Physiologically relevant assays are crucial to identify adult stem cells and understand how they self-renew and give rise to differentiated progeny. The most commonly used strategies are discussed here, as well as how they have contributed to our understanding of stem-cell biology.
Keywords: adult stem cells, lineage tracing, stemness, multipotency, self-renewal
Abstract
The maintenance of stem-cell-driven tissue homeostasis requires a balance between the generation and loss of cell mass. Adult stem cells have a close relationship with the surrounding tissue—known as their niche—and thus, stem-cell studies should preferably be performed in a physiological context, rather than outside their natural environment. The mouse is an attractive model in which to study adult mammalian stem cells, as numerous experimental systems and genetic tools are available. In this review, we describe strategies commonly used to identify and functionally characterize adult stem cells in mice and discuss their potential, limitations and interpretations, as well as how they have informed our understanding of adult stem-cell biology. An accurate interpretation of physiologically relevant stem-cell assays is crucial to identify adult stem cells and elucidate how they self-renew and give rise to differentiated progeny.
See Glossary for abbreviations used in this article.
Glossary.
- BrdU
bromodeoxyuridine
- CBC
crypt base columnar
- EdU
5-ethynyl-2′-deoxyuridine
- EGF
epidermal growth factor
- EGFP
enhanced green fluorescent protein
- GFP
green fluorescent protein
- FACS
fluorescence activated cell sorter
- HSC
haematopoietic stem cell
- IFE
interfollicular epidermis
- ISC
intestinal stem cell
- LRG5/6
leucine-rich repeat-containing G protein coupled receptor 5/6
- LRIG1
leucine-rich repeats and immunoglobulin-like domains 1
- NGN3
neurogenin 3
Adult stem-cell definitions
Most adult tissues are believed to contain adult stem cells that compensate for tissue loss by generating new cells. Two attributes of stem cells enable this regenerative capacity throughout life. First, stem cells divide, yet maintain themselves as a population over long periods of time; a property called self-renewal. Self-renewal is not unique to stem cells, as fully differentiated cells such as activated B and T lymphocytes can also undergo self-renewal (He et al, 2009). Second, stem cells supply all the cell types to the tissue in which they are found. This ability to generate daughter cells that can differentiate into all specific cell types of the pertinent tissue is called multipotency, or unipotency when a single cell type arises (He et al, 2009). Self-renewal and multipotency are the defining characteristics of stem cells (Potten & Loeffler, 1990). The combination of these properties is often referred to as stemness; the minimal set of features that all stem cells have in common (Mikkers & Frisen, 2005).
Embryonic stem cells and induced pluripotent stem cells are defined by expression of the triad OCT4, SOX2 and NANOG (Jaenisch & Young, 2008). It is not known whether a comparable molecular signature of stemness—such as a minimal core transcriptional programme—exists in (and might be shared between) adult stem cells. The absence of a defined adult stemness signature could imply that different types of adult stem cell use different mechanisms to achieve self-renewal and multipotency (Ivanova et al, 2002; Ramalho-Santos et al, 2002; Fortunel et al, 2003; Wong et al, 2008). Stem cells might be quiescent or actively cycling. Quiescent and proliferating stem-cell pools can reside in adjacent compartments, perhaps within the same tissue (Li & Clevers, 2010). Thus, it seems dangerous to extrapolate stem-cell characteristics such as marker expression or cell-cycle behaviour from one tissue to another. The dual capacity of self-renewal and multipotency should be the only criteria for stemness, independent of mechanism or signature.
The way in which stem cells balance self-renewal with the production of daughter cells is not known and might be unique to each tissue. The most prevalent view is that stem cells only divide symmetrically when their numbers need to be expanded, such as during embryonic development or after tissue injury. In the steady state, asymmetrical division—if executed perfectly—would allow stem cells to maintain their numbers while sustaining the production of transit-amplifying daughter cells (Morrison & Kimble, 2006).
Asymmetrical stem-cell division results in daughter cells with different fates. This is often shown by an asymmetrical distribution of cellular content. As a consequence of this intrinsic asymmetry, one daughter cell obtains the molecular cues to maintain stemness, whereas the other differentiates (Neumuller & Knoblich, 2009). The term 'asymmetrical division' also refers to division that results in two daughter cells that are positioned in different signalling environments, for example if the mitotic spindle has a pre-imposed perpendicular orientation to the original stem-cell niche (Fuller & Spradling, 2007). Although this extrinsically governed asymmetry has been reported in mammals (Huttner & Kosodo, 2005; Lechler & Fuchs, 2005; Kuang et al, 2007; Quyn et al, 2010), the significance of a pre-destined outcome is not always clear. In a hierarchical model of tissue homeostasis, adult stem cells follow a strict pattern of invariant asymmetry to self-renew while generating progeny.
An alternative view states that cells initially divide into intrinsically equal stem-cell daughters through symmetrical stem-cell division. After division, the fate of the individual daughter cells is determined independently and stochastically such that two stem cells, two daughter cells, or one stem cell and one daughter cell could result. Homeostasis is maintained at the population level through regulatory mechanisms exerted by the microenvironment in which the stem cells reside—known as the stem-cell niche. The location of the daughter cells in relation to the stem-cell niche is important, but it is not predetermined (Morrison & Kimble, 2006). The niche has a crucial role in the homeostatic maintenance of the stem-cell pool, by supplying essential factors and adhesion anchors (Voog & Jones, 2010). In a stochastic model of tissue homeostasis, an equipotent population of stem cells follows a pattern of population asymmetry in which symmetrical self-renewal of stem cells compensates for the loss of neighbouring stem cells.
There are many types of adult stem cells and they might use different mechanisms to ensure homeostatic self-renewal. As argued above, the only commonality between adult stem-cell types is their capacity for self-renewal and multipotency. It is therefore essential that new candidate stem-cell populations are tested for these defining criteria, rather than for other characteristics that have proven useful in identifying a particular type of adult stem cell, such as quiescence or the expression of certain molecules.
Finally, cells might alter their characteristics due to experimentally induced stress (Potten & Loeffler, 1990). Therefore, actual stemness—or real stem-cell behaviour—needs to be distinguished from stemness potential—or the ability of a cell population to obtain actual stemness. For example, transplantation could activate stem-cell behaviour in cells that in normal situation do not behave as stem cells.
We describe four assays that are commonly used in mouse adult stem-cell biology. Examples are taken from different tissues, particularly the small intestine and the skin. We emphasize the advantages and limitations of each assay and discuss interpretation of the data (Table 1).
Table 1. Assays in adult stem-cell biology.
| Advantages | Limitations | |
|---|---|---|
| Label retention | Fluorescent label allows isolation of label-retaining cells | Not a feature of all adult stem cells |
| Not specific to stem cells (for example, memory B and T cells) | ||
| Unable to discriminate between the 'immortal strand hypothesis' and quiescence when using DNA analogues | ||
| In vitro culture | Enables manipulation of stemness potential in a controlled setting | Measures stemness potential, rather than actual stemness |
| Ideal for high-throughput studies | No physiological context | |
| Ideal for live-imaging studies | Knowledge is required for culturing cells/tissues | |
| Transplantation | Measures stem-cell behaviour in vivo | Measures stemness potential, rather than actual stemnessLimited physiological contextKnowledge is required for isolating rare cell populations |
| Limited physiological context | ||
| Knowledge is required for isolating rare cell populations | ||
| Lineage tracing | Reveals in vivo stem-cell behaviour in its physiological context; actual stemness | Actual stem cells might be limited to a subset of the marked population |
| Bicistronic knock-in with fluorescent marker allows isolation of cell population | Interpretation is only accurate in solid tissues | |
| Reproducible experimentation/readout | Knowledge is required to find stem-cell-specific marker genes |
Quiescence and label retention
In addition to self-renewal and multipotency, a feature commonly associated with stemness is quiescence—stem cells dividing infrequently to prevent stem-cell 'exhaustion' (Arai & Suda, 2007; Orford & Scadden, 2008). Examples of slow cycling adult stem cells are hair follicle bulge cells and haematopoietic stem cells (HSCs; Cotsarelis et al, 1990; Tumbar et al, 2004; Wilson et al, 2008; Foudi et al, 2009). However, the well-defined germ stem cells in the fly actively divide (Xie et al, 2005). Furthermore, the best-characterized of all stem cells—mammalian embryonic stem cells—proliferate rapidly while being able to self-renew and maintain pluripotency (He et al, 2009). Indeed, actively proliferating stem cells have been documented in the stomach, small intestine and colon (Barker et al, 2007, 2010), indicating that quiescence is not a prerequisite of stemness.
The incorporation of DNA analogues—such as BrdU, tritiated thymidine or EdU—during S-phase is often used to study cell-cycle kinetics. Actively dividing stem cells can be efficiently labelled with a short pulse of such DNA labels (Barker et al, 2007), whereas labelling quiescent stem cells requires prolonged exposure to the DNA label or their temporary activation, for example by tissue injury (Potten et al, 1978). During the subsequent chase, quiescent cells slowly dilute the label, in contrast to cells that go through successive rounds of cell division. As a result, quiescence can be visualized as DNA-label retention after long chase periods (Cotsarelis et al, 1990). Unfortunately, the chemical DNA labels can only be visualized in fixed and permeabilized cells. The demonstration of DNA-label retention is not sufficient to identify stem cells. In many tissues, fully differentiated cells have long lifetimes and do not undergo cell division (Kiel et al, 2007; Foudi et al, 2009); such cells efficiently retain DNA labels.
As an alternative strategy, chromatin can be labelled in vivo with a pulse of transgenically expressed EGFP-tagged histone 2B (H2B-EGFP; Tumbar et al, 2004). This approach enables cell fractionation by FACS—in addition to label retention and cell visualization—and has been used in several tissues (Tumbar et al, 2004; Wilson et al, 2008; Foudi et al, 2009). As H2B-EGFP-retaining cells can be visualized and isolated alive, additional experimental strategies can be used to prove that stem cells were labelled.
Another possibility that would lead to the retention of DNA labels involves asymmetrical segregation of DNA strands; the 'immortal strand hypothesis' (Cairns, 1975). Dividing stem cells would retain the template DNA strands and the newly synthesized chromatids would be passed on to the daughter cells, presumably to maintain genome integrity. Potten postulated that an intestinal stem cell (ISC) is found on average at position +4 relative to the crypt bottom and divides every 24 h, but retains DNA labels, possibly through this mechanism (Potten et al, 2002). There are other examples of well-described adult stem cells—such as muscle satellite cells—that asymmetrically segregate their DNA strands (Shinin et al, 2006), whereas other adult stem cells—such as HSCs and hair follicle stem cells—have been shown not to use this mechanism (Kiel et al, 2007; Waghmare et al, 2008).
Overall, it is clear that cell-cycle properties differ between stem cells and should not be used as principal determinants of stemness.
In vitro culture
The study of isolated adult stem cells in culture has practical advantages for their study in situ, for example to obtain a detailed description of stem-cell behaviour or growth factor requirement (Schroeder, 2008; Lutolf et al, 2009). However, the main downside of studying stem cells in culture is that changes might be induced when cells are disconnected from their physiological surroundings. Despite this caveat, the development of cell-culture methods for adult stem cells has made important contributions to the stem-cell field. For example, heterogeneity was observed between epidermal cells—after extensive culture periods—when different types of clonal behaviour became apparent, which led to the definition of epidermal stem cells (Barrandon & Green, 1987). Furthermore, innovations in in vitro keratinocyte cultures improved long-term regeneration of human epidermis through autologous cultured skin engraftments (Pellegrini et al, 1999; Ronfard et al, 2000). More recently, the successful transplantation of clones derived from single epidermal hair follicle cells, was used to demonstrate the multipotency of the original cell (Blanpain et al, 2004; Claudinot et al, 2005).
Growing individual clones of cells derived from different progenitor populations became a widely used method to define the identity and behaviour of the relevant stem cells. Such cultures were named after the tissues they were derived from, for example neurospheres (Reynolds & Weiss, 1992), pancreatospheres (Rovira et al, 2010), mammospheres (Shackleton et al, 2006; Stingl et al, 2006), prostaspheres (Lawson et al, 2007) and tracheospheres (Rock et al, 2009). From muscles, single myofibres could be cultured in vitro, allowing the analysis of satellite-cell behaviour (Shinin et al, 2006; Kuang et al, 2007).
In addition to spheres, three-dimensional asymmetrical cell-culture conditions have been developed for the small intestine that allow the generation of long-lived organoids from adult crypts, and even from single ISCs. The resulting 'mini-guts' have all the characteristics of normal gut epithelium (Sato et al, 2009). The intestinal organoids do not contain mesenchymal niche elements; instead, specialized daughters of the stem cells—the Paneth cells—are interspersed between the stem cells and provide essential niche signals such as Wnt, EGF and Notch (Sato et al, 2010). With small adaptations, the protocol allows the robust outgrowth of stomach organoids from single stomach epithelial stem cells (Barker et al, 2010). Alternatively, three-dimensional intestinal organ cultures have been initiated by using neonatal tissue; in this case, both the mesenchymal niche architecture and the multilineage epithelial lining could be preserved during long-term culture (Ootani et al, 2009).
Interestingly, some tissues give rise to sphere formation in culture, whereas intestine and stomach cultures establish an asymmetry in which proliferative and differentiated cell types are positioned in accordance to their natural locations in the epithelial lining. It is tempting to speculate that the presence of niche cells in intestinal cultures—either mesenchymal structures and/or specialized daughter cells such as Paneth cells (Ootani et al, 2009; Sato et al, 2010)—create local morphogen gradients, thereby generating progenitor zones that are separate from areas with differentiated cells. The ability of single stem cells to grow into structures in vitro will facilitate analysis of the influence of growth factors on multipotency and self-renewal, and constitutes a simple and unambiguous in vitro test for stemness potential.
Transplantation
One of the most common assays in stem-cell biology is the transplantation of putative stem cells into recipient mice. Historically, this has been the 'gold standard', because it functionally measures the two criteria of stemness: it can directly assess if a candidate stem cell persists for long periods of time (self-renewal) and can produce all types of cell for the tissue in which it resides (multipotency).
Bone marrow stem cells were the first to be studied; the first attempt to transplant bone marrow in a clinical setting was in 1957 (Thomas et al, 1957). Through the enrichment of progenitor populations and sophisticated limiting dilution experiments, the hierarchical characteristics of the haematopoietic system were subsequently revealed (Spangrude et al, 1988; Chao et al, 2008). Ultimately, a single transplanted haematopoietic stem cell was successfully used to repopulate the entire haematopoietic system (Osawa et al, 1996).
Other adult stem-cell fields have adopted the transplantation assay to identify candidate stem cells. For example, it has long been known that injured muscle can initiate a strong regenerative response through the activation of satellite cells (Moss & Leblond, 1971). However, the self-renewal capacity and multipotency of satellite cells was only shown after transplantation of either single intact myofibres (Collins et al, 2005) or directly purified satellite cells (Montarras et al, 2005). The most stringent proof of stemness was provided when single, transplanted satellite cells repopulated new muscle fibres (Sacco et al, 2008). Despite this success, it remains controversial whether all satellite cells show equal stemness (Kuang et al, 2007). In the mammary gland, the development of the 'cleared fat pad'-transplantation assay allowed the definitive demonstration of stemness, as single, transplanted cells could grow into complete mammary glands (Shackleton et al, 2006; Stingl et al, 2006).
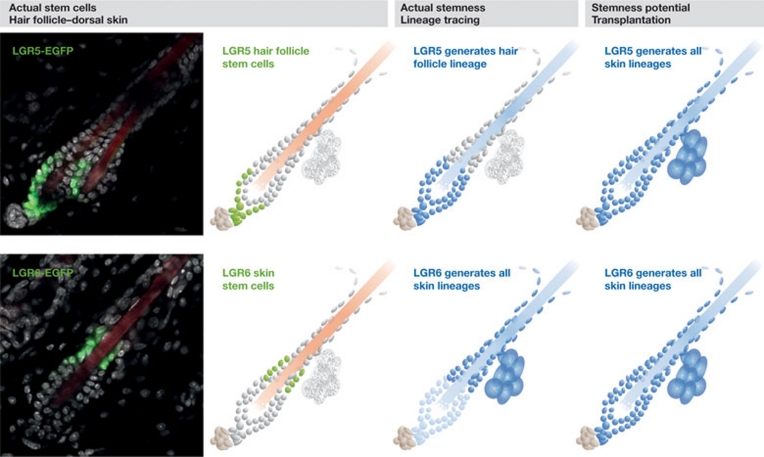
Although the ability of a single cell to repopulate a tissue is a dramatic example of its developmental potential, it is questionable whether the same potential could have been observed if the cell had been studied in its endogenous environment, before isolation and transplantation. Transplantation assays in cutaneous stem-cell biology illustrate this point. Label-retaining stem cells were first identified as potential stem cells in the bulge region of hair follicles (Cotsarelis et al, 1990). Once these cells were isolated from adult mice and transplanted, they gave rise to all three of the main structures of the skin: all cell lineages of the hair follicle, sebaceous gland and interfollicular epidermis (IFE; Morris et al, 2004). However, when the same bulge cells were observed in normal homeostasis, the majority generated hair follicles and only occasionally sebaceous gland or IFE (Morris et al, 2004). These counterintuitive results were confirmed when neighbouring cell populations of the hair follicle bulge were isolated using LRIG1, LGR5 or LGR6 expression. Under normal homeostasis, LGR5+ cells exclusively give rise to hair follicles (Jaks et al, 2008). LRIG1 and LGR6+ cells predominantly give rise to sebaceous glands and IFE, whereas LGR6 hair-follicle potential diminishes with age (Jensen et al, 2009; Snippert et al, 2010a). However, after transplantation, they all generated all three components of the skin (Jaks et al, 2008; Jensen et al, 2009; Snippert et al, 2010a; Fig 1).
Figure 1.
Actual stemness compared with stemness potential in the skin. LGR5 and LGR6 indicate different stem-cell populations along hair follicles. Stem cells (green), nuclei (grey) and hair follicles (red) are shown in confocal pictures (left) and in the cartoon panels (second from the left). Lineage tracing reveals the actual stemness of a population. LGR5 stem cells generate progeny that repopulate the hair follicle (blue), whereas LGR6 stem cells predominantly generate progeny for sebaceous gland and IFE (blue) and, to a lesser extent, hair follicle (light blue). Transplantation of both stem-cell populations reveals equal stemness potential towards all lineages (blue). EGFP, enhanced green fluorescent protein; IFE, interfollicular epidermis.
It could be argued that stemness potential, as shown after transplantation, might be fulfilled after injury. Indeed, hair follicle bulge cells demonstrate the same complete stemness potential after transplantation assays, as these cells can heal full-thickness wounds in the dorsal skin of mice by generating progeny for all three skin lineages (Morris et al, 2004; Blanpain et al, 2004; Jaks et al, 2008; Jensen et al, 2009), although their contribution to wound healing is only transient (Ito et al, 2007). Long-term healing of full-thickness wounds requires cells from above the hair follicle bulge, in particular LGR6+ cells, which can heal wounds efficiently by generating permanent residents for all three skin lineages (Ito et al, 2007; Levy et al, 2007; Snippert et al, 2010a).
Similar discrepancies were observed in the testes, in which normal spermatogenesis is maintained by a small subset of undifferentiated NANOS2+ spermatogonia cells that self-renew. However, during normal spermatogenesis and in regenerating tissue, a second NGN3+ subpopulation that normally differentiates is able to self-renew and therefore probably has stemness potential (Nakagawa et al, 2007, 2010; Sada et al, 2009; Klein et al, 2010).
These studies might illustrate a more general principle in transplantation strategies. Different stem-cell populations in the skin are fully competent in the generation of all skin lineages after transplantation—stemness potential—however during normal homeostasis, their 'actual stemness' is more restricted (Watt & Jensen, 2009). In a recent example of such plasticity, thymic epithelial cells were shown to adopt adult hair-follicle stem-cell fate after translantation into the skin microenvironment (Bonfanti et al, 2010).
In vivo lineage tracing
In vivo lineage tracing has evolved in recent years into a powerful technique for the experimental testing of actual stemness. Central to lineage-tracing strategies is the genetic marking of stem cells, which allows the tracing of daughter populations. Table 2 summarizes inducible genetic-tracing studies performed in adult mice that have elucidated either stem-cell identity or a mechanism of self-renewal.
Table 2. Inducible genetic fate mapping experiments in mice with implication to adult stem biology.
| Direct analysis | |||||
|---|---|---|---|---|---|
| Organ | Promoter | Expression pattern | Downstream lineages/implications | Longevity | Reference |
| Adipose tissue | Pparγ | Adipose vasculature | Adipocytes | 30 days | Tang et al, 2008 |
| Blood | Foxp3 | Foxp3+ TREG cells | Only FOXP3+ Treg cells | 8 months | Rubtsov et al, 2010 |
| Brain | Gli1 | SVZ and DG | Neurons, oligodendrocytes, astrocytes | 1 year | Ahn & Joyner, 2005 |
| Nestin | SVZ and DG | Neurons, astrocytes | 2 months | Lagace et al, 2007 | |
| Colon | Lgr5 | Base of colonic crypts | Complete colonic epithelium | 14 months | Barker et al, 2007 |
| Incisor | Gli1 | Proximal ameloblasts | Ameloblasts, str. intermedium, stel. reticulum | 15 months | Seidel et al, 2010 |
| Intestine | Lgr5 | CBCs | Complete intestinal epithelium | 14 months | Barker et al, 2007 |
| Bmi1 | '+4' cells | Complete intestinal epithelium | 9 months | Sangiorgi et al, 2008 | |
| Prom1 | CBCs | Presence of complete epithelium | 2 months | Zhu et al, 2009 | |
| Prom1 | CBCs and early progenitors | Occasional complete epithelium | >2 months | Snippert et al, 2009 | |
| Sox9 | CBCs, Paneth cells and early prog. | Presence of complete epithelium | 12 months | Furuyama et al, 2010 | |
| Liver | Sox9 | SOX9+ prog. in biliary duct | Biliary ducts and hepatocytes | 12 months | Furuyama et al, 2010 |
| Lung/trachea | Scgb1a1 | Clara cells, putative BASCs | Bronchiole; Clara cells, BASCs and ciliated cells | 1 year | Rawlins et al, 2009 |
| Krt5 | Basal cells | Basal cells, Clara cells and ciliated cells | 15 weeks | Rock et al, 2009 | |
| Muscle | Pax7 | Satellite cells | Myofibres + satellite cells, including 2 × injury | 23 days | Lepper et al, 2009 |
| Pancreas | Insulin (RIP) | β-cells | Only β-cell lineage | 1 year | Dor et al, 2004 |
| Elastase | Acinar cells | Only acinar cell lineage | 6 weeks | Desai et al, 2007 | |
| Caii | Ductal cells | Ducts and acinar cells | 3 weeks | Inada et al, 2008 | |
| Bmi1 | Acinar cells | Only acinar cells | 1 year | Sangiorgi et al, 2009 | |
| Hnf1β | HNF1β+ duct/centroacinar cells | Ducts and centroacinar cells | 6 months | Solar et al, 2009 | |
| Sox9 | SOX9+ duct/centroacinar cells | Ducts, centroacinar and acinar cells | 12 months | Furuyama et al, 2010 | |
| Prostate | Nkx3-1 | Luminal (CARNs) | Luminal, occasionally basal after injury | 10 months | Wang et al, 2009 |
| Skin | K15 | HF bulge | Predominantly HF, occasionally SG and IFE | 1 month | Morris et al, 2004 |
| Lgr5 | HF germ, lower bulge | HF | 14 months | Jaks et al, 2008 | |
| Lgr6 | HF central isthmus | Predominantly SG and IFE, occasionally HF | 12 months | Snippert et al, 2010a | |
| Stomach | Lgr5 | Base of pyloric glands | Complete stomach epithelium | 20 months | Barker et al, 2010 |
| Testis | Ngn3 | Undif. spermatogonia Aal | Differentiated spermatogonia | 14 months | Nakagawa et al, 2007 |
| Nanos2 | Undif. spermatogonia As and Apr | Differentiated spermatogonia | 5 months | Sada et al, 2009 | |
| Indirect analysis | |||||
| Intestine | v | Epithelial cells, except Paneth cells | Neutral drift dynamics in intestinal self-renewal | 12 months | Lopez-Garcia et al, 2010 |
| Cyp1a1 | Epithelial cells, except Paneth cells | Neutral drift dynamics in intestinal self-renewal | 7 months | Snippert et al, 2010b | |
| Lgr5 | CBCs at entire crypt base | ISCs divide symmetrically | 2 weeks | Snippert et al, 2010b | |
| Skin/tail | Cyp1a1 | Basal cells of tail epidermis | Homeostasis involves one progenitor cell type | 12 months | Clayton et al, 2007 |
| Skin/back | K14 | Epithelial cells | HF upper isthmus clones, including SG and IFE | nd | Jensen et al, 2009 |
| Skin/ear | Cyp1a1 | Basal cells of ear epidermis | Homeostasis involves one progenitor cell type | 12 months | Doupe et al, 2010 |
| Testis | Ngn3/Nanos2 | Undif. spermatogonia As, Apr and Aal | Stochastic turnover germ-line stem cells | 14 months | Klein et al, 2010 |
BASC, bronchioalveolar stem cells; CARN, castration-resistant Nkx3-1-expressing cells; CBC, crypt base columnar; DG, dentate gyrus; HF, hair follicle, IFE, interfollicular epidermis; ISC, intestinal stem cell; nd, not determined; prog., progenitor; SG, sebaceous gland; str., stratum; stel., stellatum; SVZ, subventricular zone; undif., undifferentiated.
In mouse intestine, such genetic marking was obtained by using the Dlb-1 locus. Its inactivation by random mutagenesis resulted in stem-cell-derived clones lacking the ability to be stained by the lectin Dolichos biflorus agglutin (Winton et al, 1988). By inverting the original strategy, the Dlb-1 locus could be used to draw a lineage hierarchy of intestinal progenitor compartments (Bjerknes & Cheng, 1999).
These strategies do not usually allow the exact cell-of-origin for the mutant clone to be identified. However, this limitation was circumvented in the haematopoietic system by combining clonal marking with single-cell transplantation: the stem-cell population was isolated before genetic marking and the contribution of single stem cells to various blood lineages was scored over time after transplantation (Jordan & Lemischka, 1990). In another approach, HSC mixtures from different genetic backgrounds were transplanted to determine the contribution of single HSCs to different lineages (Smith et al, 1991).
To minimize interference in normal physiology, Cotsarelis and colleagues introduced inducible genetic marking of a defined putative stem-cell population (Morris et al, 2004). A hormone-inducible version of the Cre enzyme was expressed in the putative stem-cell population of the hair follicle. The Cre enzyme remained inactive in the cytoplasm, but entered the nucleus on activation with a progesterone antagonist. When crossed with the uniform Cre-reporter mouse R26R-LacZ (Soriano, 1999), the active Cre enzyme could excise a transcriptional roadblock in front of the LacZ gene, leading to irreversible genetic marking of the Cre-expressing cell and its offspring. This could be visualized by an enzymatic (blue) staining reaction. Examination of individual blue clones over time readily revealed the growth kinetics, longevity and multipotentiality of the original marked cell (Fig 2). Intriguingly, as discussed above, marked bulge stem cells were developmentally more restricted in situ as scored by lineage tracing, than when analysed after transplantation (Fig 1; Morris et al, 2004).
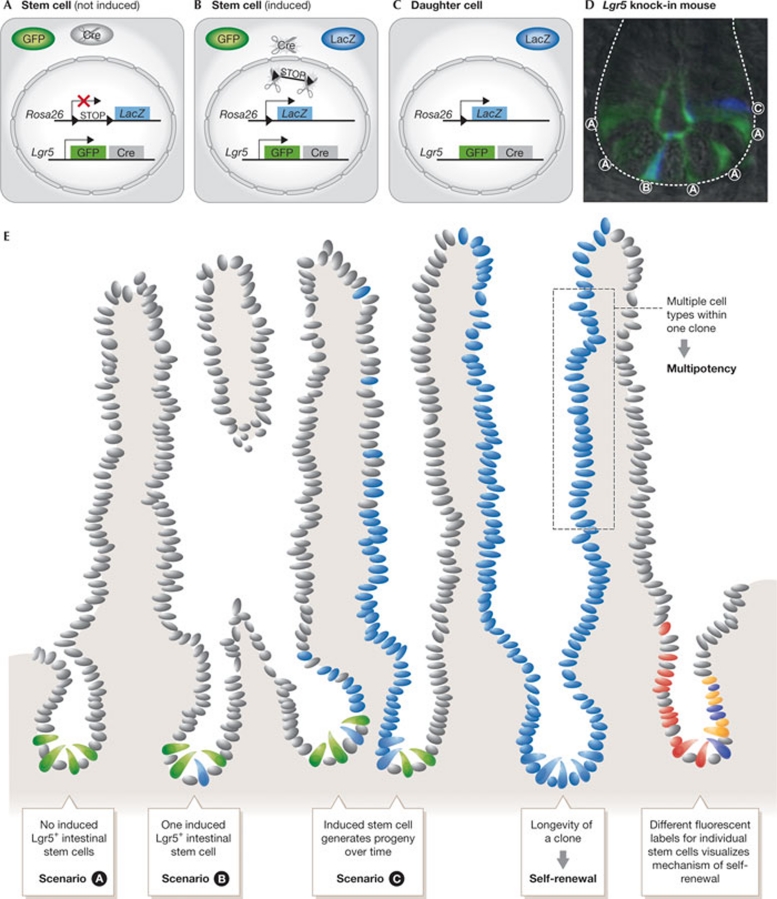
Figure 2.
In vivo lineage tracing in the small intestine. (A) Stem cells exclusively express GFP and the inactive version of Cre. (B) On activation of Cre, LacZ can be transcribed. (C) After cells differentiate, the GFP and inducible Cre are no longer produced. LacZ expression will be maintained. (D) In vivo small intestinal crypt of Lgr5-EGFP-Ires-CreERT2 mouse with all of the above scenarios. (E) Cartoon of small intestine in which lineage tracings (blue) are visualized at different stages originating from LGR5+ISCs (green; Barker et al, 2007). Over time, true stem cells generate clones that are long-lived (self-renewal) and contain different cell types (multipotency). The crypt to the right shows lineage tracing with R26R-Confetti in which stem cells, and subsequently their progeny, are marked with different colours (Snippert et al, 2010b). EGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; ISC, intestinal stem cell; LGR5, leucine-rich repeat-containing G protein coupled receptor 5.
A recent refinement of in vivo lineage tracing involves a bicistronic message being knocked into the genomic locus of a candidate gut-stem-cell gene Lgr5 (Barker et al, 2007). This allows the expression of two different proteins from the same locus in the cell, such as an inducible version of Cre to start lineage tracings and a fluorescent protein for visualization of the potential stem cell (Fig 2). The ISC marker gene Lgr5 was shown to be expressed in rare cells in several organs. By using the Lgr5 knock-in mouse, actual stemness has been shown for LGR5+ cells in the stomach, small intestine, colon and the hair follicle (Barker et al, 2007, 2010; Jaks et al, 2008). The same in vivo lineage-tracing strategy was used to document actual stemness for LGR6+ cells, which marks cells above the bulge region where hair follicle stem cells reside (Snippert et al, 2010a). Intriguingly, the LGR6 and LRIG1 stem-cell populations located high in the hair follicle clearly generate IFE (Jensen et al, 2009; Snippert et al, 2010a), whereas uninjured IFE has been reported to self-maintain without the need for hair follicles (Levy et al, 2005; Ito et al, 2005; Clayton et al, 2007). This indicates that the LGR6 and LRIG1 stem cells are not essential in epidermis maintenance, yet both populations generate progenitors that migrate into the IFE (Jensen et al, 2009; Snippert et al, 2010a).
Although most genetic lineage tracings are initiated in defined populations, it is difficult to exclude the possibility that actual stemness is found in an even smaller subpopulation. It is therefore essential that the cell-of-origin of traced clones is carefully mapped, and that the tracing efficiencies are quantitatively measured over time, as this can indirectly score the self-renewal capacity of a population. For example, the first reported genetic-fate mappings in the small intestine were initiated in LGR5+ crypt base columnar (CBC) cells at the base of intestinal crypts. The traced lineages were long-lived and included all intestinal cell types—the first identification of intestinal stem cells (Barker et al, 2007). However, BMI1 was subsequently reported to mark cells just above the LGR5+ CBC population. These so-called '+4 cells' were capable of initiating lineage tracings with the same kinetics as LGR5+ CBC cells, thereby refuelling the ISC identity debate (Sangiorgi & Capecchi, 2008). These tracing studies seem contradictory in terms of their cell-of-origin. We have since studied gene expression in crypts in greater detail and found that Bmi1 expression levels are highest in LGR5+ CBC cells (van der Flier et al, 2009). Thus, both tracings probably derive from overlapping—or even identical—cells at the crypt base. Another example that illustrates the need for quantitative measurements involves PROM1/ CD133, which was postulated to mark mouse ISCs. Lineage tracings initiated in PROM1+ cells indicated the presence of long-lived, multilineage clones (Zhu et al, 2009). However, by using a comparable approach, the number of clones traced from PROM1+ cells reduced by almost ten times after the first week, as PROM1/ CD133 marks not only ISCs, but also the more abundant early progenitor cells (Snippert et al, 2009).
A further refinement of lineage tracing has been made possible by the construction of multicolour Cre reporters by Jeff Lichtman and colleagues. The original Brainbow mice were designed to allow colour-coding of neurons with up to 90 distinguishable colour codes (Livet et al, 2007). Driven by a strong, ubiquitous promoter and inserted into the Rosa locus, this cassette yielded the R26R-Confetti allele, a general multicolour Cre-reporter (Snippert et al, 2010b). Using the R26R-Confetti allele, the individual behaviour of multiple stem cells in the same niche can be recorded (Fig 2). One such study showed that stem-cell fate in the intestine is stochastically determined: the observed stem-cell dynamics were consistent with a model in which the number of LGR5+ stem cells doubles each day by symmetrical divisions, after which all daughters undergo a neutral competition for residency in the niche (Snippert et al, 2010b). We recently documented that Paneth cells constitute the niche for neighbouring LGR5+ ISCs (Sato et al, 2010). Thus, it is likely that equal stem-cell daughters compete for available Paneth cell surface and that loss of direct Paneth cell contact—which happens to half of the stem cells each day—drives differentiation. A similar pattern of neutral drift in the intestine was deduced from quantitative analysis of the size and number of clonal tracing events on villi over time (Lopez-Garcia et al, 2010).
Other studies performed in unipotent-stem-cell systems similarly propose that homeostasis can be governed through a stochastic rather than a hierarchical mechanism. Lineage tracing in tail and ear epidermis revealed that basal progenitor cells are equal, but their fates are balanced such that equal numbers of progenitors and differentiated cells are produced in a population, thereby ensuring tissue homeostasis (Clayton et al, 2007; Doupe et al, 2010). Similar stochastic patterns of extinction and expansion of stem-cell clones were found in the mouse testes (Klein et al, 2010). We speculate that simultaneous tracing of multiple individual stem cells in other tissues might reveal similar unexpected insights into mechanisms of self-renewal.
Actual stemness compared with stemness potential
The defining characteristics of adult stem cells—their ability to generate all the cell types of the pertinent tissue and to do so for the lifetime of an organism—are fundamental principles in biology. Recent technical developments, especially pertaining to in vivo lineage-tracing strategies, have revealed discrepancies in the previous 'gold standards' of ex vivo culture and transplantation. Most approaches measure different aspects of stem-cell biology; lineage tracing measures the actual stemness of cells in their physiological context, whereas culture and transplantation strategies focus on stemness potential (Fig 1).
Discriminating between actual stemness and stemness potential is not always straightforward. For example, stem-cell identity and function in the endocrine pancreas are still debated, despite the fact that many lineage-tracing studies have been performed. By using a rat insulin promoter to initiate genetic-lineage tracing in differentiated islet β-cells, these cells were found to maintain their population by self-duplication during homeostasis and during regeneration after partial pancreatectomy (Dor et al, 2004). More recently, it was reported that new NGN3+ progenitor cells arise near the ducts to give rise to new β-cells after another type of injury—pancreatic duct ligation (Xu et al, 2008). Although embryonic duct progenitors have the plasticity to generate exocrine as well as endocrine lineages, genetic-lineage tracing with adult duct structures showed that adult duct progenitors generate acinar cells but not new β-cells (Solar et al, 2009; Furuyama et al, 2010). Thus, although the formation of new endocrine cells occurs in the proximity of ductal structures, they are not of ductal origin.
The identity of stem cells in lung tissue is also controversial. Bronchioalveolar stem cells have been shown to have self-renewal and multipotent abilities in vitro (Kim et al, 2005), but genetic labelling of bronchioalveolar stem cells and columnar Clara cells revealed no contribution to alveoli lineages during normal homeostasis and regeneration (Rawlins et al, 2009). This further illustrates the differences between actual stemness—of Clara and bronchioalveolar cells—and stemness potential in vitro. Similar discrepancies have been reported in the mammary gland (Shackelton et al, 2006; Stingl et al, 2006; Bai & Rohrschneider, 2010) and prostate (Goldstein et al, 2008; Wang et al 2009).
As explained above, the distinction between actual stemness and stemness potential is not always clear and, in some tissues, populations with both properties could coexist and have different functions. The physiological role of cells with stemness potential is unknown; they might be able to acquire actual stemness in situ during tissue repair or regeneration. Future research should address the nature of plasticity in actual stemness and stemness potential. In vitro culture systems in which single stem cells can grow into three-dimensional tissues are likely to provide the experimental platform from which to obtain such molecular insights.
Many types of adult stem cell exist, and experimental assays reveal different aspects of stem-cell behaviour. A combinatorial approach is obviously required for a complete picture of adult stem-cell biology. Nevertheless, as adult stem cells are functionally defined by two criteria—self-renewal and multipotency—testing these should always be the primary focus when investigating new stem-cell populations. So far, genetic lineage-tracing approaches have provided the most definitive in vivo demonstrations of stem cells in action.
See Sidebar A.
Sidebar A | In need of answers.
Self-renewal and multipotency are two functionally defined criteria of stemness. Are there other characteristics common to all types of adult stem cells?
What is the relationship between the states of actual stemness and stemness potential? Are these cellular states interchangeable?
What is the role of cell populations with stemness potential in vivo, both during steady-state homeostasis as well as after injury?
Acknowledgments
We thank Laurens van der Flier and Marc van de Wetering for helpful discussions and critical reading of the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahn S, Joyner AL (2005) In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437: 894–897 [DOI] [PubMed] [Google Scholar]
- Arai F, Suda T (2007) Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann NY Acad Sci 1106: 41–53 [DOI] [PubMed] [Google Scholar]
- Bai L, Rohrschneider LR (2010) s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 24: 1882–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Barker N et al. (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36 [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H (1987) Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA 84: 2302–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H (1999) Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116: 7–14 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648 [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y (2010) Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 466: 978–982 [DOI] [PubMed] [Google Scholar]
- Cairns J (1975) Mutation selection and the natural history of cancer. Nature 255: 197–200 [DOI] [PubMed] [Google Scholar]
- Chao MP, Seita J, Weissman IL (2008) Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb Symp Quant Biol 73: 439–449 [DOI] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y (2005) Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci USA 102: 14677–14682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH (2007) A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189 [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122: 289–301 [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: 1329–1337 [DOI] [PubMed] [Google Scholar]
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA (2007) Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 117: 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA (2004) Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46 [DOI] [PubMed] [Google Scholar]
- Doupe DP, Klein AM, Simons BD, Jones PH (2010) The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell 18: 317–323 [DOI] [PubMed] [Google Scholar]
- Fortunel NO et al. (2003) Comment on “'Stemness': transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science 302: 393; author reply 393 [DOI] [PubMed] [Google Scholar]
- Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H (2009) Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 27: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC (2007) Male and female Drosophila germline stem cells: two versions of immortality. Science 316: 402–404 [DOI] [PubMed] [Google Scholar]
- Furuyama K et al. (2010) Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet [Epub 28 Nov] doi:10.1038/ng.722 [DOI] [PubMed] [Google Scholar]
- Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte OW (2008) Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci USA 105: 20882–20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Nakada D, Morrison SJ (2009) Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 25: 377–406 [DOI] [PubMed] [Google Scholar]
- Huttner WB, Kosodo Y (2005) Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol 17: 648–657 [DOI] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S (2008) Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 105: 19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354 [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320 [DOI] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR (2002) A stem cell molecular signature. Science 298: 601–604 [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young R (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132: 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM (2009) Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CT, Lemischka IR (1990) Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev 4: 220–232 [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ (2007) Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature 449: 238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835 [DOI] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, Simons BD (2010) Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7: 214–224 [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC et al. (2007) Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci 27: 12623–12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON (2007) Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA 104: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM (2009) Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460: 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA (2005) Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9: 855–861 [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA (2007) Epidermal stem cells arise from the hair follicle after wounding. FASEB J 21: 1358–1366 [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H (2010) Coexistence of quiescent and active adult stem cells in mammals. Science 327: 542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450: 56–62 [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ (2010) Intestinal stem cell replacement follows a pattern of neutral drift. Science 330: 822–825 [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Gilbert PM, Blau HM (2009) Designing materials to direct stem-cell fate. Nature 462: 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkers H, Frisen J (2005) Deconstructing stemness. EMBO J 24: 2715–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M (2005) Direct isolation of satellite cells for skeletal muscle regeneration. Science 309: 2064–2067 [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J (2006) Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441: 1068–1074 [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP (1971) Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170: 421–435 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S (2007) Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 12: 195–206 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S (2010) Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller RA, Knoblich JA (2009) Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23: 2675–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootani A et al. (2009) Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford KW, Scadden DT (2008) Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 9: 115–128 [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H (1996) Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273: 242–245 [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Ranno R, Stracuzzi G, Bondanza S, Guerra L, Zambruno G, Micali G, De Luca M (1999) The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation 68: 868–879 [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M (1990) Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110: 1001–1020 [DOI] [PubMed] [Google Scholar]
- Potten CS, Hume WJ, Reid P, Cairns J (1978) The segregation of DNA in epithelial stem cells. Cell 15: 899–906 [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D (2002) Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115: 2381–2388 [DOI] [PubMed] [Google Scholar]
- Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, Ridgway RA, Sansom OJ, Nathke IS (2010) Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 6: 175–181 [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA (2002) “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science 298: 597–600 [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL (2009) The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4: 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710 [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106: 12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronfard V, Rives JM, Neveux Y, Carsin H, Barrandon Y (2000) Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation 70: 1588–1598 [DOI] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD (2010) Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA 107: 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY (2010) Stability of the regulatory T cell lineage in vivo. Science 329: 1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM (2008) Self-renewal and expansion of single transplanted muscle stem cells. Nature 456: 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A, Suzuki A, Suzuki H, Saga Y (2009) The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 325: 1394–1398 [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR (2009) Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci USA 106: 7101–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2010) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature [Epub 28 Nov] doi:10.1038/nature09637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T (2008) Imaging stem-cell-driven regeneration in mammals. Nature 453: 345–351 [DOI] [PubMed] [Google Scholar]
- Seidel K et al. (2010) Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development 137: 3753–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE (2006) Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S (2006) Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol 8: 677–687 [DOI] [PubMed] [Google Scholar]
- Smith LG, Weissman IL, Heimfeld S (1991) Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci USA 88: 2788–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, Clevers H (2009) Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 136: 2187–2194 [DOI] [PubMed] [Google Scholar]
- Snippert HJ et al. (2010) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389 [DOI] [PubMed] [Google Scholar]
- Snippert HJ et al. (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144 [DOI] [PubMed] [Google Scholar]
- Solar M et al. (2009) Pancreatic exocrine duct cells give rise to insulin-producing β cells during embryogenesis but not after birth. Dev Cell 17: 849–860 [DOI] [PubMed] [Google Scholar]
- Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71 [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241: 58–62 [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997 [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM (2008) White fat progenitor cells reside in the adipose vasculature. Science 322: 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL Jr, Lu WC, Ferrebee JW (1957) Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med 257: 491–496 [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG et al. (2009) Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136: 903–912 [DOI] [PubMed] [Google Scholar]
- Voog J, Jones DL (2010) Stem cells and the niche: a dynamic duo. Cell Stem Cell 6: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare SK, Bansal R, Lee J, Zhang YV, McDermitt DJ, Tumbar T (2008) Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J 27: 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461: 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Jensen KB (2009) Epidermal stem cell diversity and quiescence. EMBO Mol Med 1: 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A et al. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129 [DOI] [PubMed] [Google Scholar]
- Winton DJ, Blount MA, Ponder BA (1988) A clonal marker induced by mutation in mouse intestinal epithelium. Nature 333: 463–466 [DOI] [PubMed] [Google Scholar]
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY (2008) Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Kawase E, Kirilly D, Wong MD (2005) Intimate relationships with their neighbors: tales of stem cells in Drosophila reproductive systems. Dev Dyn 232: 775–790 [DOI] [PubMed] [Google Scholar]
- Xu X et al. (2008) Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207 [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ (2009) Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]