Abstract
Plasminogen activator inhibitor (PAI)-1 is a major fibrinolytic inhibitor. High PAI-1 is associated with increased renal and cardiovascular disease risk. Previous studies demonstrated PAI-1 down-regulation by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), but the molecular mechanism remains unknown. Here we show that exposure of mouse embryonic fibroblasts to TNFα or LPS led to a marked induction of PAI-1, which was blunted by 1,25(OH)2D3, NF-κB inhibitor or p65 siRNA, suggesting the involvement of NF-κB in 1,25(OH)2D3-induced repression. In mouse Pai-1 promoter a putative cis-κB element was identified at −299. EMSA and ChIP assays showed that TNF-α increased p65/p50 binding to this κB site, which was disrupted by 1,25(OH)2D3. Luciferase reporter assays showed that PAI-1 promoter activity was induced by TNFα or LPS, and the induction was blocked by 1,25(OH)2D3. Mutation of the κB site blunted TNFα, LPS or 1,25(OH)2D3 effects. 1,25(OH)2D3 blocked IκBα degradation and arrested p50/p65 nuclear translocation. In mice LPS stimulated PAI-1 expression in the heart and macrophages, and the stimulation was blunted by pre-treatment with a vitamin D analog. Together these data demonstrate that 1,25(OH)2D3 down-regulates PAI-1 by blocking NF-κB activation. Inhibition of PAI-1 production may contribute to the reno- and cardio-protective effects of vitamin D.
Keywords: vitamin D, PAI-1, NF-κB, gene regulation
Introduction
Plasminogen activator inhibitor-1 (PAI-1), a 50 kDa glycoprotein, is the principal inhibitor of tissue-type plasminogen activators (t-PAs) and urokinase-type plasminogen activators (u-PAs), which convert inactive plasminogen to active plasmin. Plasmin is a serine protease and involved in the degradation of clot fibrin and extracellular matrix. Elevated plasma PAI-1 levels are associated with and contribute to thrombotic diseases including hyperthrombosis and myocardial infarction as well as fibrotic disorders such as atherosclerosis, pulmonary fibrosis and nephropathy [1, 2], thus PAI-1 has long been considered an important therapeutic target. PAI-1 is produced by a broad range of cell types, including hepatocytes, glomerular mesangial cells, tubular epithelial cells, fibroblasts, vascular endothelial cells, smooth muscle cells, macrophages and adipocytes. Under normal conditions, PAI-1 is present in plasma at very low concentrations. High levels of PAI-1 are caused by a number of mechanisms, including stimulation by pro-fibrotic factors such as transforming growth factor β (TGFβ) [3] and inflammatory factors such as tumor necrosis factor α (TNFα), interleukin-1 β (IL-1β) and lipopolysaccharide (LPS) [4–8]. PAI-1 is thus considered as an inflammatory response gene. Given the important contribution of inflammation to the development of chronic renal and cardiovascular diseases [9–14], inflammatory stimulation of PAI-1 has important pathological implications. The mechanism underlying the inflammatory regulation of PAI-1, however, remains poorly defined.
1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the hormonal metabolite of vitamin D, is a pleiotropic hormone that exerts its actions by activating the vitamin D receptor (VDR), a member of the nuclear receptor superfamily [15]. Vitamin D-deficiency is now recognized as a global health issue with adverse consequences [16]. Accumulating epidemiological and clinical evidence has demonstrated an association of vitamin D-deficiency with increased risk of renal and cardiovascular diseases. For example, in patients with chronic kidney disease (CKD), vitamin D-deficiency is an independent risk factor for cardiovascular disease [17]. In hypertensive patients, low serum vitamin D levels markedly increase the risk of cardiovascular disease [18]. Vitamin D therapy reduces mortality in CKD patients including cardiovascular mortality and infectious mortality [19, 20]. The molecular basis of these observations remains to be defined, but given the crucial role of PAI-1 in the development of renal and cardiovascular disorders, there is a good possibility for vitamin D to target PAI-1 in its renal and cardiovascular protection. Indeed, several previous studies have reported regulatory effects of vitamin D on PAI-1 production in a number of cell types. For example, 1,25(OH)2D3 was shown to enhance plasminogen activator activity and decrease PAI-1 production in rat calvarial osteoblast-like cells and osteogenic sarcoma cells [21]. Activated vitamin D analogs were reported to suppress PAI-1 in human coronary artery smooth muscle cells [22, 23]. While these observations appear to be important, the mechanism underlying vitamin D repression of PAI-1 remains to be defined.
In this study, we investigated the mechanism whereby 1,25(OH)2D3 counters the induction of PAI-1 by TNFα and LPS in mouse embryonic fibroblasts (MEFs). We identified a functional cis-κB element in the proximal promoter of the mouse pai-1 gene that mediates the up-regulation of PAI-1 by TNFα and LPS. We further demonstrated that 1,25(OH)2D3 down-regulates PAI-1 expression via targeting the NF-κB activation pathway.
Materials and Methods
Cell culture
MEFs were isolated from 13.5-day old VDR(+/−) and VDR(−/−) mouse embryos as described previously [24]. VDR(+/−) and VDR(−/−) MEFs were cultured in DMEM supplemented with 10% FBS. For most experiments, the cells were pretreated for 24 hours with 20 nM 1,25(OH)2D3 before being stimulated with either TNFα (20 ng/ml) or LPS (100 ng/ml). MEFs used in the experiments are VDR(+/−) unless otherwise stated. Detailed experimental conditions are described in figure legends.
RT-PCR
Total cellular RNAs were extracted using TRIzol reagent (Invitrogen). First-strand cDNAs were synthesized from total RNAs using MML-V reverse transcriptase (Invitrogen) and hexanucleotide random primers. The cDNAs were used as templates for PCR amplifications with the following primers. PAI-1: 5′TCATCCTGCCTAAGTTCTCTCT3′ (forward), and 5′GCTCTTGGTCGGAAAGACTT3′ (reverse); and p65: 5′CAGGCAGAGTGACTTCATGG3′ (forward) and 5′GCTCGTGAGAACTGCTGCTA3′ (reverse). The internal control for the PCR reaction was β2-microglobulin (B2M) with primers 5′ACCGGCCTGTATGCTATCCAGAAA3′ (forward) and 5′ATTTCAATGTGAGGCGGGTGGAAC3′ (reverse).
Western blotting
Proteins were separated by SDS-PAGE and transferred onto Immobilon membranes. Western blotting was carried out as previously described [25], using antibodies against PAI-1 or IκBα (Santa Cruz Biotechnologies).
Immunostaining
VDR(+/−) and VDR(−/−) MEFs (grown on cover slips) pretreated with 20 nM 1,25(OH)2D3 overnight were stimulated with TNFα for 30 min. The cells were fixed with 4% paraformaldehyde for 30 min and stained with anti-p65 antibody as described [26]. Cell nuclei were stained with DAPI (4′-6-diamidino-2-phenylindol).
Northern Blot
Northern blot analyses were performed as described previously [27] with 32P-labelled PAI-1 cDNA as hybridization probe.
PAI-1 promoter constructs and luciferase reporter assays
The 5′ upstream region from −362 to +10 in the mouse pai-1 gene was amplified by PCR using primers 5′ATGGCTGTCTCCAAAAAAAG3′ (forward) and 5′CGGACGCGTAGCCTGATCCAGCTGTGCT3′ (reverse), and cloned into pGL3 basic vector (Promega) to generate reporter plasmid pGL-Pai-Luc. Reporter plasmid pGL-Pai-m-Luc carrying mutations at the −299 κB site (mutated from 5′GGGAATTCCA3′ to 5′GGGcAcTCCA3′) was generated using the RapidChange mutagenesis kit (Stratagene). Mutations were confirmed by DNA sequencing. Cells were transfected using Lipofectamine 2000 (Invitrogen) in serum free media with pGL-Pai-Luc, pGL-Pai-m-Luc or pNF-κB-Luc (Promega) as indicated in the experiment. Twenty-four hours after transfection the cells were exposed to 20 ng/ml TNFα or 100 ng/ml LPS in the presence or absence of 20 nM 1,25(OH)2D3 for 24 hours. The cells were lysed and luciferase activity determined using Luciferase Assay Systems (Promega) as reported previously [28].
Electrophoretic mobility shift assays (EMSA)
Nuclear extract preparations and EMSA were performed as described previously [28]. Confluent MEFs were stimulated with 20 ng/ml TNFα for 2 hours in the presence or absence of 20 nM 1,25(OH)2D3 for 24 hours. Nuclear extracts were prepared from MEFs following an established method described previously [28]. For EMSA, 5 μg of nuclear extracts were incubated with 5×106 cpm of 32P-labeled −299 κ B probe (5′AGGAAGGGAATTCCAAACAC3′; underlined is the core NF-κB binding site) at room temperature for 20 minutes. The specificity of protein-DNA interaction was confirmed by competition with an excess amount of unlabeled probes of the same sequence, the canonical κB probe (designated as κ Bc, (5′AGTTGAGGGGCATTTCCCAGGC3′, Santa Cruz Biotechnology), or the mutated probe −299κBmut (5′AGGAAGGGcAcTCCAAACAC3′). The presence of p65 or p50 in the DNA-protein complex was confirmed with antibody supershift assays using anti-p65 or anti-p50 antibody (Santa Cruz Biotechnology).
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were carried out as described previously [28] using a commercial kit from Upstate Biotechnology (Lake Placid, NY). Briefly, MEFs were pretreated with or without 20 nM 1,25(OH)2D3 in serum-free media for 24 hours, and then stimulated with TNFα for 30 minutes. After treatment with 1% formaldehyde to cross-link histones to DNA, cells were lysed and sonicated to shear the chromatins. The sonicated chromatins were incubated with anti-p65 antibody or anti-p50 antibody and the chromatin-antibody complex was precipitated with protein-A-agarose beads. The DNA isolated from the complex was subjected to PCR amplification using the following primers flanking the −299κB site in the pai-1 gene promoter: 5′ATGGCTGTCTCCAAAAAAAG3′ (forward) and 5′GTGTGTGTACGTGTGAAAGG3′ (reverse). The PCR products were visualized by electrophoresis on a 1.5% agarose gel stained with ethidium bromide.
Animal studies
Two-months old C57BL/6 mice were injected i.p. with vehicle or paricalcitol (19-nor-1,25-dihydroxyvitamin D2, Abbott Laboratories, Abbott Park, IL, USA) at 300 ng/kg (dissolved in propylene glycol:water:ethanol =60:30:10) daily for 2 weeks. After the last paricalcitol treatment, the mice were treated with LPS at 15 mg/kg by i.p. injection, and the mice were killed after 18 hours for tissue harvest. Total RNAs were extract from the left ventricle of the heart and peritoneal macrophages, and total cell lysates were prepared from the aorta. PAI-1 mRNA transcript and protein levels were determined by Northern and Western blottings. The animal studies were approved by the Institutional Animal Care and Use Committee at The University of Chicago.
Statistical analysis
Data values are presented as means ± SEM. Statistical comparisons were carried out using unpaired two-tailed Student’s t-test or AVOVA as appropriate, with P< 0.05 being considered statistically significant.
Results
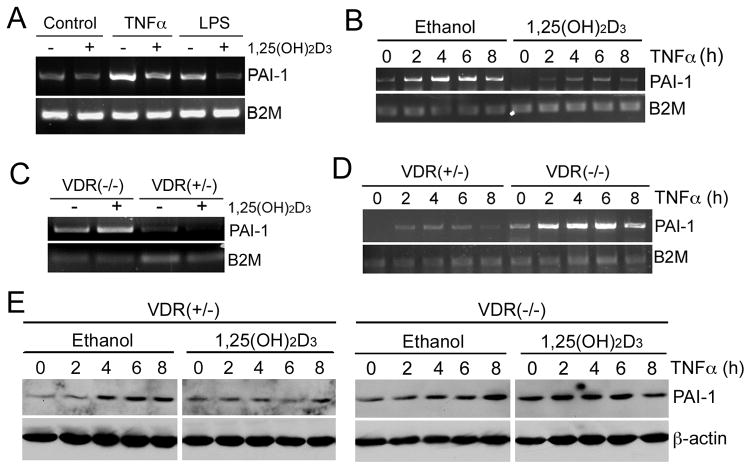
We used MEFs as a model system to investigate the mechanism whereby 1,25(OH)2D3 regulates PAI-1 expression. As shown in Figure 1, when MEFs were treated with TNF-α or LPS for 8 hours, PAI-1 mRNA was markedly induced, and the induction was blocked when the cells were pre-treated with 20 nM 1,25(OH)2D3 (Fig. 1A). Time course studies showed that TNF-α induced PAI-1 mRNA within 2 hours, and the induction peaked in 4 to 6 hours, but was dramatically attenuated in the presence of 1,25(OH)2D3 (Fig. 1B). However, 1,25(OH)2D3 failed to suppress the induction of PAI-1 mRNA by TNF-α in VDR(−/−) MEFs (Fig. 1C and D). Western blot analyses confirmed that the time-dependent induction of PAI-1 protein by TNF-α was blocked in the presence of 1,25(OH)2D3 in VDR(+/−) MEFs, but not in VDR(−/−) MEFs (Fig. 1E). These results demonstrate that 1,25(OH)2D3 suppresses the induction of PAI-1 expression in a VDR-dependent manner.
Figure 1.
Vitamin D suppresses the induction of PAI-1 in a VDR-dependent manner. (A) VDR(+/−) MEFs were pre-incubated with ethanol (−) or 20 nM 1,25(OH)2D3 (+) overnight, followed by treatment with or without 20 ng/ml TNF-α or 100 ng/ml LPS for 8 hours; (B) VDR(+/−) MEFs were pre-incubated with ethanol or 1,25(OH)2D3 (20 nM) overnight, followed by TNF-α induction for 0, 2, 4, 6 and 8 hours; (C) VDR(+/−) and VDR(−/−) MEFs were pre-incubated with ethanol (−) or 20 nM 1,25(OH)2D3 (+) overnight, followed by 8 hours of TNF-α induction; (D) 1,25(OH)2D3 pre-treated VDR(+/−) and VDR(−/−) MEFs were induced by TNF-α for 0–8 hours. PAI-1 mRNA levels were determined by RT-PCR. (E) VDR(+/−) and VDR(−/−) MEFs were pre-incubated by ethanol or 20 nM 1,25(OH)2D3 overnight, followed by TNF-α for 0, 2, 4, 6 and 8 hours. PAI-1 protein levels were assessed by Western blotting.
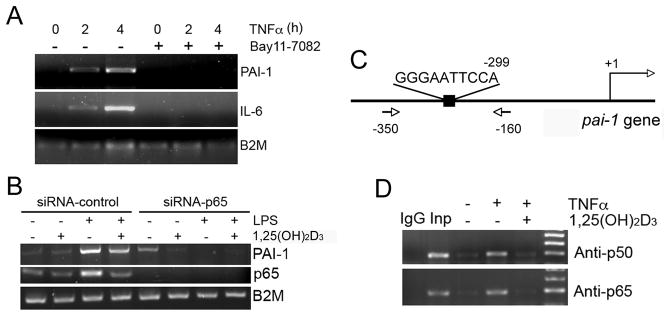
It is well known that both LPS and TNF-α can induce NF-κB activity [29, 30]. To test whether the induction of PAI-1 in MEFs was mediated by NF-κB, we stimulated PAI-1 with TNF-α in the presence of NF-κB-specific inhibitor Bay 11-7082. As shown in Figure 2, Bay 11-7082 completely blocked the induction of PAI-1 and IL-6 (Fig. 2A). The latter is known to be regulated by NF-κB [31], a family of transcription factors that play an essential role in innate and adaptive immune responses [29]. The NF-κB transcription factors are homo- or heterodimers formed by five proteins including NF-κB1 (p105/p50), NF-κB2 (p100/p52), RelA (p65), RelB and c-Rel. NF-κB dimers bind to specific DNA sequence (cis-κB element) in gene promoters to regulate gene transcription [29, 30]. When MEFs were transfected with p65-specific siRNA to silence p65 (p65 mRNA was undetectable), LPS failed to induce PAI-1, in contrast to MEFs transfected with a scramble control siRNA (Fig. 2B). These results demonstrate that NF-κB is likely to mediate the induction of PAI-1 by TNF-α or LPS in MEFs, and predict a cis-κB element in the pai-1 gene promoter.
Figure 2.
Vitamin D suppresses the induction of PAI-1 in a NF-κB-dependent manner. (A) VDR(+/−) MEFs were incubated with 20 ng/ml TNF-α for 0, 2 and 4 hours in the presence or absence of BAY11-7082, and PAI-1 and IL-6 mRNA levels were determined by RT-PCR. (B) VDR(+/−) MEFs were transfected with control siRNA or p65-specific siRNA. After 24 hours, the cells were incubated with ethanol (−) or 20 nM 1,25(OH)2D3 (+) overnight, followed by PBS (−) or LPS (+) induction for 8 hours. The mRNA levels of PAI-1 and p65 were assessed by RT-PCR. (C) Schematic illustration of the putative κB site at −299 in mouse pai-1 gene promoter. Also illustrated are the positions of the PCR primers used for the ChIP assay. (D) ChIP assays demonstrate vitamin D disrupts NF-κB interaction with PAI-1 gene promoter. VDR(+/−) MEFs were pre-incubated with ethanol (−) or 1,25(OH)2D3 (+) overnight, followed by 4 hours of PBS (−) or TNF-α (+) induction. ChIP assays were performed using anti-p65 and anti-p50 antibodies as indicated. IgG, control IgG; Inp, DNA input.
We carried out an in silico survey of the mouse pai-1 gene promoter and identified a putative κB element 5′GGGAATTCCA3′ at −299 (Fig. 2C), which shares a high degree of homology to the conserved κB site (GGGAMTTYCC). We have previously shown that 1,25(OH)2D3 is able to suppress NF-κB activity in MEFs and other cell types [24, 26, 32]. Therefore, we addressed whether 1,25(OH)2D3 suppresses PAI-1 induction by blocking NF-κB interaction with the pai-1 gene promoter. ChIP assays showed that TNF-α markedly induced p65 or p50 binding to the −299 κB site in pai-1 gene promoter in MEFs; however, in the presence of 20 nM 1,25(OH)2D3, p65 or p50 binding was markedly attenuated to the baseline levels (Fig. 2D). These results confirmed the repressive effect 1,25(OH)2D3 on NF-κB binding to the pai-1 gene promoter in MEFs.
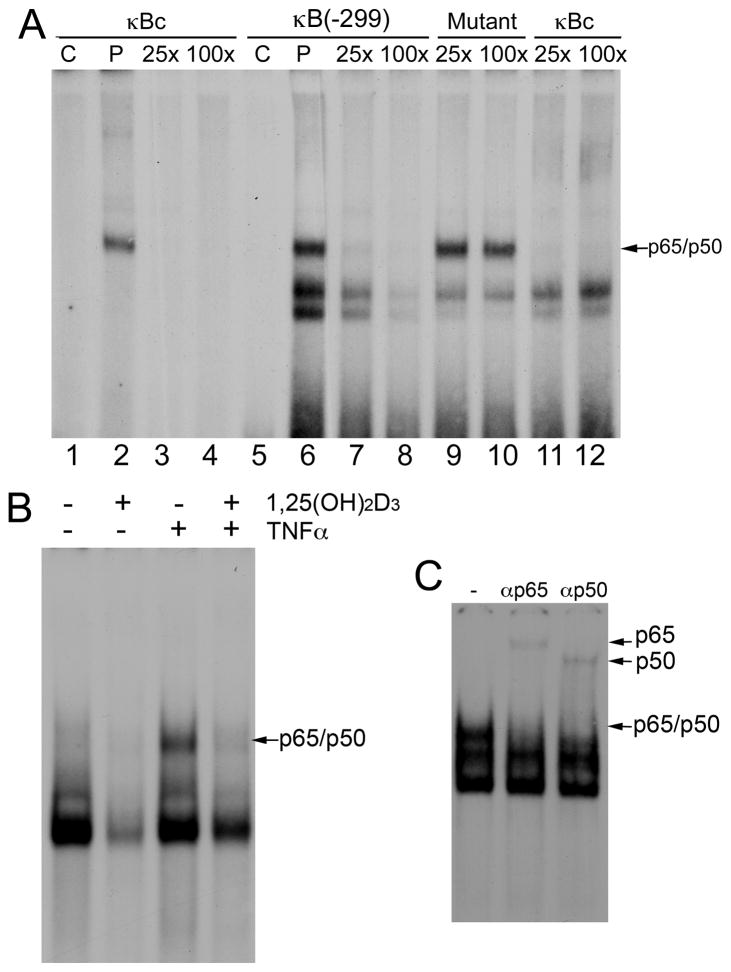
We further performed EMSAs to confirm the binding of NF-κB to the −299 κB site of the pai-1 promoter, using a 32P-labelled double-stranded probe corresponding to this κB site and its flanking sequence. As shown in Figure 3, like the canonical κB probe (5′GGGGCATTTCCC3′, designated as κBc, Fig. 3A, lanes 1–4), the −299κB probe formed a complex with nuclear proteins in MEF nuclear extracts (Fig. 3A, lane 6), and this complex was competed off by an excess amount of unlabelled −299 κB probe (Fig. 3A, lanes 7 and 8) or unlabelled canonical κBc probe (Fig. 3A, lanes 11 and 12), but not by a mutant −299 κB probe bearing mutations at the core κB site (Fig. 3A, lanes 9 and 10). The formation of this DNA-protein complex was induced by TNF-α, but the induction was inhibited in the presence of 1,25(OH)2D3 (Fig. 3B). Furthermore, the DNA-protein complex was recognized by antibodies against p65 or p50, leading to formation of supershifted bands (Fig. 3C). This confirmed the binding of p65/p50 NF-κB heterodimers to this cis-DNA site. Together, these data demonstrate that the −299 κB site in the pai-1 gene promoter interacts with p65 and p50, and the interaction can be disrupted by 1,25(OH)2D3. Therefore 1,25(OH)2D3 blunts PAI-1 induction by blocking the NF-κB binding activity.
Figure 3.
EMSAs confirm the binding of NF-κB to the −299 κB site. (A) VDR(+/−) MEFs were induced by TNF-α for 8 hours before nuclear extracts were prepared. Nuclear extracts were incubated with canonical κB probe (κBc), or −299 κB probe, and competed with 25-fold (25x) or 100-fold (100x) cold probe of its own or with cold mutant −299 κB probe or κBc probe. C, no nuclear extract control, P, probe. (B) VDR(+/−) MEFs were pre-incubated with ethanol (−) or 1,25(OH)2D3 (+) overnight, followed by 8 hours of PBS (−) or TNF-α (+) treatment. Nuclear extracts were incubated with −299κB probe. (C) TNF-α-treated MEF nuclear extracts were incubated with −299κB probe in the presence of anti-p65 (αP65) or anti-p50 (αP50) antibodies.
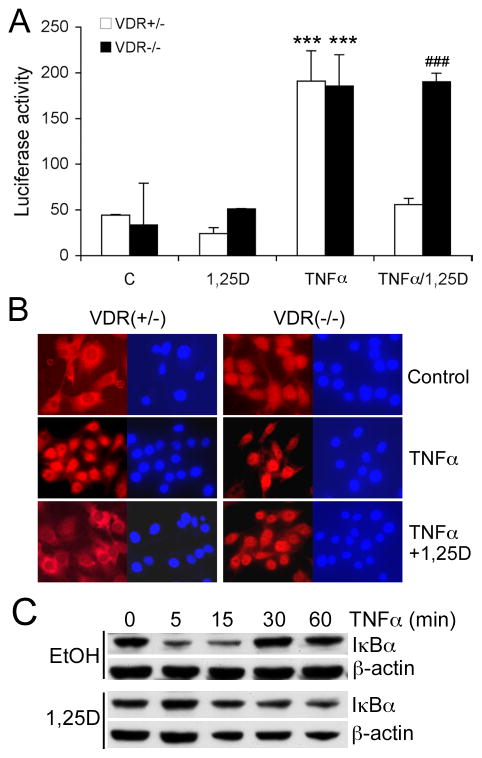
We then used luciferase reporter assays to determine the effects of vitamin D on NF-κB activity. When VDR(+/−) MEFs were transfected with standard pGL-κB-Luc reporter that contains a canonical κB element, luciferase activity was induced by TNF-α, and the induction was markedly suppressed by 1,25(OH)2D3; however, as expected, 1,25(OH)2D3 suppression was not seen in VDR(−/−) MEFs (Fig. 4A). TNFα stimulation quickly promoted p65 nuclear translocation in both VDR(+/−) and VDR(−/−) MEFs, and the nuclear translocation was blocked by 1,25(OH)2D3 in VDR(+/−) cells, but not in VDR(−/−) cells (Fig. 4B). IκBα is known to complex with p50/p65 in the cytoplasm, and IκBα degradation is required for p50/p65 nuclear translocation [29]. As expected, TNFα promoted IκBα degradation and quickly reduced the protein levels of IκBα in MEFs; however, the TNFα-induced IκBα degradation was markedly diminished with 1,25(OH)2D3 pre-treatment (Fig. 4C). These data suggest that 1,25(OH)2D3 inhibits NF-κB activity by blocking IκBα degradation and subsequently arresting p65/p50 nuclear translocation.
Figure 4.
Vitamin D suppresses NF-κB activity by blocking its nuclear translocation. (A) Luciferase reporter assays. VDR(+/−) and VDR(−/−) MEFs were transfected with pNF-κB-Luc plasmid, followed by treatment with control vehicle, 1,25(OH)2D3, TNFα or TNFα+1,25(OH)2D3 before the luciferase activity assay; *** P<0.001 vs. C; ### P<0.001 vs. VDR(+/−). (B) VDR(+/−) and VDR(−/−) MEFs were treated with TNFα in the presence or absence of 1,25(OH)2D3 pre-treatment. The cells were stained with anti-p65 antibody (red) and the nuclei were visualized with DAPI staining (blue). Note the cytoplasmic and nuclear distribution of p65 in untreated VDR(−/−) MEFs, and the blockade of p65 nuclear translocation in VDR(+/−) cells, but not in VDR(−/−) cells. (C) Stabilization of IκBα by 1,25(OH)2D3. Ethanol- or 1,25(OH)2D3-pre-treated VDR(+/−) MEFs were stimulated with 20 ng/ml TNFα for 0, 5, 15, 30 and 60 minutes, and IκBα levels were determined by Western blotting.
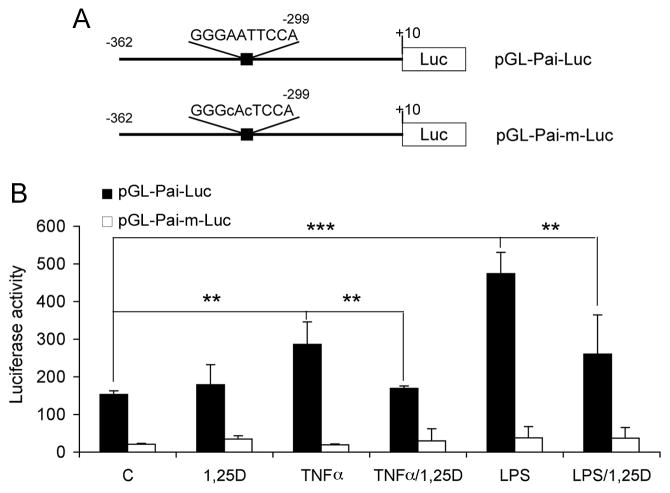
To measure PAI-1 gene promoter activity, we cloned a 5′ flanking fragment of the mouse pai-1 gene spanning −362 to +10 into pGL3 vector. This promoter region contained the −299 κB element (Fig. 5A), and a construct bearing mutations in the κB core sequence was also generated (Fig. 5A). When MEFs were transfected with pGL-Pai-Luc, luciferase activity was induced by TNF-α or LPS, and 1,25(OH)2D3 treatment significantly reduced the induction of the promoter activity by 40–50% (Fig. 5B). In contrast, in MEFs transfected with the mutant construct pGL-Pai-m-Luc, neither TNFα nor LPS was able to increase the promoter activity, and the activity was no longer affected by 1,25(OH)2D3 treatment (Fig. 5B). These results demonstrate that the −299κB element in pai-1 gene promoter is indeed functional, and it mediates the inhibition of PAI-1 induction by 1,25(OH)2D3.
Figure 5.
Vitamin D suppression of pai-1 gene promoter activity requires NF-κB binding site. (A) Schematic illustration of wild-type and mutant mouse pai-1 gene promoter luciferase reporter constructs; (B) Luciferase reporter assays. VDR(+/−) MEFs were transfected with the wild-type or mutant luciferase reporter, followed by treatment with TNFα or LPS in the presence or absence of 1,25(OH)2D3 as indicated. ** P<0.01; *** P<0.001.
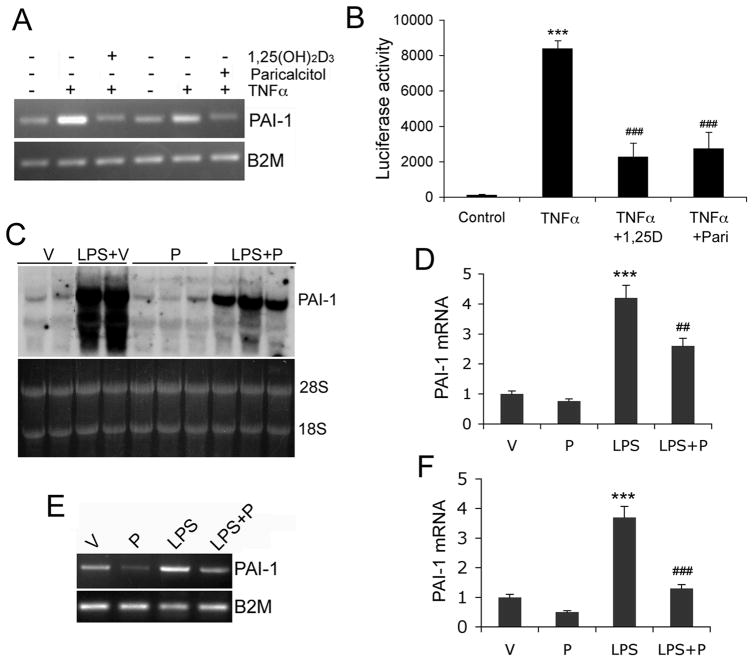
Finally we investigated whether paricalcitol (an activated vitamin D analog) suppresses LPS-induced PAI-1 expression in mice. We used paricalcitol because it does not have strong hypercalcemic effects in vivo as opposed to 1,25(OH)2D3. To confirm that paricalcitol is as active as 1,25(OH)2D3 in suppressing PAI-1 induction, we treated VDR(+/−) MEFs with TNFα in the presence of 1,25(OH)2D3 or paricalcitol. We also performed luciferase reporter assays using the pGL-Pai-Luc reporter. As shown in Figure 6A and B, paricalcitol suppressed TNFα-induced PAI-1 mRNA expression (Fig. 6A) and pai-1 promoter activity (Fig. 6B) as effectively as 1,25(OH)2D3 at the same dose (20 nM) in VDR(+/−) MEFs. In the in vivo experiment, mice were pre-treated with vehicle or paricalcitol for one week before one dose of intraperitoneal LPS administration. As shown in Figure 6C-F, 16 hours after LPS injection PAI-1 mRNA was markedly induced in the heart (Fig. 6C and D) and in peritoneal macrophages (Fig. 6E and F) in mice pre-treated with vehicle, but paricalcitol pre-treatment blunted the induction of PAI-1 in these tissues (Fig. 6C-F). Thus we conclude that vitamin D analogs are able to inhibit LPS-induced PAI-1 in vivo.
Figure 6.
Vitamin D analog suppresses LPS-induced PAI-1 expression in mice. (A-B) In vitro confirmation of paricalcitol activity. (A) VDR(+/−) MEFs were pre-treated with 1,25(OH)2D3 (20 nM) or paricalcitol (20 nM) before being stimulated with TNFα as indicated. (B) VDR(+/−) MEFs were transfected with pGL-Pai-Luc reporter followed by TNFα stimulation in the presence of 1,25(OH)2D3 (1,25D) or paricalcitol (Pari) as indicated. Note both 1,25(OH)2D3 and paricalcitol were able to block TNFα-induced PAI-1 mRNA expression or pai-1 promoter activity. ***, P<0.001 vs. control; ### P<0.001 vs. TNFα. (C-F) C57B/L6 mice were pretreated with paricalcitol (300 ng/kg) for one week before challenged by peritoneal injection of LPS (15 mg/kg). After 24 hours, the heart and peritoneal macrophages were harvested for RNA extraction. (C and D) PAI-1 expression in the heart. Northern blot (C) and PhosphoImaging quantification (D) show that LPS-induced PAI-1 mRNA in the heart was suppressed by paricalcitol (P) treatment. Each lane represents one mouse. (E and F) PAI-1 expression in macrophages. RT-PCR (E) and quantification (F) show that LPS-induced PAI-1 mRNA in peritoneal macrophages was suppressed by P treatment. V, vehicle-treated control. ***, P<0.001 vs. Vehicle; ## P<0.01, ### P<0.001 vs. LPS.
Discussion
In this study, we identified a functional NF-κB binding site within the mouse pai-1 gene promoter and demonstrated that 1,25(OH)2D3 suppressed the up-regulation of PAI-1 induced by pro-inflammatory factors (TNFα and LPS) by blocking NF-κB activation. Given the involvement of PAI-1 in the development of a variety of renal and cardiovascular disorders [1, 2], it is not unreasonable to speculate that the suppression of PAI-1 contributes to the reno-protective and cardio-protective effects of vitamin D.
As a major transcription factor in response to inflammatory stimulation, NF-κB mediates the effects of TNFα and LPS. PAI-1 is highly stimulated by TNFα and LPS [6], thus NF-κB is thought to be a crucial transcriptional regulator for PAI-1. A NF-κB element has been identified in the distal promoter region (−15 kb) of the human PAI-1 gene [4]; however, whether this cis DNA element is functional was not demonstrated. On other hand, direct binding of Nur77/NAK-1 to the proximal human PAI-1 promoter was shown to mediate the TNFα-induced PAI-1 expression [33]. The mechanism involved in TNFα-stimulation of mouse PAI-1, however, remains unknown. Here we identified a functional NF-κB element in the proximal promoter of mouse pai-1 gene that mediates the stimulatory effect of pro-inflammatory factors. We demonstrated by EMSA, ChIP and functional luciferase reporter assays that this cis-κB element interacts with the p65/p50 heterodimer upon TNFα treatment and is required for the stimulatory effect of TNFα on PAI-1. To our knowledge this is the first demonstration for the involvement of NF-κB in the regulation of mouse PAI-1 production.
High PAI-1 levels are associated with an increased risk of renal and vascular complications such as thrombosis, myocardial infarction [1] and atherosclerosis [14, 34]. Thus, PAI-1 is a potential therapeutic target for inflammation-induced renal and cardiovascular diseases [1]. In this regard, the finding that vitamin D and its analogs inhibit PAI-1 is significant. In this report we demonstrated that the inhibitory effect of vitamin D on PAI-1 involved the blockade of NF-κB. 1,25(OH)2D3 treatment disrupted the interaction of p65/p50 with the cis-κB element in the pai-1 gene promoter, and this appears to result from stabilization of IκBα (blockade of degradation) and thus arrest of p65/p50 nuclear translocation. We also showed that VDR is required to mediate this inhibitory effect, as 1,25(OH)2D3 cannot suppress PAI-1 expression nor NF-κB activity in VDR(−/−) MEFs. It is noticeable that the induction of PAI-1 by TNFα was more robust in VDR(−/−) cells (Figure 1), consistent with a repressive role of VDR in NF-κB regulation. We further demonstrated that an activated, low-calcemic vitamin D analog, paricalcitol, was able to inhibit the PAI-1 induction in vitro and in LPS-stimulated mice. Together these data from the in vitro and in vivo models provide good evidence that vitamin D is a physiological inhibitor of PAI-1 production and its low calcemic analogs can potentially be used to inhibit PAI-1 production for therapeutic purposes.
Targeting of NF-κB appears to be a general mechanism for vitamin D to regulate a variety of important genes. We have previously shown that 1,25(OH)2D3 uses this mechanism to regulate MCP-1 and angiotensinogen in kidney cells [26, 32]. In dendritic cells, 1,25(OH)2D3 inhibits IL-12 expression through targeting the NF-κB pathway [35]. The exact molecular basis underlying vitamin D repression of NF-κB remains poorly defined and controversial. Some studies reported a physical interaction between liganded VDR and p65 [36], suggesting that sequestration of p65 by the VDR prevents p65 nuclear translocation or its binding to DNA [37]. Other studies, including the present one, demonstrated stabilization of IκBα protein by 1,25(OH)2D3, leading to arrest of p65 nuclear translocation and thus attenuation of NF-κB activity [26, 38, 39]. 1,25(OH)2D3 can also directly suppress RelB transcription [40], as well as suppress the increase in p50 and its precursor, p105, and c-rel proteins [41]. Given the paramount importance of NF-κB in inflammation and disease development, more studies are warranted to fully elucidate the molecular mechanism whereby vitamin D regulates NF-κB.
Many recent studies have demonstrated impressive therapeutic benefits of vitamin D analogs in renal and cardiovascular diseases. For example, therapies with vitamin D analogs can prevent the development of left ventricular hypertrophy in Dahl salt-sensitive rats, spontaneously hypertensive rats and in hemodialysis patients [42, 43]. Vitamin D analogs have also been shown to reduce proteinuria and prevent renal injury in animal models of kidney disease such as renal failure [44, 45], renal fibrosis [37, 46] and diabetic nephropathy [47, 48], and in patients with chronic kidney disease [49]. Studies have also suggested beneficial impact of vitamin D on the development of atherosclerosis in humans [50]. The effect of vitamin D on PAI-1 and whether PAI-1 inhibition contributes to the therapeutic outcome were not examined in these studies. Because of the important pathological roles of PAI-1 in the renal and cardiovascular systems, the data obtained from the present study now provide a good basis to investigate the impact of vitamin D inhibition of PAI-1 by translational and clinical investigations.
Acknowledgments
This work was supported in part by NIH grant HL085793, a research grant from the Center for D-Receptor Activation Research (to YCL), and a research grant from Abbott Laboratory (to RT). JK is support by American Heart Association Scientist Development Grant (10SDG4280066).
Abbreviations
- VDR
vitamin D receptor
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- NF-κB
nuclear factor-κB
- EMSA
electrophoretic mobility shift assay
- ChIP
chromatin immunoprecipitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ha H, Oh EY, Lee HB. Nat Rev Nephrol. 2009;5:203–211. doi: 10.1038/nrneph.2009.15. [DOI] [PubMed] [Google Scholar]
- 2.Gramling MW, Church FC. Thrombosis research. 2010;125:377–381. doi: 10.1016/j.thromres.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westerhausen DR, Jr, Hopkins WE, Billadello JJ. J Biol Chem. 1991;266:1092–1100. [PubMed] [Google Scholar]
- 4.Hou B, Eren M, Painter CA, Covington JW, Dixon JD, Schoenhard JA, Vaughan DE. J Biol Chem. 2004;279:18127–18136. doi: 10.1074/jbc.M310438200. [DOI] [PubMed] [Google Scholar]
- 5.Kasza A, Kiss DL, Gopalan S, Xu W, Rydel RE, Koj A, Kordula T. Journal of neurochemistry. 2002;83:696–703. doi: 10.1046/j.1471-4159.2002.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawdey MS, Loskutoff DJ. J Clin Invest. 1991;88:1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emeis JJ, Kooistra T. J Exp Med. 1986;163:1260–1266. doi: 10.1084/jem.163.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hinsbergh VW, Kooistra T, van den Berg EA, Princen HM, Fiers W, Emeis JJ. Blood. 1988;72:1467–1473. [PubMed] [Google Scholar]
- 9.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimburger O, Cederholm T, Girndt M. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. J Am Soc Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 11.Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, Yoshinaga K. Am J Kidney Dis. 1993;21:480–485. doi: 10.1016/s0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Nephrology (Carlton) 2006;11:226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 13.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 14.Glass CK, Witztum JL. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 15.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. J Bone and Mineral Research. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 17.Levin A, Li YC. Kidney Int. 2005;68:1973–1981. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, Thadhani R. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 20.Kovesdy CP, Kalantar-Zadeh K. Kidney Int. 2008;73:1355–1363. doi: 10.1038/ki.2008.35. [DOI] [PubMed] [Google Scholar]
- 21.Fukumoto S, Allan EH, Martin TJ. Biochim Biophys Acta. 1994;1201:223–228. doi: 10.1016/0304-4165(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 22.Wu-Wong JR, Nakane M, Ma J. Thrombosis research. 2006;118:709–714. doi: 10.1016/j.thromres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Wu-Wong JR, Nakane M, Ma J. Journal of vascular research. 2007;44:11–18. doi: 10.1159/000097812. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC. Am J Physiol Endocrinol Metab. 2006;291:E315–322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 25.Li YC, Bolt MJG, Cao LP, Sitrin MD. Am J Physiol Endocrinol Metab. 2001;281:E558–E564. doi: 10.1152/ajpendo.2001.281.3.E558. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 27.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. J Biol Chem. 2007;282:29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 29.Bonizzi G, Karin M. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Vallabhapurapu S, Karin M. Annual review of immunology. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 31.Libermann TA, Baltimore D. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. Am J Physiol Renal Physiol. 2009;296:F1212–1218. doi: 10.1152/ajprenal.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruber F, Hufnagl P, Hofer-Warbinek R, Schmid JA, Breuss JM, Huber-Beckmann R, Lucerna M, Papac N, Harant H, Lindley I, de Martin R, Binder BR. Blood. 2003;101:3042–3048. doi: 10.1182/blood-2002-07-2331. [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Ridker PM, Maseri A. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 35.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X, Farmer P, Rubin J, Nanes MS. J Cell Biochem. 2004;92:833–848. doi: 10.1002/jcb.20143. [DOI] [PubMed] [Google Scholar]
- 37.Tan X, Wen X, Liu Y. J Am Soc Nephrol. 2008;19:1741–1752. doi: 10.1681/ASN.2007060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riis JL, Johansen C, Gesser B, Moller K, Larsen CG, Kragballe K, Iversen L. Arch Dermatol Res. 2004;296:195–202. doi: 10.1007/s00403-004-0509-9. [DOI] [PubMed] [Google Scholar]
- 39.Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. J Immunol. 2004;173:2280–2287. doi: 10.4049/jimmunol.173.4.2280. [DOI] [PubMed] [Google Scholar]
- 40.Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, McKean DJ, Kumar R, Griffin MD. J Biol Chem. 2003;278:49378–49385. doi: 10.1074/jbc.M308448200. [DOI] [PubMed] [Google Scholar]
- 41.Yu XP, Bellido T, Manolagas SC. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Proc Natl Acad Sci U S A. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong J, Kim GH, Wei M, Sun T, Li G, Liu SQ, Li X, Bhan I, Zhao Q, Thadhani R, Li YC. Am J Pathol. 2010;177:622–631. doi: 10.2353/ajpath.2010.091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. J Am Soc Nephrol. 2007;18:1796–1806. doi: 10.1681/ASN.2006091028. [DOI] [PubMed] [Google Scholar]
- 45.Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B. Kidney Int. 2008;74:1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- 46.Tan X, Li Y, Liu Y. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, DDK, Kong J, Ning G, Wong Y, Li G, Chen Y, Zhang Z, Strugnell S, Sabbagh Y, Arbeeny C, Li YC. American Journal of Physiology Renal Physiology. 2009;297:F791–F801. doi: 10.1152/ajprenal.00247.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Proc Natl Acad Sci U S A. 2008;105:15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 50.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]