Abstract
We used the C57BL/6J (B6) and PWD/PhJ (PWD) mouse strains to investigate the controls of calcium intake. Relative to the B6 strain, the PWD strain had higher preferences in two-bottle choice tests for CaCl2, calcium lactate (CaLa), MgCl2, citric acid and quinine hydrochloride, but not for sucrose, KCl or NaCl. We also measured taste-evoked chorda tympani (CT) nerve activity in response to oral application of these compounds. Electrophysiological results paralleled the preference test results, with larger responses in PWD than in B6 mice for those compounds that were more highly preferred for the former strain. The strain differences were especially large for tonic, rather than phasic, chorda tympani activity. These data establish the PWD strain as a “calcium-preferring” strain and suggest that differences between B6 and PWD mice in taste transduction or a related peripheral event contributes to the differences between the strains in preferences for calcium solutions.
Keywords: mouse, gustatory, electrophysiology, calcium, mineral
1. INTRODUCTION
Calcium is an essential nutrient required by the body for diverse physiological activities, and it is therefore not surprising that animals consume calcium when they need it [32]. The sense of taste plays an important role in this behavior. For example, calcium-deprived rats have elevated intakes of CaCl2, even if there is minimal absorption of the solution [7,20,23]. Calcium deprivation also changes taste-evoked responses and measures of taste reactivity, so the taste of calcium appears to change based on the need for the mineral [14,21,23].
In human subjects, the acceptance of green vegetables is inversely related to their calcium content [35], presumably due to calcium ions contributing a bitter taste to the foods. However, calcium, does not always taste purely bitter or unpleasant. Some mouse strains avidly consume solutions or vegetables with a high calcium content [2,36,37], and people with disturbances of calcium metabolism prefer foods high in calcium [18,33]. Human subjects attribute bitter, salty, sour, and sweet tastes to CaCl2 and calcium lactate when asked to make ratings based on these primary taste qualities [17,31], but there is also evidence that calcium-containing compounds have a unique taste quality that is distinct from bitterness, saltiness, sourness, sweetness, or umami taste [28]. This psychophysical evidence implies that there may be a specific gustatory transduction mechanism for calcium that is distinct from those for other taste qualities. This view is also supported by work using inbred mouse strains and their segregating generations, which show low correlations between preferences for CaCl2 and for representatives of primary taste qualities [2,34,36,37] and by the presence of a population of chorda tympani nerve fibers in mice that respond more vigorously to calcium and magnesium than to exemplars of bitter, salty, sour, or sweet taste qualities [24].
One possible taste transduction mechanism for calcium involves the calcium-sensing receptor (CaSR). CaSR is functional in amphibian taste [26] and is expressed in rodent taste bud cells [9,37]. Binding of Ca2+ ions to CaSR in taste bud cells could be responsible for the aspects of calcium’s taste that are shared with magnesium but not other minerals, because the receptor is selective for Ca2+ and Mg2+ ions [4]. The gene, Tas1r3, which codes for the protein T1R3, is also hypothesized to be important for calcium taste. Recent work suggests that the pronounced preference for calcium by the PWK/PhJ (PWK) strain is due to polymorphisms of Tas1r3, and mice that lack Tas1r3 show less avoidance of CaCl2 than do B6 mice with an intact gene, possibly because binding of Ca2+ to T1R3 imparts a negative taste [38]. There is also a possibility that CaSR and T1R3 dimerize to form a gustatory receptor for calcium, given that T1R3 is known to dimerize with the T1R1 and T1R2 proteins to form receptors for umami and sweet taste, respectively [39].
Nonetheless, there remain unanswered questions related to calcium taste transduction and appetite. For example, although T1R3 may be involved in the unpleasant aspect of calcium’s taste, Tas1r3-knockouts prefer some concentrations of CaCl2, implying there is an alternate mechanism involved in the positive taste of calcium [38]. In addition, QTL mapping using the B6 and PWK strains has identified several additional markers involved in preferences for calcium [37]. Moreover, the involvement of taste sensation in guiding calcium intake does not rule out additional contributions from non-gustatory mechanisms, such as postingestive feedback.
In previous work, we have demonstrated that the PWK/PhJ strain has a strong preference for calcium-containing solutions [2,37]. In the current work, we use PWD/PhJ (PWD) mice which, like the PWK/PhJ strain, are of Mus m. musculus origin as opposed to more commonly used laboratory mouse strains derived from Mus m. domesticus origin [11]. However, the PWD mice offer an advantage over PWK/PhJ mice in having available consomic strains on a B6 background [12], which will facilitate future genetic analyses. Here, we characterize preferences for taste solutions in PWD mice for the first time. We also measure taste-evoked responses in the chorda tympani (CT) nerve in these strains, in order to examine the contribution of peripheral gustatory mechanisms in isolation.
2. METHODS
2.1 Experiment 1: Behavioral testing
2.1.1 Subjects
Two-bottle tests were performed in 18 C57BL/6J (B6) and 17 PWD/PhJ (PWD) mice (8 males for each strain and the rest females). All animals were purchased from The Jackson Laboratory (Bar Harbor, ME). Procedures were approved by the Animal Care and Use Committee of the Monell Chemical Senses Center. Mice were kept at 23–26°C with a 12:12 h light:dark cycle (lights off at 7 PM) and were maintained on AIN-76A diet (Dyets, Bethlehem, PA, cat. no. 100000) and tap water ad libitum.
2.1.2 Preference tests
Details of drinking tube construction, cage layout, and the position of drinking tubes during tests are described elsewhere (http://www.monell.org/MMTPP/). The mice were given 48-h access to two drinking tubes, one of which contained water and the other a taste solution. The positions of the tubes were switched at 24 h. Preference scores were defined as the total solution consumed in 48 h divided by the total fluid (solution plus water) consumed. Although our main interest in this experiment was calcium-containing stimuli, we used a broader array of compounds in order to examine the specificity of any observed strain differences, and we chose concentrations that would be unlikely to cause extremes of avoidance or preference, but that would be likely to reveal differences between mouse strains. Solutions used were 50 mM CaCl2, 50 mM calcium lactate (CaLa), 50 mM MgCl2, 50 mM KCl, 100 mM NH4Cl, 100 mM NaCl, 5 mM citric acid, 30 μM quinine hydrochloride (QHCl), and 8% sucrose. A day with access to a single bottle of water was interposed between each 48-h test.
2.1.3 Analysis
A two-way mixed ANOVA was performed to assess whether the strains differed in general in their taste preferences (effect of strain) and whether they differed on preferences for certain solutions (strain × concentration interaction). Preliminary analyses also included Sex as a factor, but there were no differences between males and females so the results presented here are for both sexes combined. Post-hoc t-tests were used to assess strain differences in preferences for individual compounds. For all tests p < 0.05 was considered to be significant.
Within each strain, preference scores for each compound were also assessed relative to 50% (i.e., indifference relative to water) by calculating the 95% confidence interval around the mean. Overlap between the confidence interval and 50% indicated indifference, whereas intervals that were higher or lower than 50% indicated significant preference or avoidance of the compounds, respectively.
2.2 Experiment 2: Electrophysiology
2.2.1 Subjects
Measurements of CT activity were made in 6 male B6 and 6 male PWD mice. All animals were purchased from The Jackson Laboratory (Bar Harbor, ME). Procedures were approved by the Animal Care and Use Committee of Ball State University. Mice were kept at 23–26°C with a 12:12 h light:dark cycle (lights off at 7 PM) and were maintained on AIN-76A diet (Dyets, Bethlehem, PA, cat. no. 100000) and tap water ad libitum. Mice did not receive stimulus solutions prior to electrophysiology to ensure that they had similar gustatory backgrounds.
2.2.2 Surgery
Each animal was anesthetized with a mixture of ketamine, xylazine and acepromazine (90, 20, and 3 mg/kg, respectively; i.p., with further doses as necessary). A tracheotomy was performed to prevent suffocation, and the animal was placed supine with the head secured in a non-traumatic head holder. In all animals, the CT nerve was accessed through the right ear by puncturing the tympanic membrane and exposing the right CT nerve adjacent to the malleus [6]. An electrode made of platinum/iridium wire was placed on the nerve, and the multiunit signal was amplified, filtered, rectified and integrated with a time constant of 1.0 s. A few drops of mineral oil were placed in the wound site at the vicinity of contact of the nerve with the electrode to prevent desiccation of the nerve. An indifferent electrode was positioned in nearby muscle tissue.
In previous experiments we used a different surgical approach to access the CT ventrally through the neck. We were interested in whether type of surgery might affect the size of the taste-evoked responses, and so we compared the results for B6 mice in the current experiment with those of B6 mice from a prior study [38]. Identical taste compounds and stimulation procedures were used in the two experiments. We performed a two-way ANOVA and did not observe a significant difference in the size of responses (effect of surgical method and method × chemical interaction, n.s.).
2.2.3 Stimuli and delivery
The following taste stimuli (mixed in distilled water) were applied on the tongue with deionized water as background rinse: CaCl2 at 0.1, 1, 10, 100 mM; CaLa, MgCl2, NaCl and KCl, all at 10 and 100 mM; 500 mM sucrose; 10 mM citric acid; 20 mM QHCl. Concentrations were not matched exactly with those in experiment 1, but rather were chosen to be more comprehensive and so that they would include at least one for each compound that would evoke a robust multiunit CT response. The order of application of compounds was random, except that different concentrations of a given compound were applied in ascending order. In addition, 100 mM NH4Cl mixed in distilled water was applied at regular intervals throughout the entire process to serve as a reference stimulus. The anterior tongue was placed in a flow chamber, and deionized water rinse and stimulus solutions were applied by continuous flow with a rate of 0.5 ml/s. The rinse and all stimulus solutions were presented at room temperature. Each stimulus presentation lasted for 20 s and was followed by at least 60 s of rinse.
When possible, stimuli were reapplied for a given CT preparation, and relative response sizes were averaged across all applications. Across the entire experiment, the Pearson product moment correlation between the responses sizes of the first and second applications was +0.92, indicating that there was a high degree of stability in our preparations.
2.2.4 Analysis
Three response types were calculated for each stimulus application: 10-s net, peak, and tonic. Ten-s net values were based on the area-under-the-curve of the integrated voltage for 10 s after stimulus onset (evoked) minus the area for 10 s before onset (baseline). Peak values were calculated by subtracting the mean voltage level for the 10-s baseline period from the maximum voltage obtained within 3 s of stimulus onset. Tonic responses were calculated by subtracting the mean voltage level for the 10-s baseline period from the voltage level at 10 s after stimulus onset. In all cases, relative response sizes were calculated for each stimulus application and each response type based on the size of the corresponding NH4Cl reference responses. Differences between the B6 and PWD mice were assessed using t-tests for compounds presented at only one concentration. For compounds presented at more than one concentration, two-way, mixed ANOVAs were used, and if there was a significant effect of strain or a strain × concentration interaction, then post hoc t-tests were conducted to examine differences for individual concentrations. For all tests p < 0.05 was considered to be significant.
3. RESULTS
3.1 Experiment 1
Preferences were significantly higher in PWD mice than in B6 mice for CaCl2, CaLa, MgCl2, citric acid and QHCl (Fig. 1; effect of strain, F[1,32] = 54.6, p < 0.001; strain × chemical interaction, F[8,256] = 27.3, p < 0.001; t [33] ≥ 2.9, p ≤ 0.007 in all cases). However, the two strains did not differ in preference scores for KCl, NH4Cl, NaCl, or sucrose. The PWD strain significantly preferred CaCl2 and CaLa relative to water, but the B6 mice showed significant avoidance of these compounds relative to water. B6 mice also significantly avoided MgCl2 and QHCl, whereas the scores for PWD animals indicated indifference. The two strains were similar, though, in showing indifference to NH4Cl, significant avoidance of citric acid, and significant preferences for NaCl and sucrose. Although preferences scores for KCl were a little higher than 50% in both strains, a significant preference occurred only for B6 mice.
Figure 1.

Mean (± SEM) preference scores in 48-h 2-bottle tests with water of PWD (closed bars) and B6 mice (open bars) in Experiment 1. CaCl, 50 mM calcium chloride; CaLa, 50 mM calcium lactate; Mg, 50 mM MgCl2; Ci, 5 mM citric acid; Q, 30 μM QHCl; KCl, 50 mM KCl; NH4, 100 mM NH4Cl; Na, 100 mM NaCl; Suc, 8% sucrose. *, p < 0.05 vs. B6.
3.2 Experiment 2
3.2.1 10-s net responses
Mean 10-s net CT responses to CaCl2 were generally higher in PWD than in B6 mice (Fig. 2; effect of strain, F[1,10] = 18.4, p = 0.002), and this difference was especially large for some concentrations (strain × concentration interaction, F[3,10] = 5.9, p = 0.003). Post-hoc tests revealed that responses to 10 and 100 mM CaCl2 were significantly larger in PWD than in B6 mice (t[10] ≥ 3.2, p ≤ 0.01 in both cases). For CaLa, responses in PWD mice were larger overall relative to B6 mice (Fig 3; effect of strain, F[1,10] = 5.0, p = 0.049), but post-hoc comparisons did not indicate a significant difference for individual concentrations. The PWD mice also had significantly larger responses to MgCl2 relative to those of B6 mice (Fig. 3; effect of strain, F[1,10] = 14.8, p = 0.003; strain × concentration interaction, F[1,10] = 7.2, p = 0.02), and post-hoc tests were significant for both 10 and 100 mM MgCl2 (t[10] ≥ 2.3, p < 0.05 in both cases). No strain differences in taste-evoked activity were observed for NaCl or KCl (Fig. 3). Among the chemicals representative of the basic taste qualities, QHCl and citric acid evoked significantly larger responses in PWD than in B6 mice (t[10] ≥ 3.5, p ≤ 0.002 in both cases), but responses to sucrose did not differ between the strains (Fig. 4).
Figure 2.
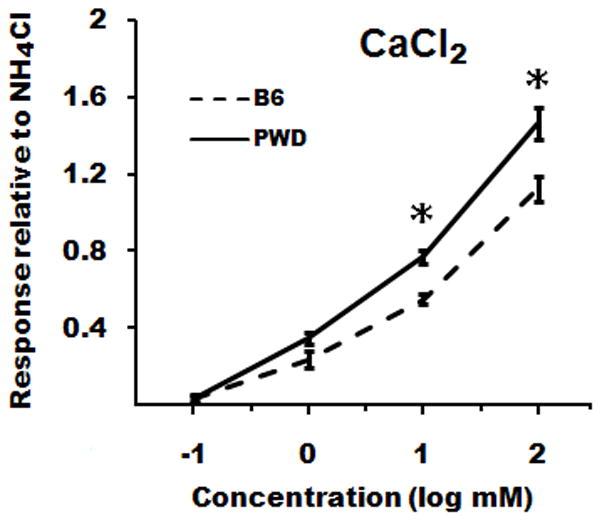
Mean (± SEM) whole-nerve chorda tympani responses to CaCl2 in PWD (solid line) and B6 (dashed line) mice in Experiment 2. * p < 0.05, PWD versus B6.
Figure 3.
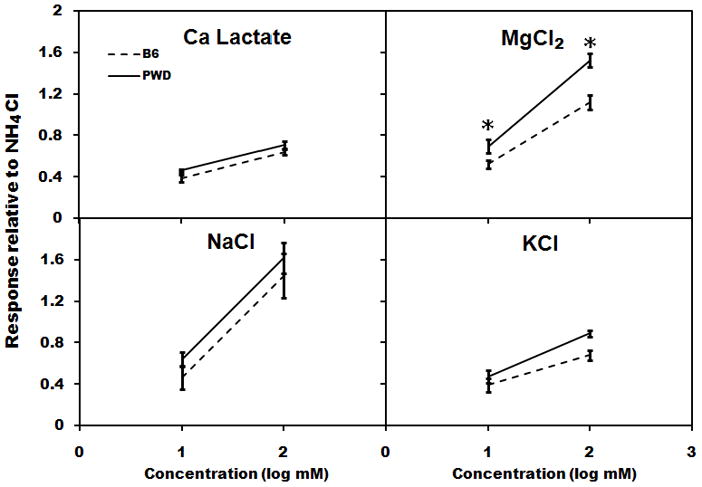
Mean (± SEM) whole-nerve chorda tympani responses in PWD (solid line) and B6 (dashed line) mice in Experiment 2 to CaLa, KCl, MgCl2, and NaCl. * p < 0.05, PWD versus B6.
Figure 4.
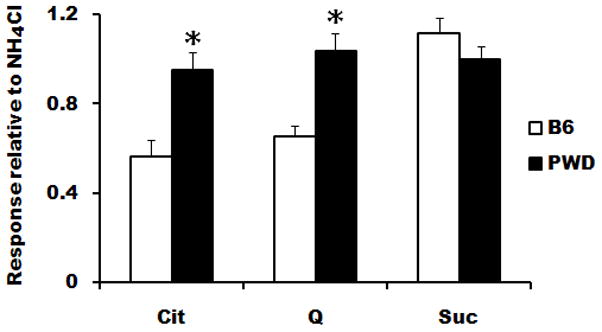
Mean (± SEM) whole-nerve chorda tympani responses to basic taste qualities in PWD (closed bars) and B6 (open bars) mice in Experiment 2. * p < 0.05, PWD versus B6. Cit, 10 mM citric acid; Q, 20 mM QHCl; Suc, 500 mM sucrose.
Responses to a wide range of taste stimuli were larger in PWD than in B6 mice. Naturally, this raises the issue of whether the strains differed in their responsiveness to the NH4Cl reference stimulus. In order to investigate this issue, we compared strains on their raw scores for NH4Cl. Such scores would be expected to vary based on several non-taste factors, such as thickness or dryness of the nerve, but such variation should be distributed evenly between the B6 and PWD strains. We found that the two mouse strains did not differ in their raw NH4Cl responses. When considered together with the finding in Experiment 1 that B6 and PWD mice prefer NH4Cl equally, it is likely that NH4Cl was an appropriate reference stimulus that gave equivalent responses in the two mouse strains, and the strain differences described earlier for relative response sizes can be considered to reflect variation in gustatory responsiveness for individual chemicals.
3.2.2 Time course of responses
The 10-s net values described above provide a summation of the overall level of multiunit responses, but they do not provide insight into their time courses and how such temporal patterns might vary between the strains. Examination of individual responses, though, indicated that tonic activity (i.e., the response portion occurring after the initial voltage peak) tended to be large in PWD mice, whereas responses in B6 mice declined more quickly after peaking (Fig. 5). In order to quantify this apparent strain difference, we calculated separate phasic and tonic responses; the former represented the maximum increase within 3 s of stimulus onset, and the latter was based on the response level at 10 s after onset (see Methods for more details). In general, the strain differences described above for 10-s net responses arose due to differences in the tonic, rather than phasic, portion of the response (Table 1). Mean 10-s tonic responses were significantly larger in PWD than in B6 mice for 10 and 100 mM CaCl2; 10 mM Ca lactate; 10 and 100 mM MgCl2; and citric acid (t[10] ≥ 2.4, p ≤ 0.04 in all cases). No differences were observed for QHCl, sucrose, NaCl and KCl.
Figure 5.
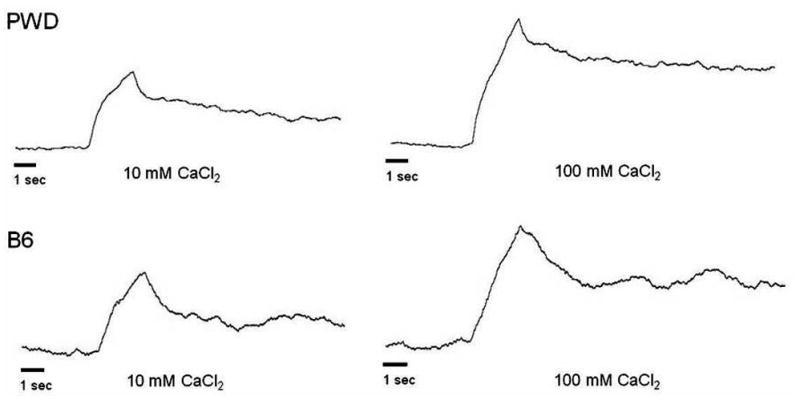
Sample recordings of integrated whole nerve chorda tympani responses to CaCl2 in a PWD (top) and a B6 (bottom) mouse in Experiment 2. The mice differed in the temporal patterns of their responses, with more sustained activity in the PWD mouse than in the B6.
Table 1.
Mean (± SEM) 3-sec phasic and 10-sec tonic chorda tympani responses of B6 and PWD mice. Values represent responses relative to 100 mM NH4Cl.
| STIMULUS | 3-Sec phasic | 10-Sec tonic | ||
|---|---|---|---|---|
| B6 | PWD | B6 | PWD | |
| 0.1 mM CaCl2 | 0.12 ± 0.02 | 0.07 ± 0.01 | 0.05 ± 0.04 | 0.02 ± 0.02 |
| 1 mM CaCl2 | 0.37 ± 0.06 | 0.46 ± 0.04 | 0.17 ± 0.03 | 0.25 ± 0.04 |
| 10 mM CaCl2 | 0.73 ± 0.05 | 0.88 ± 0.05 * | 0.46 ± 0.06 | 0.71 ± 0.04 * |
| 100 mM CaCl2 | 1.11 ± 0.09 | 1.38 ± 0.08 * | 1.18 ± 0.06 | 1.64 ± 0.14 * |
| 10 mM Ca lactate | 0.56 ± 0.04 | 0.64 ± 0.02 | 0.18 ± 0.04 | 0.36 ± 0.03 * |
| 100 mM Ca lactate | 0.79 ± 0.05 | 0.86 ± 0.03 | 0.56 ± 0.02 | 0.60 ± 0.05 |
| 10 mM MgCl2 | 0.65 ± 0.06 | 0.85 ± 0.09 | 0.34 ± 0.09 | 0.58 ± 0.05 * |
| 100 mM MgCl2 | 1.17 ± 0.07 | 1.39 ± 0.08 | 1.16 ± 0.13 | 1.68 ± 0.10 * |
| Citric acid | 0.59 ± 0.04 | 0.89 ± 0.08 * | 0.58 ± 0.06 | 0.93 ± 0.13 * |
| Quinine | 0.78 ± 0.12 | 1.00 ± 0.09 | 0.84 ± 0.16 | 1.05 ± 0.13 |
| Sucrose | 0.73 ± 0.11 | 0.72 ± 0.07 | 1.29 ± 0.27 | 1.31 ± 0.14 |
| 10 mM NaCl | 0.55 ± 0.08 | 0.77 ± 0.07 | 0.45 ± 0.19 | 0.61 ± 0.10 |
| 100 mM NaCl | 1.41 ± 0.15 | 1.60 ± 0.18 | 1.57 ± 0.20 | 1.69 ± 0.15 |
| 10 mM KCl | 0.51 ± 0.11 | 0.77 ± 0.07 | 0.29 ± 0.06 | 0.45 ± 0.08 |
| 100 mM KCl | 0.75 ± 0.05 | 0.84 ± 0.04 | 0.71 ± 0.12 | 0.93 ± 0.06 |
p < 0.05, PWD versus B6.
In contrast, there were few strain differences when only the phasic response portion was considered (Table 1). Mean 3-s phasic responses were significantly larger for 10 mM and 100 mM CaCl2and for citric acid (t[10] ≥ 2.3, p < 0.05 in all cases). No strain differences in phasic activity were seen for CaLa, MgCl2, KCl, NaCl, QHCl or sucrose. Although strain differences in CaCl2 responses were significant for both phasic and tonic responding, the difference was larger for the latter. Tonic responses to 10 and 100 mM CaCl2 in PWD mice were 54 and 39% larger, respectively, than those of B6 mice, whereas phasic responses were only 21 and 24% larger in the PWD strain.
4. DISCUSSION
4.1 Summary
We observed significant differences between the PWD and B6 strains in their preferences for some of the solutions tested, and, in general, solutions that were preferred to a greater extent by PWD mice also evoked larger CT responses in that strain relative to responses in B6 mice. PWD mice had significantly higher preferences for CaCl2, CaLa, MgCl2, citric acid and QHCl relative to the B6 strain. CT responses were significantly larger in PWD mice for all of these compounds, though in the case of CaLa there was a significant effect of strain, but differences for the two individual concentrations were not significant. The strains did not differ in their preferences for or CT responses to NaCl, KCl, NH4Cl and sucrose.
The preferences for CaCl2 and CaLa by the PWD strain were similar to those reported previously for PWK mice [37]. Both strains avidly preferred these solutions, which are avoided by B6 mice and most other inbred mouse strains that have been tested [2]. It appears, then, that PWD mice will provide a good model for studying calcium appetite, and B6-Chr nPWD consomic mice will be useful in helping to identify genes responsible for the positive taste of calcium. PWD mice differed from the PWK strain, though, in having significantly higher preferences for citric acid and QHCl compared with B6 mice [37].
4.2 The role of the chorda tympani in determining taste preferences
Preferences for taste solutions were measured over 48 h in experiment 1, and thus they were influenced by multiple factors, including learning about post-ingestive consequences. Measurements of chorda tympani activity were conducted in experiment 2 in order to examine the role of taste sensation more specifically. If one considers previous strain comparisons involving multiunit CT responses and behavioral tests, the former measure seems to provide insight into the perceived taste intensity of compounds in mice. For example, larger CT responses to sweeteners have been associated with higher preferences in two-bottle tests when mouse strains have been compared with each other [1,16]. In contrast, larger CT responses to bitter compounds have been associated with greater behavioral avoidance [8]. CT responses to the bitter compound SOA were found to be larger in the “taster” SWR/J strain, which avoids the compound, than in the “nontaster” C57BL/6J strain, which is indifferent to the compound in two-bottle tests with water [15]; presumably then, the larger whole-nerve response in SWR/J mice resulted in a more intense perception of bitterness in that strain, which resulted in less consumption of SOA relative to that found in the B6 mice.
Multiunit CT responses, however, provide poor insight into the perceived taste quality or palatability of compounds, given the broad tuning of the whole-nerve response. That is, the CT evokes a multiunit response in mice that is robust for both preferred and avoided taste stimuli, and for stimuli that evoke different taste qualities. As a result, the relationship between CT and behavioral data may be especially complex for compounds whose taste quality or palatability vary widely across concentrations, such as NaCl and CaCl2. For example, there is an inconsistent pattern between studies as to whether larger CT responses to NaCl are associated with sodium appetite. Pharmacological blockage or genetic knockout of the epithelial sodium channels (ENaCs), both of which reduce the size of taste-evoked CT responses to NaCl, have been reported to reduce sodium appetite [3,5,27]. However, a reduction in the size of taste-evoked neural responses to NaCl has also accompanied treatments that induce sodium appetite in rats [19].
Calcium is similar to sodium, in that both may be preferred by rodents at moderate concentrations but strongly avoided at higher ones. Moreover, human subjects report changes in the perceived taste quality of calcium solutions depending on concentration [31]. Thus, it is difficult to predict the effect on taste perception caused by larger multiunit CT responses to calcium-containing stimuli, even if one assumes that there is merely a shift in taste sensitivity. In the current work, greater taste-evoked neural activity was associated with higher preferences for CaCl2 and CaLa when comparing our two mouse strains. This contrasts with prior work [38], in which Tas1r3-KO mice had significantly lower CT responses to CaCl2, but higher behavioral preferences, compared with B6 littermate controls (see section 4.3 for a possible explanation of this apparent discrepancy). These considerations are not meant to discount the importance of the current CT data. However, additional work will be needed to illuminate the connection between the large CT responses to the calcium and magnesium stimuli and the high preference for them in the PWD strain. Insight into the perceived taste quality of these compounds will be especially useful.
Our data for quinine and citric acid are puzzling, in that the larger CT responses to these compounds in PWD than B6 mice may result in more intense perception of aversive bitter and sour tastes in the former strain. However, we observed that PWD mice had higher preferences for citric acid and quinine than did B6 mice. A possible explanation is that the strain differences in CT responses are offset by differences in the opposite direction in other nerves, such as the glossopharyngeal, which is more sensitive to bitter compounds than is the CT [30]. Another factor to consider is that different concentrations of QHCl needed to be used in experiments 1 and 2, and there could be strain differences in perceived intensity of this stimulus that change direction as its concentration is increased.
4.3 Time course of neural responses
In addition to quantifying the overall level of the whole-nerve response by integrating the activity and measuring the area-under-the-curve, we examined the temporal patterns of taste-evoked responses. These analyses indicated that strain differences in CT responses were especially pronounced for tonic responding, with more sustained taste-evoked activity in PWD than B6 mice. In our prior study involving B6 and Tas1r3-KO mice [38], in which the latter strain preferred calcium solutions more highly but had smaller multiunit CT responses to them, we did not look originally at the time course of neural responses. However, we considered it important to re-analyze the older data to look more closely at this issue, given the outcome of the present study. In the previous experiment, we reported that 10-s net responses in the CT were significantly higher in B6 than in Tas1r3-KO mice for CaCl2 (significant effect of strain) and for 100 mM CaLa. In our re-analysis, these strain differences arose primarily due to differences in phasic, rather than tonic, responding.
This finding provides a possible reconciliation of the two data sets, if the positive aspects of calcium’s taste are most active during the tonic period, but the negative aspects of its taste are most active during its phasic response. This explanation is consistent with the fact that Tas1r3-KO mice (i.e., a strain with low phasic responses to CaCl2 but not unusually high tonic responses) do not prefer CaCl2 over water, but merely show less avoidance of it than do B6 mice [38]. PWD mice, in contrast, appear to find calcium solutions highly palatable, which may be related to their large tonic responses for these compounds.
Electrophysiological recordings from the nucleus of the solitary tract (NST), the destination for CT fibers, have also indicated that the temporal patterns of taste-evoked responses play a major role in influencing perceptions of palatability in rats. For example, the phasic-tonic ratio of NST responses correlated positively with the toxicity of taste stimuli [29]. In addition, when rats were given a conditioned taste preference for citric acid, the NST responses evoked by that compound included a smaller peak response, but similar tonic responding, compared with responses in unconditioned animals [10]. These data are consistent with ours in suggesting that perceptions of aversive taste may be generally associated with the initial, phasic portion of taste responses, at least for early stages of gustatory processing.
Naturally, we are not claiming that calcium evokes a solely negative taste during its first three seconds, but then tastes palatable at 10 s after a solution is tasted. For one thing, all aspects of calcium’s complex taste quality are probably perceived within less than a second [13]. Rather, we would like to suggest that multiunit CT responses incorporate the activity of different classes of nerve fibers, which vary in their temporal patterns of responding to calcium-containing stimuli, and that those fibers that are most responsible for the positive aspects of calcium’s taste have delayed but sustained responses compared to those fibers most responsible for the negative aspects. A candidate to be involved in such an effect would be sugar-best CT fibers, some of which can also respond to CaCl2 and MgCl2 in B6 mice [25]. That is, our behavioral and neural data can be reconciled if the CT response evoked by calcium stimuli were carried to a greater extent by sugar-best fibers in PWD than in B6 mice, with these fibers being responsible for elevations in both tonic neural activity and palatability in the former strain. Such an effect would be analogous to prior results in calcium-deprived rats, who show elevations relative to replete rats in both ingestion of calcium and the taste-evoked responses of sugar-best cells in the NST [21]. In any case, resolving these questions will depend on techniques such as single-fiber recording from the CT, which offers significant technical challenges compared to multiunit recording in mice.
4.4 Underlying mechanisms
As mentioned earlier, 48-h two-bottle tests are influenced by multiple factors. We therefore measured chorda tympani responses in order to provide greater insight into the causes underlying any strain differences in preference behavior. We found that PWD mice not only preferred calcium solutions and MgCl2 to a greater extent than did B6 mice, but they also had larger taste-evoked CT responses to these solutions. These data certainly suggest the involvement of a taste-related mechanism, though they do not explain exactly how these solutions are perceived by the animals, nor do they preclude other mechanisms, especially given that the magnitudes of strain differences in chorda tympani responding to the test solutions were generally smaller than the magnitude of the associated behavioral differences. For example, PWD mice demonstrated a strong preference for 50 mM CaLa and B6 mice clearly avoided it, but the strain differences in CT responses to 10 and 100 mM CaLa were not large enough to reach significance in post-hoc tests. There are several possible explanations: Our results do not rule out non-gustatory factors as being important in contributing to the strain differences in preference behavior. It is possible that taste signals carried by nerves other than the CT contribute to the high preferences of PWD mice for certain compounds. Furthermore, our neural data were based on the response of the whole chorda tympani, whereas behavioral data may be influenced by responding in primarily a subset of the nerve’s fibers.
We do not know the mechanisms underlying the strain differences in CT activity, though they must be found in the periphery (i.e., in taste buds or in the CT itself). Ninomiya and colleagues [24,25] found individual CT fibers tuned to Ca2+/Mg2+ salts in some, although not other, strains of mice, and whether they exist in PWD mice is unknown. Certainly, one possibility is that the CT of PWD mice contains a larger percentage of these Ca-Mg fibers, which respond robustly to CaCl2, MgCl2, and QHCl, than is the case for the CT of B6 mice. However, such fibers also respond well to KCl and NH4Cl, and we did not observe strain differences in CT responses to those taste stimuli.
Another possible cause is strain variation in taste transduction mechanisms. The G-protein coupled receptors CaSR and T1R3 have been proposed to mediate the unique taste of calcium. With respect to CaSR, the B6 and PWD strains have SNPs in exons 6 and 7 of the Casr gene, and these polymorphisms could be responsible for the strain differences in preferences for and CT responses to CaCl2 and CaLa. Magnesium ions can also bind to CaSR [4], and MgCl2 shares taste properties with CaCl2 and CaLa [37], so strain variation in Casr sequence may also explain our results for MgCl2. Nonetheless, rats are able to discriminate between MgCl2 and CaCl2 [22], and so there remains a possibility that different mechanisms mediated our results for the two compounds. PWD and B6 mice also differ in their alleles of Tas1r3, which has been implicated in sweet, umami, and calcium taste [38,39], and so this variation provides another potential basis for the observed behavioral and neural differences for CaCl2 and CaLa. The fact that we did not observe strain differences for sucrose does not rule out this mechanism, as different SNPs in Tas1r3 appear to mediate taste transduction for sweeteners and calcium [38].
It seems highly unlikely that a single mechanism that could explain the strain differences in responses to citric acid, QHCl, CaCl2, CaLa, and MgCl2, given their disparate taste qualities. The finding that PWD but not PWK mice show similar strong avidity for calcium but only the PWD strain prefers citric acid (relative to the B6 strain) argues that at least two mechanisms are involved, with the one for calcium evolving in the common Mus m. musculus ancestor, whereas the one for citric acid originating since the PWD and PWK strains diverged. It will require additional work to determine the genetic loci responsible for these behaviors.
Acknowledgments
This work was supported by NIH R01 DK-46791 to M. G. T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–13. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2, and NH4Cl solutions by 28 mouse strains. Behav Genet. 2002;32:445–57. doi: 10.1023/a:1020832327983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand JG, Teeter JH, Silver WL. Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res. 1985;334:207–14. doi: 10.1016/0006-8993(85)90212-4. [DOI] [PubMed] [Google Scholar]
- 4.Brown EM. Mutations in the calcium-sensing receptor and their clinical implications. Horm Res Paediatr. 1997;48:199–208. doi: 10.1159/000185516. [DOI] [PubMed] [Google Scholar]
- 5.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheal M. Taste responses of the chorda tympani nerve in the mouse. Physiol Behav. 1977;19:175–7. doi: 10.1016/0031-9384(77)90178-0. [DOI] [PubMed] [Google Scholar]
- 7.Coldwell SE, Tordoff MG. Immediate acceptance of minerals and HCl by calcium-deprived rats: brief exposure tests. Am J Physiol. 1996;271:R11–17. doi: 10.1152/ajpregu.1996.271.1.R11. [DOI] [PubMed] [Google Scholar]
- 8.Frank ME, Blizard DA. Chorda tympani responses in two inbred strains of mice with different taste preferences. Physiol Behav. 1999;67:287–97. doi: 10.1016/s0031-9384(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 9.San Gabriel A, Uneyama H, Maekawa T, Torii K. The calcium-sensing receptor in taste tissue. Biochem Biophys Res Commun. 2009;378:414–18. doi: 10.1016/j.bbrc.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 10.Giza BK, Ackroff K, McCaughey SA, Sclafani A, Scott TR. Preference conditioning alters taste responses in the nucleus of the solitary tract of the rat. Am J Physiol. 1997;273:R1230–40. doi: 10.1152/ajpregu.1997.273.4.R1230. [DOI] [PubMed] [Google Scholar]
- 11.Gregorová S, Forejt J. PWD/Ph and PWK/Ph inbred mouse strains of Mus m. musculus subspecies--a valuable resource of phenotypic variations and genomic polymorphisms. Folia Biol (Praha) 2000;46:31–41. [PubMed] [Google Scholar]
- 12.Gregorová S, Divina P, Storchova R, Trachtulec Z, Fotopulosova V, Svenson KL, Donahue LR, Paigen B, Forejt J. Mouse consomic strains: Exploiting genetic divergence between Mus m. musculus and Mus m. domesticus subspecies. Genome Res. 2008;18:509–15. doi: 10.1101/gr.7160508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpern BP, Tapper DN. Taste stimuli: Quality coding time. Science. 1971;171:1256–58. doi: 10.1126/science.171.3977.1256. [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, Tordoff MG. Calcium deficiency alters chorda tympani nerve responses to oral calcium chloride. Physiol Behav. 1998;63:297–303. doi: 10.1016/s0031-9384(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Li X, McCaughey SA, Beauchamp GK, Bachmanov AA. Soa genotype selectively affects mouse gustatory neural responses to sucrose octaacetate. Physiol Genomics. 2001;5:181–6. doi: 10.1152/physiolgenomics.2001.5.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:915–23. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawless HT, Rapacki F, Horne J, Hayes A. The taste of calcium and magnesium salts and anionic modifications. Food Qual Prefer. 2003;14:319–25. [Google Scholar]
- 18.Leshem M, Rudoy J, Schulkin J. Calcium taste preference and sensitivity in humans: II. Hemodialysis patients. Physiol Behav. 2003;78:409–14. doi: 10.1016/s0031-9384(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 19.McCaughey SA, Scott TR. The taste of sodium. Neurosci Biobehav Rev. 1998;22:663–76. doi: 10.1016/s0149-7634(97)00067-5. [DOI] [PubMed] [Google Scholar]
- 20.McCaughey SA, Tordoff MG. Calcium-deprived rats sham-drink CaCl2 and NaCl. Appetite. 2000;34:305–11. doi: 10.1006/appe.1999.0317. [DOI] [PubMed] [Google Scholar]
- 21.McCaughey SA, Tordoff MG. Calcium deprivation alters gustatory-evoked activity in the rat nucleus of the solitary tract. Am J Physiol. 2001;281:R971–78. doi: 10.1152/ajpregu.2001.281.3.R971. [DOI] [PubMed] [Google Scholar]
- 22.McCaughey SA, Tordoff MG. Magnesium appetite in the rat. Appetite. 2002;38:29–38. doi: 10.1006/appe.2001.0443. [DOI] [PubMed] [Google Scholar]
- 23.McCaughey SA, Forestell CA, Tordoff MG. Calcium deprivation increases the palatability of calcium solutions in rats. Physiol Behav. 2005;84:335–42. doi: 10.1016/j.physbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Ninomiya Y, Tonosaki K, Funakoshi M. Gustatory neural response in the mouse. Brain Res. 1982;244:370–73. doi: 10.1016/0006-8993(82)90100-7. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya Y, Mizukoshi T, Higashi T, Katsukawa H, Funakoshi M. Gustatory neural responses in three different strains of mice. Brain Res. 1984;302:305–14. doi: 10.1016/0006-8993(84)90244-0. [DOI] [PubMed] [Google Scholar]
- 26.Okada Y, Imendra KG, Miyazaki T, Hotokezaka H, Fujiyama R, Zeredo JL, Toda K. A calcium-receptor agonist induces gustatory neural responses in bullfrogs. Cell Mol Neurobiol. 2007;27:771–81. doi: 10.1007/s10571-007-9171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roitman MF, Bernstein IL. Amiloride-sensitive sodium signals and salt appetite: multiple gustatory pathways. Am J Physiol. 1999;276:R1732–38. doi: 10.1152/ajpregu.1999.276.6.R1732. [DOI] [PubMed] [Google Scholar]
- 28.Schiffman SS, Erickson RP. A psychophysical model for gustatory quality. Physiol Behav. 1971;7:617–33. doi: 10.1016/0031-9384(71)90117-x. [DOI] [PubMed] [Google Scholar]
- 29.Scott TR, Mark GP. The taste system encodes stimulus toxicity. Brain Res. 1987;414:197–203. doi: 10.1016/0006-8993(87)91347-3. [DOI] [PubMed] [Google Scholar]
- 30.Tanimura S, Shibuya T, Ishibashi T. Neural responses of the glossopharyngeal nerve to several bitter stimuli in mice. Comp Biochem Physiol A Physiol. 1994;108:189–94. [PubMed] [Google Scholar]
- 31.Tordoff MG. Some basic psychophysics of calcium salt solutions. Chem Senses. 1996;21:417–24. doi: 10.1093/chemse/21.4.417. [DOI] [PubMed] [Google Scholar]
- 32.Tordoff MG. Calcium: Taste, intake, and appetite. Physiol Rev. 2001;81:1567–97. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- 33.Tordoff MG. The case for a calcium appetite in humans. In: Weaver CM, Heaney RP, editors. Calcium in human health. Totowa, NJ: Humana Press; 2005. pp. 247–66. [Google Scholar]
- 34.Tordoff MG, Bachmanov AA. Survey of calcium and sodium intake and metabolism with bone and body composition data (MPD:103): Mouse Phenome Project. 2002 Retrieved Sep. 9, 2010 from http://phenome.jax.org/db/q?rtn=projects/details&sym=Tordoff3.
- 35.Tordoff MG, Sandell MA. Vegetable bitterness is related to calcium content. Appetite. 2009;52:498–504. doi: 10.1016/j.appet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of voluntary calcium intake, blood calcium, and bone mineral content. Physiol Behav. 2007;91:632–43. doi: 10.1016/j.physbeh.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tordoff MG, Reed DR, Shao H. Calcium taste preferences: genetic analysis and genome screen of C57BL/6J × PWK/PhJ hybrid mice. Genes Brain Behav. 2008;7:618–28. doi: 10.1111/j.1601-183X.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tordoff MG, Shao H, Alarcón LK, Margolskee RF, Mosinger B, Bachmanov AA, Reed DR, McCaughey S. Involvement of T1R3 in calcium-magnesium taste. Physiol Genomics. 2008;34:338–48. doi: 10.1152/physiolgenomics.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–66. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]


