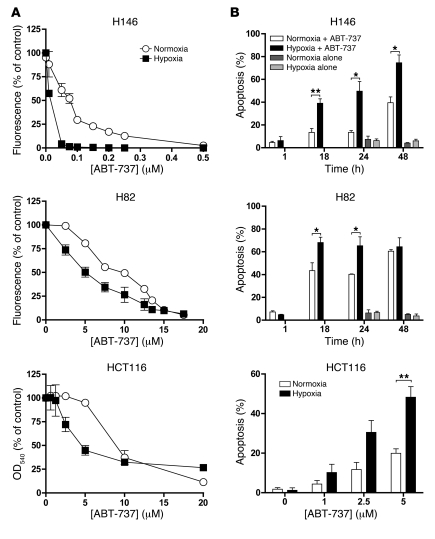
Cells were more sensitive to ABT-737 in hypoxia than normoxia.
Hypoxia, prevalent in solid human tumors, causes drug resistance (23), and consistent with this hypoxic resistance was also observed with the conventional cytotoxic agents and cell lines used in this study (ref. 23 and Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI43505DS1). The effect of hypoxia on the response of SCLC (H146 and H82) and CRC (HCT116) cells to ABT-737 was measured by resazurin or sulforhodamine B (SRB) assays. The concentration-response curves for the 3 cell lines are shown in Figure 1A, and resultant IC50 values are shown in Supplemental Table 2. In stark contrast to conventional cytotoxic agents, ABT-737 was significantly more potent in hypoxic compared with normoxic cells in all 3 cancer cell lines (P < 0.005, 2-way ANOVA; Figure 1A). In normoxic H82 and HCT116 cells, IC50 values for ABT-737 were similar, in the low micromolar range, and they were reduced 1.7- to 2.0-fold under hypoxia. The IC50 of ABT-737 for normoxic H146 cells was 82.1 nM, approximately 100-fold lower than for the other cell lines (consistent with previous assessments; ref. 9), and the degree of hypoxic sensitization was greatest for H526 cells: 21.5-fold more sensitive in hypoxia (Supplemental Table 1 and Figure 1A).
Figure 1. Effects of ABT-737 in cancer cell lines under normoxic and hypoxic conditions.
(A) H146, H82 SCLC, and HCT116 CRC cells were incubated in normoxia or hypoxia (1% O2) for 18 hours, after which they were exposed to a range of ABT-737 concentrations under continuous normoxia or hypoxia for 72 hours prior to determination of IC50 values using the resazurin assay. (B) H146 SCLC and H82 SCLC cells were preincubated for 18 hours in normoxia or hypoxia (1% O2) before treatment with ABT-737 at 89.1 nM and 12.2 μM, respectively, for the indicated times. Control cells were exposed to an equivalent concentration of drug diluent (DMSO) in normoxia or hypoxia at selected time points. HCT116 CRC cancer cells were preincubated for 18 hours in normoxia or hypoxia and treated with the indicated ABT-737 concentrations or DMSO vehicle with maintained hypoxia or normoxia and assessed 24 hours later. The percentage of cells undergoing apoptosis was determined by DAPI staining and counting at least 100 cells per field in duplicate. Data are mean ± SEM of 3 independent experiments. *P < 0.05, **P < 0.01; (Student’s 2-tailed t test).
The generality of this observation is demonstrated in Supplemental Table 2, which reports increased efficacy under hypoxia versus normoxia in 3 additional CRC cell lines, DLD-1 (IC50 of 2.4 μM and 8.0 μM, respectively), HT29 (IC50 of 2.9 μM and 9.2 μM, respectively), and CaCo2 (IC50 of 2.7 μM and 8.5 μM, respectively), and 3 other SCLC cell lines, H526 (IC50 of 0.26 μM and 5.6 μM, respectively; see below), H1048 (IC50 of 0.23 μM and 1.3 μM, respectively), and H345 (IC50 of 0.049 μM and 0.85 μM, respectively). A similar hypoxic sensitization to ABT-737 was also seen in two neuroblastoma cell lines (SH-SY5Y and SHEP1; data not shown). Hypoxic sensitization to ABT-737 has been observed in every cell line investigated so far.
Hypoxic cells were sensitized to ABT-737–induced apoptosis.
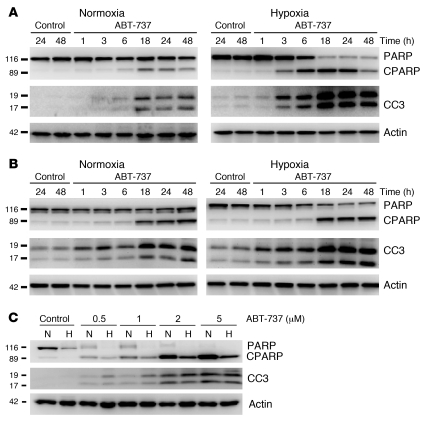
The sensitization in hypoxia to ABT-737 observed could have resulted from increased cell death and/or reduced cell proliferation. In the absence of ABT-737, hypoxia slowed the cell population growth kinetics in H82, H146, and HCT116 cell lines (Supplemental Figure 1) but did not alter basal levels of apoptosis per se (Figure 1B). Following 18 hours preincubation in normoxia or hypoxia, ABT-737–induced apoptosis was assessed by evaluation of nuclear morphology in H146, H82, and HCT116 cells maintained in hypoxic and normoxic conditions (Figure 1B). H146 and H82 cells exhibited a time-dependent induction of apoptosis in response to ABT-737 in both hypoxia and normoxia, with significantly increased apoptosis under hypoxia (P < 0.05, Student’s t test). Consistent with these data, Figure 2A (H146) and Figure 2B (H82) demonstrate the more rapid ABT-737–induced cleavage of PARP (CPARP) and caspase-3 (CC3) (biomarkers of apoptosis) in hypoxia compared with normoxia.
Figure 2. Western blot analysis of CC3 and CPARP in response to ABT-737 in cancer cell lines under hypoxic and normoxic conditions.
Cells were preincubated in normoxia or hypoxia (1% O2) for 18 hours prior to treatment with ABT-737, after which cells were harvested for protein analysis by Western blot. (A) H146 cells (treated with 89.1 nM ABT-737). (B) H82 cells (treated with 12.2 μM ABT-737). “Time” indicates the number of hours exposed to ABT-737 or control (DMSO). (C) HCT116 cells were treated with ABT-737 for 24 hours; concentration of ABT-737 is shown. Actin was used as a protein loading control.
After 18 hours preincubation in hypoxia or normoxia, treatment of HCT116 cells with ABT-737 resulted in a concentration-dependent apoptotic response in both hypoxia and normoxia at 24 hours (Figure 1B). Significantly increased apoptosis was seen for hypoxic HCT116 cells treated with 5 μM ABT-737 compared with normoxic cells (P < 0.05, Student’s t test) (Figure 1B).
Increased CC3 was seen in hypoxic HCT116 cells treated with 0.5 μM and 1 μM ABT-737 compared with normoxic counterparts, although at higher concentrations no difference between CC3 levels was detectable (Figure 2C). Hypoxia alone did not induce apoptosis but decreased full-length PARP levels in HCT116, confounding interpretation of the comparisons of ABT-737 treatment on CPARP in hypoxia and normoxia; even so, at the lower ABT-737 concentrations (0.5 μM and 1 μM), full-length PARP was detected in normoxic but not hypoxic cells, again suggesting increased apoptosis in the latter. No full-length PARP available for cleavage was detected in hypoxic HCT116 cells treated with ABT-737. It was barely detectable in normoxic cells treated with ABT-737 concentrations below 2 μM and undetectable at higher concentrations where the increased cleaved PARP was observed. To investigate whether the decrease in full-length PARP seen in hypoxic HCT116 cells was a caspase-dependent event, we treated cells in the absence and presence of the pan-caspase inhibitor QVD (20 μM) and incubated them in normoxia or hypoxia for 24 hours. Supplemental Figure 2 shows that PARP was lower in hypoxia in comparison to normoxia regardless of whether QVD was present. As a control for apoptosis and activity of QVD, cells were also treated with ABT-737 (10 μM) for 24 hours; this caused cleavage of PARP, which was prevented by QVD.
Overall these data show that while hypoxic cells proliferate more slowly than normoxic cells (Supplemental Figure 1), they are also, in comparison to normoxic cells, more sensitive to ABT-737–induced apoptosis (Figures 1 and 2).
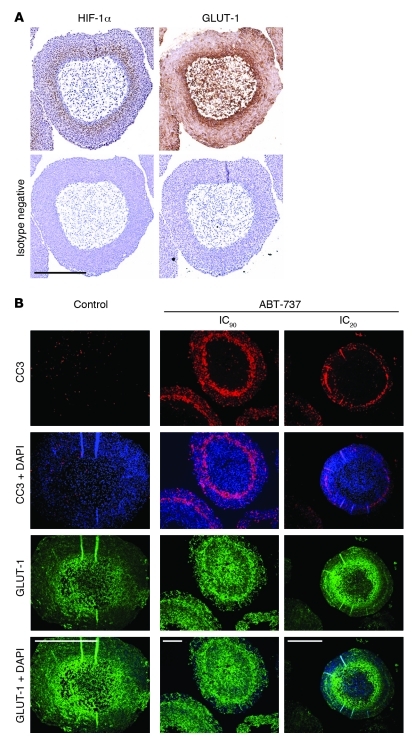
ABT-737–induced apoptosis in tumor spheroids.
We have previously shown that hypoxic regions of HCT116 spheroids were less prone to apoptosis induced by the conventional cytotoxic agent oxaliplatin when compared with normoxic regions (24). Expression of a dominant negative (DN) HIF-1 prevents upregulation of the glucose transporter GLUT-1 in hypoxic regions of HCT116 spheroids (24). GLUT-1 and HIF1-α colocalized in these spheroids (Figure 3A).The same 3D culture model was used here to investigate further the hypoxic sensitization to ABT-737, where CC3 was used to report apoptosis and GLUT-1 was used to report hypoxia. Spheroids were treated with an IC20 (2.8 μM) or IC90 (14.4 μM) concentration of ABT-737 for 24 hours before serial sectioning and immunofluorescence analysis. GLUT-1 staining (green) revealed a hypoxic rim between the necrotic core and normoxic periphery (Figure 3B). Although sporadic apoptotic cells could be seen in the outermost layers of the spheroids, ABT-737 treatment resulted in a sharply defined “ring” of CC3 staining (red) several cell layers deep into the spheroid. This CC3-positive region overlapped the region that stained positively for the HIF-1 target GLUT-1. The spheroid data are consistent with Figure 1B and Figure 2 and show that ABT-737 is most potent at inducing apoptosis in an oxygen tension at which the HIF-1 target GLUT-1 is upregulated.
Figure 3. ABT -737–induced apoptosis in HCT116 tumor spheroids.
(A) HCT116 cells were grown as spheroids, and serial sections were stained for GLUT1 or HIF-1α and then the appropriate secondary antibody or isotype negative as described in Methods. Scale bar: 500 μm. (B) Spheroids were treated with ABT-737 at IC20 (2.8 μM) or IC90 (14.4 μM) for 24 hours as described in Methods. DAPI: blue; GLUT-1: green; CC3: red. Data are representative of 3 independent experiments (n > 12 for both drug-treated and untreated spheroids). Scale bar: 500 μm.
Mcl-1 was downregulated in hypoxia.
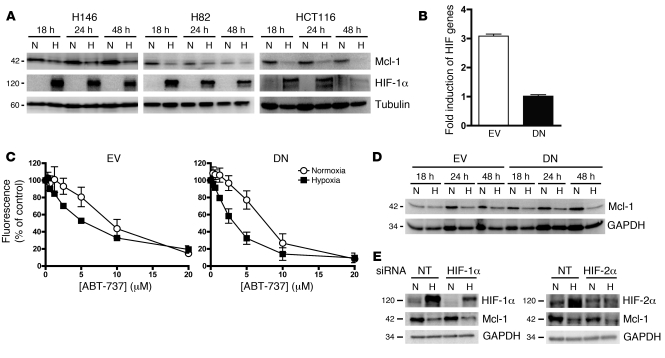
As Mcl-1 expression correlates with ABT-737 resistance (15) and increased potency of ABT-737 was observed in hypoxia, the impact of hypoxia in H146, H82, and HCT116 cells was investigated. Using the protocol used for the ABT-737 treatment studies, we found that Mcl-1 levels were consistently lower in hypoxic cells (which exhibited expression of the hypoxia marker, HIF-1α) compared to normoxic counterparts (Figure 4A and see below). Downregulation of Mcl-1 in hypoxia was observed in every cell line tested (including DLD-1 and CaCo2; Supplemental Figure 4). No other consistent changes in antiapoptotic Bcl-2 family members were observed across the cell line panel in hypoxia (data not shown). No consistent changes in proapoptotic family members were seen in normoxic or hypoxic cells before or after treatment with ABT-737, including the Mcl-1 expression modulating family member Noxa (data not shown).
Figure 4. Effect of hypoxia and HIF-1 on Mcl-1 protein expression levels.
(A) Effect of hypoxia on Mcl-1 and HIF-1α protein levels after 18, 24, or 48 hours hypoxia (1% O2) or normoxia. (B) Validation of HCT116 cell line expressing DN HIF-1α protein. HCT116 EV control and HCT116 DN HIF-1α cells were incubated in hypoxia (1% O2) or normoxia for 18 hours, after which the fold induction of firefly luciferase in hypoxia was calculated over that of Renilla luciferase (see Methods). (C) HCT116 EV or HCT116 DN HIF-1α cells were incubated in normoxia or hypoxia (1% O2) for 18 hours, after which they were exposed to a range of ABT-737 concentrations under continuous normoxia or hypoxia for 72 hours prior to determination of IC50 values using the SRB assay. (D) Western blot analysis of Mcl-1 expression level in HCT116 EV and HCT116 DN cells after 18, 24, or 48 hours hypoxia (1% O2) or normoxia. (E) HCT116 cells were treated with HIF-1α or HIF-2α or NT siRNA for 24 hours, and then siRNA was removed and cells were incubated in normoxia or hypoxia for 24 hours, after which cells were harvested and levels of HIF-1α, HIF-2α, Mcl-1, and GAPDH were determined by Western blot. Data are mean ± SEM of 3 independent experiments.
Mcl-1 downregulation in hypoxia was caspase and HIF independent.
The data so far demonstrate that increased sensitivity to ABT-737 in hypoxia was associated with decreased Mcl-1 level and that ABT-737–induced apoptosis in cells that upregulated the HIF-1 target GLUT-1. Two approaches were taken to determine whether hypoxic downregulation of Mcl-1 was HIF dependent (as previously noted with HIF-1 for the Bcl-2 family member Bid; ref. 16). First, Mcl-1 levels were examined in hypoxic and normoxic HCT116 cells containing stably overexpressed DN HIF-1α or empty vector (EV; ref. 25). Second, transient transfection of RNAi constructs to HIF-1α and HIF-2α was employed.
HCT116 DN cells express a truncated form of HIF-1α with a deleted oxygen-dependent degradation domain (ODDD) that is able to bind to HIF-1β and hypoxia-response elements (HREs) in target promoters; however, in contrast to wild-type HIF-1α, it cannot activate transcriptional machinery. These cells have been characterized previously (25). Hypoxic HCT116 EV control cells exhibited a 3-fold induction of firefly luciferase compared with that observed in normoxia, whereas hypoxia did not induce firefly luciferase in hypoxic HCT116 DN cells (Figure 4B), validating the model. Both HCT116 EV and HCT116 DN cells were significantly more sensitive to ABT-737 in hypoxia than normoxia, as evaluated by growth assay (P < 0.001 and P < 0.0001, respectively, 2-way ANOVA) (Figure 4C). Furthermore, Mcl-1 levels were downregulated in hypoxic compared with normoxic conditions regardless of HIF-1α function (Figure 4D). These data show that hypoxic sensitization to ABT-737 and Mcl-1 downregulation in hypoxia was a HIF-1–independent processes. To examine whether loss of Mcl-1 in hypoxia was due to either HIF-1α or HIF-2α, we knocked down these two proteins with RNAi in normoxia and hypoxia and measured levels of Mcl-1 by Western blot. Figure 4E shows that both HIF-1α and HIF-2α were stabilized in hypoxia and that their knockdown did not prevent Mcl-1 loss in hypoxia, indicating that Mcl-1 loss in hypoxia was a HIF-1α– and HIF-2α–independent effect.
Mcl-1 can be cleaved by caspase-3 for form two degradation products of 26 and 18 kDa (26). Only basal levels of apoptosis (<3% cells) were detected in hypoxia in HCT116 cells between 24 and 48 hours (Figure 1B), and no degradation products of Mcl-1 were observed when cells were incubated in hypoxia, suggesting that loss of Mcl-1 was not due to its cleavage by caspase-3 (Supplemental Figure 3A). To rule out the possibility that Mcl-1 loss in hypoxia was due to caspase-3 activation, cells were treated in the absence and presence of the pan-caspase inhibitor QVD (20 μM) and then incubated in normoxia or hypoxia for 24 hours before being harvested, and Mcl-1 levels were measured by Western blot. Mcl-1 levels were reduced in hypoxia in comparison to normoxia regardless of QVD exposure (Supplemental Figure 3B), confirming that Mcl-1 loss was a caspase-independent process.
Hypoxic sensitization to ABT-737 was Mcl-1 dependent.
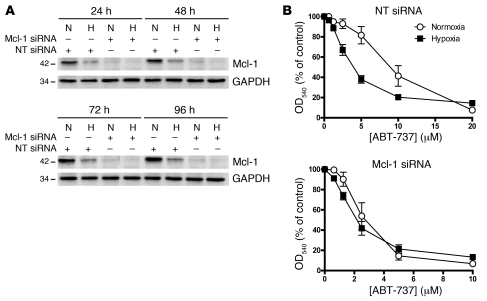
To examine whether hypoxic sensitization to ABT-737 was Mcl-1 dependent, we treated cells with siRNA targeted to Mcl-1. Figure 5A reconfirms the reduced expression of Mcl-1 in hypoxia compared with normoxia in HCT116 cells and demonstrates effective downregulation of Mcl-1 expression with targeted siRNA. Consistent with previous findings, cells treated with nontargeting (NT) siRNA showed significant hypoxic sensitization to ABT-737 (P < 0.001, 2-way ANOVA) (Figure 5B). When cells were treated with Mcl-1–targeted siRNA, two observations were made. First, both hypoxic and normoxic cell were more sensitive to ABT-737 when Mcl-1 was knocked down, indicating that decreased levels of Mcl-1 were sufficient to sensitize cells to ABT-737 (note different x-axis scales). Second, cells treated with Mcl-1 siRNA showed no significant sensitization to ABT-737 under hypoxic conditions (P = 0.74, 2-way ANOVA) (Figure 5B). An identical experiment performed in DLD-1 and CaCo2 cells gave identical results (Supplemental Figure 4), confirming that hypoxic sensitization was Mcl-1 dependent.
Figure 5. Role of Mcl-1 in hypoxic sensitization to ABT-737.
(A) HCT116 cells were transfected with 100 nM Mcl-1–targeted siRNA or NT control for 24 hours, after which siRNA was removed and replaced with full growth medium. Cells were then incubated in normoxia or hypoxia (1% O2) for 24, 48, 72, or 96 hours, and cells were harvested for Western blot analysis of Mcl-1 and actin levels. (B) HCT116 cells were treated with Mcl-1 or NT siRNA as described above and then incubated in normoxia or hypoxia (1% O2) for 18 hours, after which they were exposed to a range of ABT-737 concentrations under continuous normoxia or hypoxia for 72 hours prior to determination of IC50 values using the SRB assay. Data are mean ± SEM of 3 independent experiments.
The converse experiment was also performed, where HCT116 cells were transfected with a vector containing MCL1 and GFP or GFP alone (control) and subsequently cultured in normoxia and hypoxia, and their ABT-737 sensitivity was determined by SRB assay (Supplemental Figure 5). Cells expressing GFP alone were sensitized to ABT-737 in hypoxia in comparison to normoxic GFP-expressing cells as expected. In the cells that had been transfected with Mcl-1 and GFP, Mcl-1 was maintained in hypoxia, and cells were more resistant to ABT-737 than GFP control (P < 0.05, Student’s t test at 5 μM ABT-737).
Together, these function testing experiments support the hypothesis that increased sensitization of cells to ABT-737 in hypoxia was due to decreased levels of Mcl-1.
Comparison of Mcl-1 synthesis and degradation in normoxia and hypoxia.
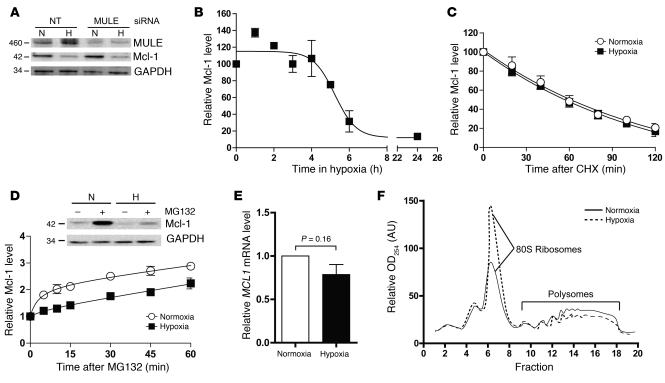
Mcl-1 ubiquitin ligase E3 (MULE), an enzyme that directly ubiquitinylates Mcl-1, causing its degradation, is one of several proteins that regulate cellular levels of Mcl-1. MULE was increased in hypoxia, and this may have explained the decrease in Mcl-1; however, knockdown of MULE did not cause Mcl-1 levels to change and did not prevent loss of Mcl-1 in hypoxia (Figure 6A). In parallel, studies were performed to investigate whether hypoxia affected the rate of Mcl-1 synthesis or degradation. Before this was done, the kinetics of Mcl-1 loss in hypoxia was assessed initially by incubation of cells in hypoxia for up to 24 hours, during which cells were harvested at various time points and the relative amount of Mcl-1 was determined by densitometric analysis of Western blots. Mcl-1 levels did not change during the first 4 hours of hypoxia, but then decreased rapidly between 4 and 6 hours and remained at a low level from that point onward (Figure 6B). To investigate whether hypoxia increased the rate of Mcl-1 degradation, we added cycloheximide, which inhibits protein synthesis, to cells after 4 hours of hypoxia, and cells were harvested every 20 minutes for the next 2 hours (i.e., during the time when Mcl-1 levels become decreased in hypoxia). Mcl-1 levels were determined by densitometric analysis of Western blots, and rate of Mcl-1 loss was compared with that in normoxic counterparts. Hypoxia did not affect the rate of Mcl-1 degradation (Figure 6C), suggesting that Mcl-1 synthesis was decreased in hypoxia. To examine whether Mcl-1 synthesis was affected by hypoxia, we added the proteasome inhibitor MG132 to cells after 6 hours in hypoxia, harvested cells at short time points thereafter, and compared the Mcl-1 rate of accumulation with that in normoxic counterparts. Hypoxia decreased the rate of accumulation of Mcl-1, indicating a decrease in rate of synthesis of Mcl-1 (Figure 6D). To further illustrate this, we incubated cells that had been exposed to hypoxia or normoxia for 24 hours in the absence and presence of MG132 for 6 hours and then blotted them for levels of Mcl-1. Whereas normoxic cells treated with MG132 showed a clear increase in Mcl-1 upon addition of MG132, hypoxic cells showed a reproducibly smaller rise in Mcl-1 levels, confirming that Mcl-1 synthesis had been decreased (Figure 6D).
Figure 6. Mechanism of Mcl-1 loss in hypoxia.
(A) HCT116 cells were treated with NT or MULE siRNA before incubation in either normoxia or hypoxia for 6 hours, after which cells were harvested and samples analyzed for expression of MULE, Mcl-1, and GAPDH by Western blot. (B) HCT116 cells were incubated in hypoxia for up to 24 hours and were harvested at various time points for measurement of Mcl-1 levels by Western blot followed by densitometry analysis of Mcl-1, results of which were then plotted as a function of time. (C) HCT116 cells were incubated in hypoxia or normoxia for 4 hours, after which cycloheximide (10 μg/ml) was added and cells harvested every 20 minutes for the next 2 hours. Samples were then analyzed as in B. (D) Cells were incubated in hypoxia or normoxia for 6 hours, after which MG132 (10 μM) was added and cells were harvested at various time points and analyzed as in B. (E) qRT-PCR analysis of MCL1 mRNA after 18 hours preincubation in normoxia and hypoxia; results were normalized to housekeeping genes. (F) Lysates from hypoxic and normoxic cells (3-hour incubation) were separated by density on a 10%–60% sucrose gradient before being fractionated into non-polysomal (fractions 1–9) or polysomal (fractions 10–18) fractions and subjected to OD254 measurement. Data are mean ± SEM of 3 independent experiments.
Quantitative RT-PCR (qRT-PCR) analysis was performed subsequently to determine whether Mcl-1 downregulation was mediated by decreased MCL1 transcription. When MCL1 mRNA levels were normalized to a panel of housekeeping genes, no significant difference could be detected between cells cultured in normoxia and hypoxia in any of the cell lines tested (P = 0.16, Student’s t test) (Figure 6E). To determine whether hypoxia affected the translation of MCL1, we incubated cells in normoxia or hypoxia for 3 hours, and cell lysates were centrifuged on a sucrose gradient and fractionated to separate free mRNA (non-polysomes) from the denser, ribosome-bound mRNA (polysomes — which represents the fraction of mRNA being actively translated into protein). Hypoxia caused a global decrease in translation after 3 hours (Figure 6F), one that was more marked after 24 hours and also observed in H82 cells (Supplemental Figure 6).
Hypoxic H526 SCLC cells were sensitized to ABT-737 in vitro and in vivo.
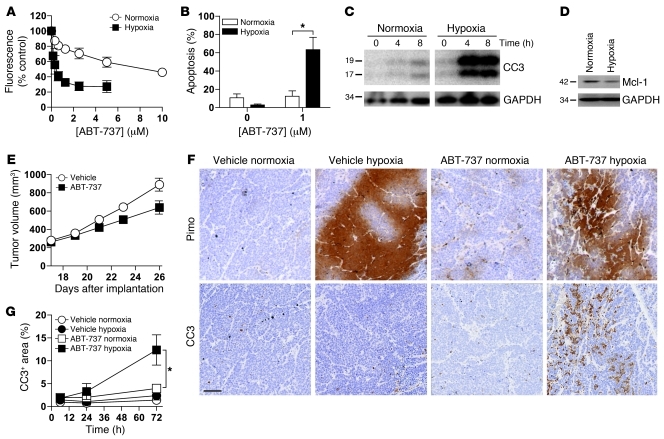
To determine whether hypoxic sensitization to ABT-737 also occurs in vivo, we assessed the effect of ABT-737 using an H526 SCLC tumor xenograft model. H526 cells have an intermediate sensitivity to ABT-737 in vitro (9). H526 cells cultured in vitro in hypoxic conditions were 21.5-fold more sensitive to ABT-737 compared with cells cultured in normoxic conditions (Figure 7A, P < 0.001, 2-way ANOVA, Supplemental Table 2). This hypoxic sensitivity was associated with increased apoptotic cell death. Specifically, after 24 hours, 1 μM ABT-737 induced 12% apoptotic cell death in normoxic cells and 63% in hypoxic cells (P < 0.05, Student’s t test), as assessed by changes in nuclear morphology (Figure 7B). Furthermore, after 4 and 8 hours of 1 μM ABT-737 treatment, there were higher levels of CC3 in H526 cells cultured in hypoxic conditions than in cells cultured in normoxic conditions (Figure 7C). Consistent with the other cell lines investigated in this study, the level of Mcl-1 was lower in hypoxic compared with normoxic H526 cells (Figure 7D). Therefore, H526 cells exhibit increased sensitivity toward ABT-737 under hypoxic conditions in vitro, consistent with the other SCLC and CRC cell lines studied (Supplemental Table 2).
Figure 7. Hypoxic sensitization to ABT-737 in H526 SCLC cells in vitro and in vivo.
(A–C) Hypoxic sensitization of H526 cells to ABT-737 in vitro. (A) H256 cell population growth (by resazurin assay) under continuous normoxia or hypoxia as in Figure 1A. (B) Apoptotic cell death (by DAPI staining and nuclear morphology) after 24 hours incubation in normoxia or hypoxia with or without ABT-737 (1 μM) or (C) as assessed by CC3 levels after 0, 4, or 8 hours incubation with ABT-737. CC3 and GAPDH protein level data shown are for noncontiguous lanes run on the same gel. (D) Expression levels of Mcl-1 in untreated H526 cells exposed to normoxia or hypoxia for up to 8 hours. GAPDH was used as a loading control. Data in A and B are mean ± SEM from 3 independent experiments. Data in C and D are representative of at least 3 independent experiments. (E–G) Hypoxic sensitization of H526 cells to ABT-737 in vivo. (E) Effect of ABT-737 (100 mg/kg/d) on tumor xenograft growth in SCID-bg mice (size-matched and dosed from day 17). Data represent the average of 6 mice per group. (F) Representative images of serial tumor sections showing staining for pimonidazole (hypoxia) and CC3 (apoptosis) from tumors harvested after 72 hours of ABT-737 treatment. Images are of identical magnification. Scale bar: 100 μm. (G) Percentage area of CC3-positive staining in 4 hypoxic or 4 normoxic tumor regions. Data are the average of 4 mice per time point and per treatment. *P < 0.05 (Student’s 2-tailed t test).
When male SCID-bg mice bearing H526 xenograft tumors were treated with 100 mg/kg/d ABT-737, there was a 26% reduction in tumor growth relative to vehicle-treated mice at 26 days (Figure 7E, P < 0.05, 2-way ANOVA). Animals bearing size-matched H526 tumors were treated with 100 mg/kg/d ABT-737 or vehicle and sacrificed 6, 24, or 72 hours after the first dose. Pimonidazole binds irreversibly to hypoxic cells and was administered to the animals 1 hour and 45 minutes prior to sacrifice to identify hypoxic tumor regions. Supplemental Figure 7 shows the overall distribution of hypoxic regions in a typical xenograft tumor. Serial sections of the tumors were analyzed (by an investigator blinded to mouse group) by immunohistochemistry (IHC) for pimonidazole binding (hypoxia) and CC3 expression (to report apoptosis). Increased apoptosis was observed in hypoxic regions of tumors from mice 72 hours after the start of ABT-737 dosing, but not those from vehicle-treated control mice (Figure 7F). To quantify this observation, we determined the percent area positive for CC3 staining in 4 hypoxic and 4 normoxic regions in each tumor (n = 4 tumors for each time point in each treatment group). The amount of CC3 staining was 3.2-fold higher in hypoxic regions of ABT-737–treated mice at 72 hours compared with the normoxic region of the same tumor, increasing from 4% to 12% (Figure 7G, P < 0.05, Student’s t test). There was no significant difference in CC3 staining between hypoxic and normoxic tumor regions from vehicle-treated mice. These proof-of-concept in vivo data demonstrate that tumor cells in a hypoxic microenvironment are preferentially killed by ABT-737.
Combination of ABT-737 with clinically relevant conventional cytotoxic agents in normoxia and hypoxia.
Hypoxic tumor cells are typically resistant to conventional cytotoxic agents (ref. 26 and Supplemental Table 1). Many of these conventional cytotoxic agents are used in combination in the clinic; for example, in SCLC etoposide and cisplatin are generally combined (27). When cisplatin and etoposide were combined in H146 SCLC cells in vitro in normoxia, the combination was synergistic, with a combination index (CI) of 0.43 determined according to the method of Chou and Talalay (28). However, when etoposide and cisplatin were combined in hypoxia, an antagonistic CI value of 1.43 was obtained (Supplemental Figure 8).
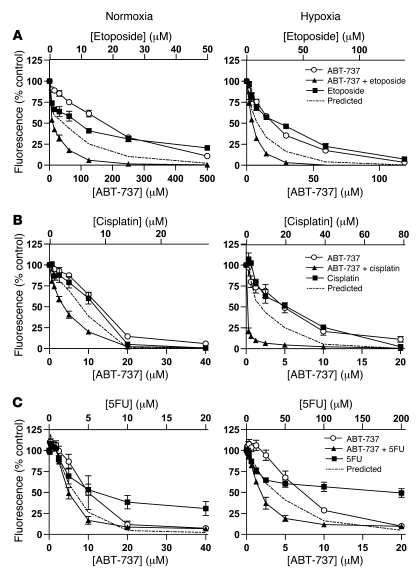
A substantial body of preclinical evidence emerging from studies of several tumor types performed in normoxia suggests that Bcl-2 family–targeted therapeutics such as ABT-737 are additive or synergistic with conventional cytotoxic agents (11). The impact of combining ABT-737 with conventional cytotoxic agents relevant to the treatment of SCLC (etoposide, cisplatin) was investigated in H146 and H82 cells and compared in hypoxic and normoxic conditions. Selected concentration response curves are shown for ABT-737 in combination with etoposide (H146 cells, Figure 8A) and cisplatin (H82 cells, Figure 8B). ABT-737 was synergistic with cisplatin and with etoposide in both hypoxic and normoxic H146 SCLC cells. A synergistic effect was also seen for H82 SCLC cells when ABT-737 was combined with either cisplatin or etoposide in normoxia, with still greater synergy in hypoxia. CI values for these drug combinations in SCLC cells are reported in Supplemental Table 3.
Figure 8. Effect of ABT-737 in combination with conventional cytotoxic agents.
(A) H146, (B) H82, and (C) HCT116 cells were treated with the indicated concentrations of ABT-737, the indicated cytotoxic drugs, or both drugs added simultaneously for 72 hours under normoxia or hypoxia, after which the effect on cell population growth was determined by resazurin assay (A and B) or SRB assay (C). Data are mean ± SEM from 3 independent experiments.
We reported previously conventional cytotoxic agent resistance in hypoxic HCT116 CRC cells (ref. 16 and Supplemental Table 1). Here, in HCT116 cells, ABT-737 was combined with fluorouracil (5FU), oxaliplatin, or SN-38 (the active metabolite of irinotecan) — drugs routinely used in the clinic to treat CRC. Synergy was seen in normoxic HCT116 when ABT-737 was combined with 5FU or SN-38, but not with oxaliplatin. However, all 3 cytotoxic drugs were synergistic with ABT-737 in hypoxic conditions, and for 5FU (as an example in Figure 8C) and SN-38 the synergistic interaction with ABT-737 was greater in hypoxia. CI values for these ABT-737 conventional cytotoxic combinations in CRC cells are reported in Supplemental Table 3.
Overall, in stark contrast to the hypoxic drug resistance profiles commonly observed for single or combined conventional cytotoxic agents, combinations of these drugs with ABT-737 show synergy in hypoxia.
Discussion
Solid tumors are generally characterized by regions of chronic or acute hypoxia that create a hostile cellular microenvironment characterized by limited delivery of nutrients and low pH (29). Hypoxia is recognized as a major obstacle in cancer treatment including chemo- and radiotherapy (30, 31). Consequently, there is continuing interest in the evaluation of drugs that are designed to have enhanced activity in conditions of limited oxygen (32) or that maintain their activity in hypoxic tumor cells. Hypoxia may also modulate drug response at the level of the cellular threshold for apoptosis via modulation of Bcl-2 family proteins (10, 16–19, 33), though this appears to be cellular context dependent. We reasoned that the efficacy of ABT-737 might be modulated in hypoxic tumor cells. This study compares, for the first time to our knowledge, the efficacy of ABT-737 in hypoxia and normoxia in vitro and in vivo, and demonstrates that ABT-737 efficacy is increased in hypoxia.
All SCLC and CRC cell lines investigated showed increased sensitivity to ABT-737 following treatment in hypoxic conditions (1% O2), albeit to different degrees (Figure 1A, Figure 7A, and Supplemental Table 2), which was accounted for by increased apoptosis (Figure 1B, Figure 2, and Figure 7, B and C). In each case Mcl-1 expression was downregulated in hypoxia (Figure 4A, Figure 7D, and Supplemental Figure 4) in the absence of clear, consistent upregulation of Noxa (which targets Mcl-1 for degradation) or of any other robust and uniform changes in Bcl-2 family protein expression levels. HCT116 tumor spheroids treated with ABT-737 revealed a sharply circumscribed “ring of cell death” consistent with hypoxic sensitization to ABT-737 (Figure 3). The hypoxic sensitization of cells to ABT-737 and downregulation of Mcl-1 in hypoxia were HIF-1 independent in HCT116 cells (Figure 4, C–E), despite the presence of an HRE in the MCL1 promoter (18) and the co-incidence of CC3 and upregulation of the HIF-1 transcriptional target GLUT-1 in ABT-737–treated tumor spheroids. Enhanced drug sensitivity in hypoxia is unusual; drug resistance is commonly seen (23).
The finding that Mcl-1 is regulated by oxygen concentration in vitro is in line with previous studies, although whether Mcl-1 is up- or downregulated may be cell type– and oxygen concentration–dependent (17–19). Our data contrast with those of Piret et al., who showed a hypoxia-mediated (1% O2) and HIF-1–dependent upregulation of Mcl-1 in hepatocellular carcinoma cells (18). Mcl-1 was not downregulated in hypoxic MEFs (with hypoxia defined as 1.5% O2 rather than the 1% O2 used in the current study) (19). The regulation of Mcl-1 by hypoxia thus seems cell type dependent. There are reports that hypoxia can increase NF-κB signaling (34, 35) and that activation of NF-κB can upregulate Mcl-1 levels (36), which would drive resistance to ABT-737. Although NF-κB was not explored in this study, the reduced expression of Mcl-1 in hypoxia in SCLC and CRC cells would suggest that this pathway, if operational, is overridden.
A considerable amount of evidence suggests that high expression of Mcl-1 contributes to ABT-737 resistance in many tumor cell lines (10, 15). Conversely, other investigators have shown that decreased expression of Mcl-1 confers sensitivity to ABT-737 (15). Similarly, knockdown of Mcl-1 over 96 hours in both normoxic and hypoxic HCT116, CaCo2, or DLD-1 cells by siRNA increased sensitivity of these cells to ABT-737 (Figure 5A and Supplemental Figure 4). Furthermore, Mcl-1 ablation also negated the differential response of normoxic and hypoxic cells, indicative of a causal effect in these cell lines (Figure 5B and Supplemental Figure 4). Conversely, the forced and maintained overexpression of Mcl-1 in hypoxia attenuated the previously observed comparative sensitivity to ABT-737 in hypoxic versus normoxic cells (Supplemental Figure 5).
The consistent downregulation of Mcl-1 in hypoxia associated with increased ABT-737–induced apoptosis across the cell line panel suggests that this is a common mechanism underlying hypoxic sensitivity to this BH-3 mimetic. Mcl-1 is regulated at the transcriptional, post-transcriptional, and post-translational levels (reviewed in ref. 37). The data presented here suggest that the mechanism of Mcl-1 downregulation in hypoxic HCT116 cells involved not increased degradation of the protein (Figure 6C) but rather decreased synthesis of Mcl-1 in hypoxia (Figure 6D). However, decreased synthesis was not due to reduced transcription of MCL1 in hypoxia (Figure 6E). Polysome analysis showed that hypoxic cells experienced a global downregulation of protein translation (Figure 6F). We hypothesize that the global decrease in translation in hypoxia may be sufficient to explain Mcl-1 downregulation, because a decrease in global translation will have a more dramatic effect on the levels of rapidly turned-over proteins, such as Mcl-1, in comparison to longer-lived proteins. However, to formally test this hypothesis, further studies would be warranted. The fact that tumor xenografts showed increased sensitization to ABT-737 in hypoxic regions suggests that although translation is globally decreased, Mcl-1 remains to be decreased relative to other proteins. One reason could be that tumor hypoxia is often acute and transient, causing only short-lived proteins, such as Mcl-1, to become decreased during the short window within which hypoxia occurs.
The Mcl-1 binding partner and p53 target Noxa (38) can promote Mcl-1 proteosomal degradation (39). Since p53 is stabilized in hypoxia (40), it is plausible that p53-driven Noxa upregulation could mediate Mcl-1 degradation in hypoxic cells. However, consistent upregulation of Noxa under hypoxia in the panel of cell lines studied was not observed, and it was not seen in cells (e.g., HCT116) expressing wild-type p53. Mcl-1 is targeted for proteosomal degradation by MULE (41), and MULE was upregulated in hypoxia across the cell panel (Supplemental Figure 9). However, knockdown of MULE in HCT116 cells did not affect Mcl-1 levels and did not prevent Mcl-1 loss in hypoxia (Figure 6A). Overall, the fact that hypoxia did not affect Mcl-1 degradation indicates that this was an effect independent of Noxa or MULE.
Multiple studies have reported ABT-737 synergy with chemotherapeutics and radiotherapy in a broad range of cancer cell types cultured in normoxic conditions (11). However, at the time of writing, no data exist on the efficacy of these combination therapies in hypoxia. Supplemental Table 3 and Figure 8 show the predominant synergy seen with ABT-737 and several clinically relevant conventional cytotoxic agents across the cell line panel in normoxia, consistent with and extending previous studies. Importantly, these drug synergies with ABT-737 were maintained and in some cases enhanced in hypoxic conditions. This is unusual for drug combinations. For example, even where two drugs, etoposide and cisplatin (clinically combined in SCLC), demonstrated a positive interaction in H146 SCLC cells in normoxia (and in the first cycle of therapy in patients; ref. 42), this was lost in hypoxic conditions (Supplemental Figure 8). The maintenance of ABT-737 synergies with clinically relevant cytotoxics (or synergy in hypoxia where none existed in normoxia; the case for oxaliplatin and ABT-737 in HCT116 cells) is therefore notable.
Despite its role as a potent apoptosis suppressor, the downregulation of Mcl-1 in hypoxia did not sensitize to conventional cytotoxic drugs, most likely because drug target coupling to apoptosis was countered by the other antiapoptotic proteins expressed in SCLC and CRC cells such as Bcl-2 and Bcl-xL, which were not downregulated in hypoxia. ABT-737 cannot counter the antiapoptotic function of Mcl-1 because of its poor affinity for this Bcl-2 family member, its downregulation in hypoxia selectively sensitizes to ABT-737. There are a multitude of established resistance mechanisms pertinent to the conventional cytotoxics used in this study, e.g., decreased drug uptake, decreased DNA damage and/or repair, increased drug efflux, decreased proliferation. The data in Figure 8 demonstrate hypoxic resistance to conventional cytotoxics in vitro, and thus these multiple potential resistance mechanisms were clearly not negated by and indeed might have been exacerbated by the global decrease in translation that was observed in hypoxia.
In summary, this study is the first to our knowledge to demonstrate enhanced efficacy of the novel BH-3 mimetic ABT-737 in hypoxia in vitro and in vivo. These data have potential significance for the treatment of solid tumors with ABT-263 (which is in early clinical trials in patients with solid tumors including SCLC), which, like ABT-737, inhibits Mcl-1 with reduced affinity compared with Bcl-2 and Bcl-xL (10). Of potential clinical benefit is the synergy between conventional cytotoxic agents and ABT-737 in hypoxia. These data are promising for the treatment of solid tumors, where current therapies reduce or stabilize tumor volume but where hypoxic tumor cells survive therapy and are the likely cause of tumor repopulation in relapsing cancer patients. Combination strategies to increase the hypoxic tumor fraction with vascular-targeted drugs and thus enhance ABT-737–induced tumor cell death are now under investigation in preclinical tumor models in our laboratory.
Methods
Cell culture.
The human SCLC cell lines NCI-H146, NCI-H82, NCI-H526, NCI-H1048, and NCI-H345 and the human CRC cell lines HCT116, HT29, CaCo2, and DLD-1 were from ATCC. SCLC cells were cultured in RPMI 1640 medium (Gibco, Invitrogen) with 10% FCS (Biosera), 4.5 g/l d-glucose (Gibco, Invitrogen), and 1% sodium pyruvate. DLD-1 and HT29 cells were cultured in RPMI 1640 medium with 10% FCS. HCT116 and derivatives were cultured in McCoy’s 5A medium with 10% FCS. HCT116 DN HIF-1α and HCT116 EV cells were provided by Kaye Williams and Ian Stratford (University of Manchester). All cell lines were maintained at 37°C in a humidified incubator containing 5% CO2.
Chemicals and antibodies.
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. ABT-737 (Abbott Laboratories), 5FU, and SN38 (Abatra Ltd.) were dissolved in DMSO. Cisplatin was dissolved in water, oxaliplatin (Axxora) was dissolved in PBS, and etoposide was dissolved in PBS/DMSO 3:1. MG132 (final concentration, 10 μM) and cycloheximide (final concentration, 0.01 mg/ml) were dissolved in DMSO. QVD was from Calbiochem. Control treatments were performed by treating cells with the appropriate vehicle alone. The maximum concentration of DMSO used was 0.08% vol/vol. Primary antibodies utilized for Western blotting were anti–Mcl-1 (1:500; BD Biosciences — Pharmingen), anti-tubulin (1:2,500; Abcam), anti–HIF-1α (1:500; BD Biosciences — Transduction Laboratories), anti–HIF-2α (1:1,000; Novus Biologicals), anti-actin (1:2,500), anti-PARP (1:1,000; Cell Signaling Technology), anti-GAPDH (1:1,000; Abcam), anti-MULE (1:1,000; Bethyl Laboratories), and anti-CC3 (Asp175) (1:100; Cell Signaling Technology). HRP-conjugated secondary antibodies (Dako) for ECL were diluted 1:3,500.
Drug treatment and protein expression analysis.
Cell cultures were placed in 1% O2 in an Invivo2 hypoxia workstation 4000 (Biotrace, Fred Baker Ltd.) for 18 hours prior to drug treatment. Thereafter, cells were harvested at time points up to 72 hours and lysed in 2× SDS protein sample buffer (62.5 mM Tris pH 6.8, 0.5% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.125% bromophenol blue). Western blotting was carried out as described previously (43).
Analysis of cell population growth by SRB or resazurin assay.
Cells were plated in 96-well plates at a density consistent with exponential population growth during the experiment. Adherent cells were left to attach overnight prior to 18 hours hypoxic or normoxic preincubation and then treated with the indicated drugs under normoxia or hypoxia maintained for 72 hours. At the end of the experiment, cells were subjected to SRB assay (adherent cell lines) or resazurin assay (suspension cell lines). For SRB assay, all media were removed and replaced with 100 μl 10% trichloroacetic acid for 1 hour and washed with PBS, and fixed cells were stained with 0.4 % (wt/vol) SRB for 15 minutes and then washed with 1% (vol/vol) acetic acid. Stained protein was then resuspended with 100 μl 1.5 M Tris-HCl (pH 8.8), and OD540 was measured using a 96-well plate reader (Labsystems). For resazurin assay, hypoxic cells were re-oxygenated for 2–48 hours and then incubated with resazurin solution (0.1 mM) at 37°C for 3 hours before measurement of resorufin fluorescence (BMG Lab Technologies; λex 530 nm, λem 590 nm). Cell survival was expressed as percentage of vehicle-treated control.
Assessment of apoptosis.
Cell suspensions were centrifuged and cell pellets fixed in formalin (10% vol/vol) for 30 minutes at room temperature. Pellets were resuspended in ProLong Gold Antifade with DAPI (Molecular Probes). Apoptotic nuclear morphology was determined by examining cells under UV illumination (Axioplan fluorescence microscope; Zeiss). The percentage of apoptotic cells was determined as the average of 2 independent analyses of at least 100 cells.
Analysis of tumor spheroids.
HCT116 cells were placed in agarose-coated 10-cm2 diameter dishes at 2 × 105 cells/ml for 72 hours. Spheroids ranging from 70 to 100 μm in diameter were selected and placed in spinner flasks maintained at 37°C and 5% CO2 and allowed to reach 500 μm in diameter before incubation with ABT-737 for 24 hours at the IC20 (2.8 μM) or IC90 (14.4 μM) concentrations derived from monolayer culture studies. Spheroids were then formalin fixed (10%) and cut into 4-μm sections. Sections were deparaffinized and rehydrated, then microwaved (700 W for 25 minutes) in citrate buffer (0.01 M, pH 6.0). After PBS wash, sections were blocked (5% normal goat serum/PBS/Triton) for 60 minutes. Sections were incubated overnight at 4°C with primary antibodies against CC3 (Cell Signaling Technology) and GLUT-1 (Alpha Diagnostic International). After further washes, goat anti-rabbit Alexa Fluor 568 (Molecular Probes, Invitrogen) or donkey anti-mouse Alexa Fluor 488 (Molecular Probes, Invitrogen) was applied for 2 hours, followed by consecutive PBS washes. Slides were viewed using either a ×10 or ×20 objective (Olympus BX51 microscope) and images captured (Colourview 12 camera and AnalySIS software, Olympus).
qRT-PCR.
Total RNA was isolated using an RNeasy Kit (QIAGEN). RNA was eluted and quantified using a Nanodrop spectrometer (ThermoScientific). The reverse transcription step was performed using the TaqMan Reverse Transcription Reagent Kit (Applied Biosystems) according to the manufacturer’s guidelines. TaqMan real-time PCR was designed using the Universal Probe Library (Roche Diagnostics). MCL1 was amplified using the following primer sequences (shown 5′ to 3′): AAGCCAATGGGCAGGTCT (forward) and TGTCCAGTTTCCGAAGCAT (reverse) and universal probe number 4. Succinate dehydrogenate complex A (SDHA) and actin were selected as housekeeping genes. RT-PCR was performed with 20 ng template cDNA using TaqMan Master Mix (Applied Biosystems) and an ABI Prism 7900HT sequence detection system (Applied Biosystems).
Polysome analysis.
Cells were exposed to hypoxia or normoxia for 3 hours, after which cycloheximide (0.1 mg/ml) was added for 3 minutes. Cells were then scraped into cold lysis buffer (20 mM HEPES, pH 7.4, 2 mM magnesium acetate, 100 mM potassium acetate, 0.1 mg/ml cycloheximide) and centrifuged to pellet cells. Cells were then washed and centrifuged to form a pellet, which was transferred into an Eppendorf tube containing 0.1 g glass beads (600 μm, Sigma-Aldrich). Cells were lysed by vortexing for 20 seconds every minute for 6 minutes and then centrifuged for 1 minute at 6,000 g, and supernatant was transferred to a clean Eppendorf tube and stored at –80°C. Lysates were loaded onto a 10%–60% sucrose gradient and centrifuged for 2.5 hours at 40,000 g. The absorbance at 254 nm as a function of depth was measured.
Validation of abrogated HIF-1 function in HCT116 DN cells by luciferase reporter assay.
HCT116 DN and HCT116 EV cells were cotransfected with luciferase reporter constructs under expression of an HRE, and Renilla reporter under expression of a CMV promoter (25) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s guidelines. Eighteen hours after transfection, cells were seeded and (after reattachment) exposed to either hypoxia or normoxia for 18 hours before measurement of firefly and Renilla luciferase activity (Dual-Luciferase Reporter Assay System, Promega). Fold induction of firefly luciferase in hypoxia was calculated using the following formula: (firefly hypoxia/Renilla hypoxia)/(firefly normoxia/Renilla normoxia).
siRNA transfection.
The siRNAs targeted to Mcl-1, MULE, HIF-1α, HIF-2α, and NT control siRNA were from Dharmacon SMARTpool. Each siRNA set was designed to target 4 different regions of the specific gene. Cells were transfected with the siRNA at 100 nM using the DharmaFECT 2 siRNA transfection reagent according to the manufacturer’s instructions. After 24 hours siRNA was replaced with full growth medium.
Transient transfection.
HCT116 cells growing in a 6-well plate were transfected with 5 μg of vector containing MCL1 and GFP or GFP alone in 1 ml Opti-MEM and 10 μl Lipofectamine 2000. Expression of MCL1/GFP and GFP alone was under the control of a CMV promoter. Six hours after transfection, Opti-MEM was removed and replaced with full growth medium. Eighteen hours later, cells were harvested and GFP-expressing cells were sorted using a FACSCalibur cell sorter (BD). GFP-expressing cells were used to examine the impact of forced and maintained expression of Mcl-1 on ABT-737 response in hypoxia.
Tumor xenografts.
All experiments were conducted according to Home Office Regulations (UK) and protocols approved under project license 40-2804. NCI-H526 xenografts were grown by subcutaneous injection of 5 × 106 cells in 0.2 ml of 1:1 serum-free RPMI/Matrigel (BD) into the mid-dorsal flank of 8- to 14-week-old male SCID-bg mice (Paterson Institute for Cancer Research). Mice were housed in individually vented caging systems in a 12-hour light/12-hour dark environment and maintained at uniform temperature and humidity. Tumor size was measured 3 times a week using calipers and the volume calculated as tumor length × tumor width2/2. Seventeen days after implantation, mice bearing tumors between 200 and 400 mm3 were randomized and treated with 100 mg/kg/d ABT-737 in 30% propylene glycol, 5% Tween 80, 65% 5% dextrose in water, pH 4–5, or vehicle only by i.p. injection. Measurements were continued 3 times a week to assess tumor growth kinetics and the animals culled when their tumor size reached 1,000 mm3. In order to identify regions of tumor hypoxia, animals were injected i.p. with 0.2 ml 10 mg/ml pimonidazole (dissolved in saline; Natural Pharmaceuticals) 1 hour and 45 minutes prior to culling. Thereafter, tumors were fixed immediately in 10% vol/vol formalin for subsequent sectioning and immunohistochemical analysis of pimonidazole positivity and CC3.
IHC.
Serial tumor sections were stained with anti-pimonidazole or anti-CC3 antibody as described below. Formalin-fixed tumors were paraffin embedded and sections cut, mounted, and dewaxed as previously described (44). Slides were incubated in 10 mM citric acid (pH 6.0) for 12 minutes at 98°C. Slides were then stained on an Autostainer i6000 (Biogenex Laboratories) as follows: for anti-pimonidazole staining, slides were incubated with 0.3% H2O2 for 10 minutes, serum-free solution (Dako) for 2 minutes, 1:500 FITC-conjugated mouse monoclonal anti-hydroxyprobe antibody (Chemicon)/TBS-Tween (or appropriate isotype control) for 30 minutes, 1:50 HRP-conjugated anti-FITC antibody (Chemicon)/TBS-Tween for 30 minutes, and DAB solution (Dako) for 5 minutes. For anti-CC3 staining, slides were incubated with 0.3% H2O2 for 10 minutes, serum-free solution (Dako) for 10 minutes, 1:100 rabbit anti-CC3 antibody (Cell Signaling Technology)/PBS (or appropriate isotype control) for 1 hour, prediluted EnVision HRP-conjugated goat anti-rabbit antibody (Dako) for 30 minutes, and DAB solution (Dako) for 5 minutes. Slides were then counterstained with hematoxylin, dehydrated in increasing concentrations of ethanol, and incubated in xylene for 5 minutes. This was followed by the mounting of glass coverslips and microscopic analysis.
Stained slides were scanned using an Ariol SL-50 image analysis system (Genetix, software version 3.3) using a ×5 objective for pimonidazole and a ×20 objective for CC3. Analysis was performed using customized GenSight Multistain scripts developed in-house. Eight regions (equivalent to a field of view on the ×20 objective), 4 exhibiting high levels and 4 exhibiting low levels of pimonidazole staining, were defined on each slide and the corresponding regions identified precisely on the CC3-stained slide on “slide-linked” serial sections. The total area of positive CC3 immunostaining was calculated for each region, and the average percent positive area in high- and low-pimonidazole regions was calculated.
CI.
Interactions of conventional cytotoxic agents and ABT-737 in normoxia and hypoxia were assessed using CI methodology (28). After 18 hours preincubation in normoxia or hypoxia, cells were treated either with a single drug or in fixed ratio drug combinations, where drugs were added simultaneously and cultures maintained for 72 hours in hypoxia or normoxia before resazurin assay. From the single-agent concentration-response curves in either normoxia or hypoxia, an algorithm was applied using the CalcuSyn software package (version 2.0, Biosoft) to predict the concentration of the two drugs required to inhibit growth by 50% assuming additive interaction. CI values were calculated by comparing the IC50 of the actual combination to that predicted. This was then used to determine whether the drug combinations were antagonistic, additive, or synergistic. A derived CI value of 1 indicates an additivity; CI less than 1 indicates synergy; and CI greater than 1 indicates antagonism.
Statistics.
Differences between datasets were examined for statistical significance using 2-tailed Student’s t test or 2-way ANOVA. P values less than 0.05 were considered significant. All experiments show the average of 3 independent experiments, unless otherwise stated. Western blots show a representative image of 3 independent experiments. Error bars indicate SEM.
Supplementary Material
Acknowledgments
The authors wish to thank Graham Pavitt and Lydia Castelli of the Faculty of Life Sciences, University of Manchester, for discussions and expertise with polysomes. This study was supported by Cancer Research UK (CR-UK) core funding to the Paterson Institute (grant no. C147). D. Micha and M. Brandenburg received CR-UK Ph.D. student stipends. O. Denneny was supported via funding from the Cancer Research UK Experimental Cancer Medicine Centre Grant to the Manchester Cancer Research Centre.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(3):1075–1087. doi:10.1172/JCI43505.
References
- 1. Moulder JE, Rockwell S. Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev. 1987;5(4):313–341. doi: 10.1007/BF00055376. [DOI] [PubMed] [Google Scholar]
- 2. Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15(4):678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 5. Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(suppl 1):S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8. Dive C, Hickman JA. Drug-target interactions: only the first step in the commitment to a programmed cell death? Br J Cancer. 1991;64(1):192–196. doi: 10.1038/bjc.1991.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 10. Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 11. Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16(3):360–367. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 12. Hann CL, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68(7):2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Konopleva M, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 14. van Delft MF, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67(2):782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 16. Erler JT, et al. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24(7):2875–2889. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61(18):6669–6673. [PubMed] [Google Scholar]
- 18. Piret JP, et al. Hypoxia-inducible factor-1-dependent overexpression of myeloid cell factor-1 protects hypoxic cells against tert-butyl hydroperoxide-induced apoptosis. J Biol Chem. 2005;280(10):9336–9344. doi: 10.1074/jbc.M411858200. [DOI] [PubMed] [Google Scholar]
- 19. Liu XH, Yu EZ, Li YY, Kagan E. HIF-1alpha has an anti-apoptotic effect in human airway epithelium that is mediated via Mcl-1 gene expression. J Cell Biochem. 2006;97(4):755–765. doi: 10.1002/jcb.20683. [DOI] [PubMed] [Google Scholar]
- 20. Brunelle JK, et al. Loss of Mcl-1 protein and inhibition of electron transport chain together induce anoxic cell death. Mol Cell Biol. 2007;27(4):1222–1235. doi: 10.1128/MCB.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willis SN, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19(11):1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med. 2004;199(1):113–124. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 24. Roberts DL, et al. Contribution of HIF-1 and drug penetrance to oxaliplatin resistance in hypoxic colorectal cancer cells. Br J Cancer. 2009;101(8):1290–1297. doi: 10.1038/sj.bjc.6605311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown LM, et al. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol Pharmacol. 2006;69(2):411–418. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- 26. Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280(11):10491–10500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- 27. Cooper S, Spiro SG. Small cell lung cancer: treatment review. Respirology. 2006;11(3):241–248. doi: 10.1111/j.1440-1843.2006.00850.x. [DOI] [PubMed] [Google Scholar]
- 28. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 29. Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 30. Teicher BA, Holden SA, al-Achi A, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990;50(11):3339–3344. [PubMed] [Google Scholar]
- 31. Zhivotovsky B, Joseph B, Orrenius S. Tumor radiosensitivity and apoptosis. Exp Cell Res. 1999;248(1):10–17. doi: 10.1006/excr.1999.4452. [DOI] [PubMed] [Google Scholar]
- 32. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 33. Azad MB, et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4(2):195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lai Y, et al. Activation of NFkappaB dependent apoptotic pathway in pancreatic islet cells by hypoxia. Islets. 2009;1(1):19–25. doi: 10.4161/isl.1.1.8530. [DOI] [PubMed] [Google Scholar]
- 35. Walmsley SR, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201(1):105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ricci MS, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12(1):66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 37. Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66(8):1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shibue T, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17(18):2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Opferman JT. Unraveling MCL-1 degradation. Cell Death Differ. 2006;13(8):1260–1262. doi: 10.1038/sj.cdd.4401978. [DOI] [PubMed] [Google Scholar]
- 40. Alarcon R, Koumenis C, Geyer RK, Maki CG, Giaccia AJ. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Res. 1999;59(24):6046–6051. [PubMed] [Google Scholar]
- 41. Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121(7):1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 42. Mascaux C, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer. 2000;30(1):23–36. doi: 10.1016/S0169-5002(00)00127-6. [DOI] [PubMed] [Google Scholar]
- 43. Hussein D, Estlin EJ, Dive C, Makin GW. Chronic hypoxia promotes hypoxia-inducible factor-1alpha-dependent resistance to etoposide and vincristine in neuroblastoma cells. Mol Cancer Ther. 2006;5(9):2241–2250. doi: 10.1158/1535-7163.MCT-06-0145. [DOI] [PubMed] [Google Scholar]
- 44. Barraclough J, Hodgkinson C, Hogg A, Dive C, Welman A. Increases in c-Yes expression level and activity promote motility but not proliferation of human colorectal carcinoma cells. Neoplasia. 2007;9(9):745–754. doi: 10.1593/neo.07442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.