Abstract
Advances in high-throughput screening now enable the rapid discovery of bioactive small molecules, but these primary hits almost always exhibit modest potency. We report a strategy for the transformation of these hits into much more potent inhibitors without compound optimization. Appending a derivative of Ru(II)(tris-bipyridyl)2+, an efficient photosensitizer of singlet oxygen production, to synthetic protein-binding compounds results in highly potent and specific target protein inactivation upon irradiation with visible light.
Bioactive small molecules are nowadays commonly identified via high-throughput screening campaigns1, 2. In most cases, the primary hits that emerge from these efforts have unknown selectivity and modest potency. The standard approach to improving the potency of a hit is to synthesize a large number of analogues and test each of them independently. These labor-intensive efforts are not amenable to high throughput, meaning that the current capacity to screen libraries far outstrips the capacity to mature them. For antagonists, an alternative strategy is to append to the molecule of interest a latent “warhead” able to inactivate nearby proteins when triggered. In this way, even when the inhibitor diffuses away, the target protein remains inactive, resulting in an apparent increase in potency. Chromophores that generate singlet oxygen when irradiated with visible light constitute an almost ideal warhead. Singlet oxygen modifies many different protein functional groups and it cannot diffuse more than ≈ 40-80 Å from its point of generation3. Indeed, efforts have been made to develop so-called CALI (chromophore-assisted light inactivation) reagents by linking organic chromophores such as fluorescein to protein-binding antibodies or small molecules4-6. However, these reagents have not made a significant impact as pharmacological tools because of the poor efficiency of singlet oxygen generation of many chromophores and the inability of antibodies to access intracellular targets. We show here that highly effective CALI agents can be created by appending derivatives of Ru(II)(tris-bipyridyl)2+ (Ru(II)(bpy)32+), an exceptionally efficient photocatalyst for singlet oxygen generation7-9,10 to highly selective protein-binding peptoids. These reagents are capable of targeting both extracellular and intracellular targets.
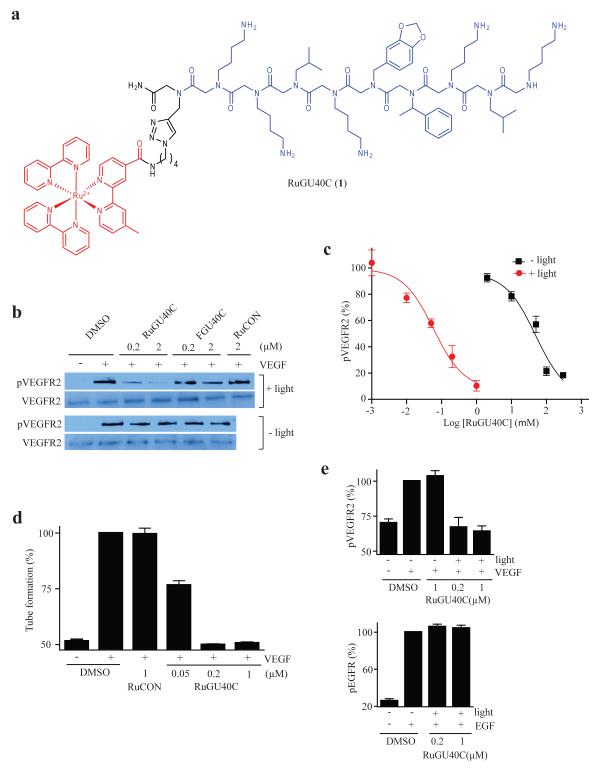
Peptoid GU40C is a weak, but highly selective, antagonist of Vascular Endothelial Growth Factor (VEGF)-induced activation of the VEGF Receptor 2 (VEGFR2)11,12. A Ru(II)(bpy)32+-GU40C conjugate (RuGU40C, Fig.1a) was constructed via click chemistry and was shown to have an affinity for the VEGFR2 extracellular domain similar to that of the GU40C parent peptoid (Supplementary Fig. 1). The activity of this compound was then tested in an assay in which cultured endothelial cells were exposed to VEGF and the activation of VEGFR2 was monitored. As shown in Figure 1b, in the absence of irradiation, RuGU40C did not inhibit VEGF-induced autophosphorylation of VEGFR2 even at the highest concentration examined (2 μM), as expected. However, with visible light (> 380 nm) irradiation (high-intensity lamp for 10 min), VEGFR2 autophosphorylation was inhibited potently. A conjugate containing Ru(II)(bpy)32+ tethered to a control peptoid that does not bind VEGFR2 (RuCON. Supplementary Fig. 2) did not show any inhibitory activity, nor did a scrambled version of RuGU40C (Supplementary Fig. 3). A titration experiment revealed that RuGU40C exhibited an IC50 of 49 μM in the absence of irradiation and 59 nM when irradiated. This represents a greater than 800-fold increase in potency (Fig. 1c). RuGU40C also inhibited the formation of vessel-like tube structures by endothelial cells in an in vitro angiogenesis assay13 when irradiated (Figure 1d and Supplementary Figure 4) with an IC50 of about 50 nM while RuCON did not.
Figure 1.
Visible light-triggered inactivation of the Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) by a ruthenium-peptoid conjugate. (a) Chemical structure of RuGU40C. The modified Ru(II)(bpy)32+ complex and the GU40C peptoid are shown in red and blue, respectively. (b) Western blots showing the level of phospho-VEGFR2 (the active form of the receptor) and total VEGFR2 after receptor-expressing cells (PAE/KDR) were incubated under the conditions indicated. The duration of irradiation was 10 minutes. FGU40C = fluorescein-conjugated GU40C (see Supplementary Fig. 2). RuCON = a Ru(II)(bpy)32+-conjugated control peptoid that does not bind VEGFR2 (see Supplementary Fig. 2). (c) Dose-dependence of the inhibition of autophosphorylation of VEGFR2 by RuGU40C with or without irradiation. (d) Effect of ruthenium-peptoid conjugates on the VEGF-induced formation of tubes by human umbilical vascular endothelial cells (HUVECs). HUVECs on Matrigel-coated plates were incubated under the conditions indicated and irradiated (10 min). 16hr after the addition of VEGF, degree of tube formation was evaluated by quantitative analysis (AngioQuant software) of images obtained using a light microscope (see Fig S3 for representative images). (e) Analysis of the specificity of RuGU40C-mediated inhibition of VEGFR2. The effect of the ruthenium-peptoid conjugate on hormone-mediated autophosphorylation (activation) of VEGFR2 or EGFR was examined by western blot in the presence or absence of irradiation (10 min) in cells that express both receptors (H441) and evaluated by quantitative analysis (Image J). Note that there is a basal level of phosph-VEGFR2 present even in the absence of VEGF treatment.
A fluorescein conjugate of GU40C also mediated the inhibition of VEGFR2 activation when irradiated, but much less efficiently than the ruthenium-peptoid conjugate (~50% at 2 μM, Fig. 1b; fluorescein itself showed inactivation at concentration higher than 2 μM (Supplementary Fig. 5)).
To examine the selectivity of VEGFR2 photo-inactivation, RuGU40C was mixed with recombinant Luciferase and the solution was irradiated. As shown in Supplementary Figure 6, RuGU40C and light had no effect on Luciferase activity, which we have shown previously to be sensitive to targeted inactivation by singlet oxygen when the ruthenium complex is attached covalently to a Luciferase fusion protein10. In addition, H441 cells, which express both Epidermal Growth Factor Receptor (EGFR; also a receptor tyrosine kinase) and VEGFR214, were irradiated in the presence of RuGU40C and hormone-induced autophosphorylation of each of the receptors was monitored. As shown in Figure 1e, at concentrations of RuGu40C that reduced VEGFR2 activation to basal levels, EGFR autophosphorylation was unaffected. Moreover, cell viability was unaffected by RuGU40C and light (Supplementary Fig. 7). Taken together, these data argue strongly that at these concentrations inactivation of VEGFR2 is highly specific and that there is little or no “bystander damage” to other membrane receptors or the cells in general.
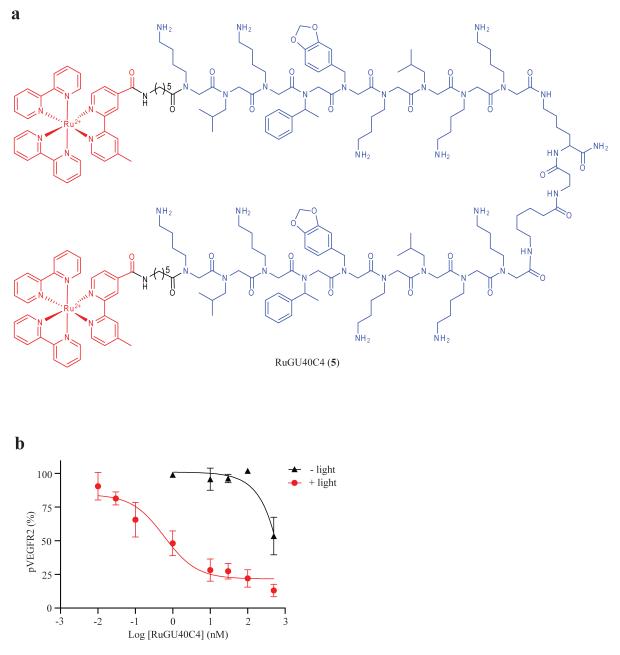
We also demonstrated that extremely potent CALI agents could be obtained by starting with a higher affinity ligand. GU40C4 contains two copies of GU40C joined by a linker, has increased binding affinity to dimeric VEGFR2 (KD = 20~30 nM) and also showed increased potency in blocking VEGF-induced autophosphorylation of VEGFR2 in cultured cells (IC50 = 1 μM) 11. After confirming that a Ru(II)(bpy)32+ conjugate of GU40C4, RuGU40C4 (see Fig, 2a), binds to the VEGFR2 extracellular domain (Supplementary Fig. 8), autophosphorylation assays were performed to determine its potency in a cell culture assay. In the absence of irradiation, RuGU40C4 showed about 50% inhibition of VEGFR2 at 500 nM, a potency similar to that of GU40C4 itself. However, with irradiation, RuGU40C4 inhibited VEGFR2 with an IC50 of 590 pM (Fig. 2b). This represents a 1700-fold increase in potency. Clearly, by starting with a higher affinity ligand, extremely potent CALI agents can be created using this method.
Figure 2.
A hyper-potent CALI inhibitor of VEGFR2. (a) Chemical structure of RuGU40C4. The modified Ru(II) (bpy) 2+3 complex and GU40C4 peptoid are shown in red and blue, respectively. (b) Dose-dependent inhibition of VEGF-induced autophosphorylation of VEGFR2 on PAE/KDR cells by RuGU40C4 with irradiation (10 min).
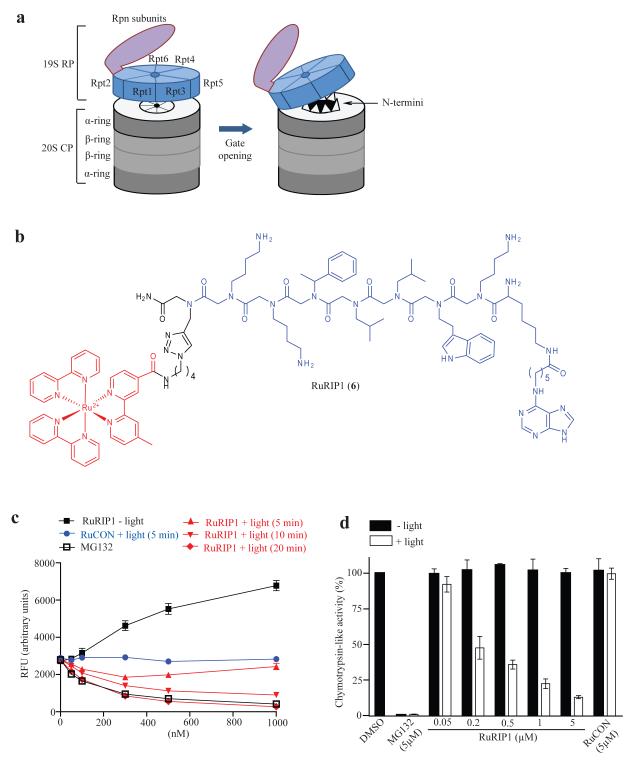
Next, we asked if this approach could be used for the inactivation of intracellular proteins. Rpt4, one of six AAA class ATPases in the 26S proteasome was chosen as a target. Rpt4 is one of a ring of six ATPases that sits atop the 20 S core particle (CP) of the proteasome and feeds substrate proteins into the barrel-like cavity of the 20 S CP15 (Fig. 3a). They also stimulate peptidolysis by holding open a “flap” on the 20S CP that otherwise restricts substrate access16, 17. Previously, a peptoid called RIP1 was isolated in a screen for specific ligands to the yeast proteasome18. RIP1 targets Rpt419 and acts as an agonist of 26 proteasome-mediated peptidase activity (chymotrypsin-like; EC50 ≈ 3 μM), presumably by locking the ATPase complex in the “flap open” conformation20. As shown in Supplementary Fig. 9, it also showed an enhancement of peptidase activity of the human 26S proteasome in living cells, but with lower potency (EC50 ≈ 50μM).
Figure 3.
Visible light-triggered inactivation of the 26S proteasome by a ruthenium-peptoid conjugate. (a) Illustration of the 26S proteasome and gate opening of the 20S proteasome. (b) Chemical structure of RuRIP1. The modified Ru(II) (bpy) 2+3 complex and RIP1 peptoid are shown in red and blue, respectively. (c) Chymotrypsin-like peptidase activity of purified, yeast 26S proteasome was measured in the presence of RuRIP1 with or without irradiation by monitoring the cleavage of fluorogenic substrate, Suc-LLVY-AMC. (d) The effect of RuRIP1 on chymotrypsin-like activity of the 26S proteasome in HeLa cells with or without irradiation (30 min) was assessed by measuring luminescence generated by substrate (Suc-LLVY-aminoluciferin) cleavage.
A Ru(II)(bpy)32+ conjugate of RIP1 (RuRIP1, Fig. 3b) was also an agonist of the chymotrypsin-like activity of the proteasome in vitro in the dark. Irradiation resulted in an inversion of RuRIP1 activity and inhibition of peptidolysis was observed, as expected (Fig. 3c). The potency increased with the irradiation time (IC50 = 300 nM and 85 nM with 10 min and 20 min irradiation, respectively) suggesting a dependence on the amount of 1O2 generated. The potency of RuRIP1 when irradiated for 20 minutes was similar to that of MG132, a direct inhibitor of the chymotrypsin-like peptidase activity of the 26S proteasome. The two other peptidase activities of the 26S proteasome (caspase-like and trypsin-like) were also inhibited by RuRIP1 and light (Supplementary Fig. 10). RuCON was neither an agonist nor a photo-antagonist of peptidolysis. Moreover, no inhibition of recombinant Luciferase was observed when this protein was irradiated in the presence of RuRIP1 (Supplementary Fig. 11). Finally, addition of imidazole, a singlet oxygen quencher, ablated the photo-antagonistic effect of RuRIP1 on the proteasome (Supplementary Figure 12).
We then asked if RuRIP1 can inactivate the human 26S proteasome inside living cells upon irradiation, requiring the compound to cross the membrane and for the oxidative chemistry to proceed efficiently even in the reducing environment of the cell. As shown in Figure 3d, a dose-dependent inhibition (IC50 ≈ 200 nM) of proteasome-mediated peptidolysis was observed when cells were incubated with RuRIP1 and irradiated for 30 minutes, whereas no effect was observed in the absence of light (note that the RuRIP1 concentration was well below that required for agonist activity). Complete inhibition of peptidase activity by RuRIP1 was not observed even at 5 μM peptoid. This may represent residual activity of 20 CP lacking an ATPase-containing cap, which are known to exist in cells21, 22.
To investigate whether RuRIP1 has off-target effects inside cells, the activity of Renilla Luciferase, which was expressed transiently in cells, was monitored. As shown in Supplementary Fig. 13, no inhibition was observed in cells irradiated in the presence of RuRIP1. Moreover, we were unable to measure a significant increase in the levels of cellular reactive oxygen species (ROS) in the irradiated cells using the ROS probe Carboxy-H2DCFDA (Supplementary figure 14).
In order for efficient ruthenium-mediated photoinactivation to occur, the “delivery molecule” must be more resistant to singlet oxygen than the protein target. Solutions containing only RuGU40C or RuRIP1 were irradiated for different lengths of time and the rate of loss of the molecular ion in the mass spectrum was monitored (Supplementary figure 15). The proteasome-binding RuRIP1 peptoid was more labile than RuGU40C, probably because of the presence of the indole side chain and the adenine ring in this molecule, though no attempt has been made to characterize the photooxidation product. Importantly though, both ruthenium-peptoid conjugates were modified at a rate significantly slower than the observed rate of protein inactivation.
In summary, we have developed a practical method that allows modest potency hits from screening efforts to be transformed into much higher potency CALI reagents for the targeted, visible light photo-triggered inactivation of proteins. Both intracellular and integral membrane proteins can be targeted using this technology. Little or no detectable inhibition of proteins not targeted by the delivery molecule was observed, though it is obviously impossible to rule out any off target effects completely. This is by no means the first report of CALI reagents containing synthetic protein ligands23,24, but the high photocatalytic efficiency of singlet oxygen formation exhibited by the Ru(II)(bpy)32+ represents an important technical advance in the design of such agents. Finally, we employed peptoid-Ru(II)(bpy)32+ conjugates in this study because screening strategies for the isolation of highly selective protein-binding peptoids have been published11,18. This is essential to limit off target effects. Moreover, peptoids are generally cell permeable25 and can be modified at their C-terminus without fear of compromising their activity. This makes them ideal “magic bullets” for the construction of CALI-based tool compounds for biological research. We believe that the combination of this powerful screening technology and the unusually efficiency of singlet oxygen generation of the Ru(II)(bpy)32+ will make the development of potent and selective CALI-based “tool compounds” far more accessible.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a contract from the National Heart, Lung, and Blood Institute (NO1-HV28185) for the UT Southwestern Center for Proteomics Research. We thank Prof. Michael Rosen (UT Southwestern) and Ben Cravatt (Scripps) for a critical reading of the manuscript.
Footnotes
ADDITIONAL INFORMNATION: A full description of the methods employed in this study is provided in the supplementary information available online at http://www.nature/naturechemicalbiology. Correspondence and requests for material should be sent to T.K. (Kodadek@scripps.edu)
REFERENCES
- 1.Diller DJ. The synergy between combinatorial chemistry and high-throughput screening. Curr Opin Drug Discov Devel. 2008;11:346–355. [PubMed] [Google Scholar]
- 2.Colas P. High-throughput screening assays to discover small-molecule inhibitors of protein interactions. Curr Drug Discov Technol. 2008;5:190–199. doi: 10.2174/157016308785739875. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 4.Liao JC, Roider J, Jay DG. Chromophore-assisted laser inactivation of proteins is mediated by the photogeneration of free radicals. Proc Natl Acad Sci U S A. 1994;91:2659–2663. doi: 10.1073/pnas.91.7.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck S, et al. Fluorophore-assisted light inactivation: a high-throughput tool for direct target validation of proteins. Proteomics. 2002;2:247–255. doi: 10.1002/1615-9861(200203)2:3<247::aid-prot247>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winterle JS, Kliger DS, Hammond GS. Mechanisms of photochemical reactions in solution. 80. Photochemical oxidation of tri(2,2′-bipyridyl)ruthenium(II) by molecular oxygen. J. Amer. Chem. Soc. 1976;98:3719–3721. [Google Scholar]
- 8.Zhang X, Rodgers MAJ. Energy and electron transfer reactions of the MLCT state of ruthenium Tris(bipyridyl) with molecular oxygen: A laser flash photolysis study. J. Phys. Chem. 1995;99:12797–12803. [Google Scholar]
- 9.Fuller ZJ, et al. Photostability of luminescent ruthenium(II) complexes in polymers and in solution. Anal Chem. 2003;75:2670–2677. doi: 10.1021/ac0261707. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Yu P, Xiao X, Kodadek T. A general system for evaluating the efficiency of chromophore-assisted light inactivation (CALI) of proteins reveals Ru(II) tris-bipyridyl as an unusually efficient “warhead”. Mol Biosyst. 2008;4:59–65. doi: 10.1039/b712307h. [DOI] [PubMed] [Google Scholar]
- 11.Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. A peptoid “antibody surrogate” that antagonizes VEGF receptor 2 activity. J Am Chem Soc. 2008;130:5744–5752. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 12.Udugamasooriya DG, Dunham G, Ritchie C, Brekken RA, Kodadek T. The pharmacophore of a peptoid VEGF receptor 2 antagonist includes both side chain and main chain residues. Bioorg Med Chem Lett. 2008;18:5892–5894. doi: 10.1016/j.bmcl.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Iwamoto Y, Tanaka K, Okuyama K, Sugioka Y. A quantitative assay using basement membrane extracts to study tumor angiogenesis in vivo. Int J Cancer. 1996;67:148–152. doi: 10.1002/(SICI)1097-0215(19960703)67:1<148::AID-IJC24>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Wu W, et al. Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther. 2007;6:471–483. doi: 10.1158/1535-7163.MCT-06-0416. [DOI] [PubMed] [Google Scholar]
- 15.Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 16.Smith DM, et al. ATP binding to PAN or the 26S ATPases causes association with the 20S proteasome, gate opening, and translocation of unfolded proteins. Mol Cell. 2005;20:687–698. doi: 10.1016/j.molcel.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Rabl J, et al. Mechanism of gate opening in the 20S proteasome by the proteasomal ATPases. Mol Cell. 2008;30:360–368. doi: 10.1016/j.molcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim HS, Archer CT, Kodadek T. Identification of a peptoid inhibitor of the proteasome 19S regulatory particle. J Am Chem Soc. 2007;129:7750–7751. doi: 10.1021/ja072027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim HS, Cai D, Archer CT, Kodadek T. Periodate-triggered cross-linking reveals Sug2/Rpt4 as the molecular target of a peptoid inhibitor of the 19S proteasome regulatory particle. J Am Chem Soc. 2007;129:12936–12937. doi: 10.1021/ja075469+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim HS, Archer CT, Kim YC, Hutchens T, Kodadek T. Rapid identification of the pharmacophore in a peptoid inhibitor of the proteasome regulatory particle. Chem Commun (Camb) 2008:1064–1066. doi: 10.1039/b717861a. [DOI] [PubMed] [Google Scholar]
- 21.Brooks P, et al. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. Biochem J. 2000;346(Pt 1):155–161. [PMC free article] [PubMed] [Google Scholar]
- 22.Peters JM, Franke WW, Kleinschmidt JA. Distinct 19 S and 20 S subcomplexes of the 26 S proteasome and their distribution in the nucleus and the cytoplasm. J Biol Chem. 1994;269:7709–7718. [PubMed] [Google Scholar]
- 23.Yogo T, et al. Modification of intracellular Ca2+ dynamics by laser inactivation of inositol 1,4,5-trisphosphate receptor using membrane-permeant probes. Chem Biol. 2004;11:1053–1058. doi: 10.1016/j.chembiol.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Marks KM, Braun PD, Nolan GP. A general approach for chemical labeling and rapid, spatially controlled protein inactivation. Proc Natl Acad Sci U S A. 2004;101:9982–9987. doi: 10.1073/pnas.0401609101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu P, Liu B, Kodadek T. A high-throughput assay for assessing the cell permeability of combinatorial libraries. Nat Biotechnol. 2005;23:746–751. doi: 10.1038/nbt1099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.