Abstract
It has been recently shown that a short sublethal brain ischemia subsequent to a prolonged harmful ischemic episode may confer ischemic neuroprotection, a phenomenon termed ischemic postconditioning. Na+/Ca2+ exchanger (NCX) isoforms, NCX1, NCX2, and NCX3, are plasma membrane ionic transporters widely distributed in the brain and involved in the control of Na+ and Ca2+ homeostasis and in the progression of stroke damage. The objective of this study was to evaluate the role of these three proteins in the postconditioning-induced neuroprotection. The NCX protein and mRNA expression was evaluated at different time points in the ischemic temporoparietal cortex of rats subjected to tMCAO alone or to tMCAO plus ischemic postconditioning. The results of this study showed that NCX3 protein and ncx3 mRNA were upregulated in those brain regions protected by postconditioning treatment. These changes in NCX3 expression were mediated by the phosphorylated form of the ubiquitously expressed serine/threonine protein kinase p-AKT, as the p-AKT inhibition prevented NCX3 upregulation. The relevant role of NCX3 during postconditioning was further confirmed by results showing that NCX3 silencing, induced by intracerebroventricular infusion of small interfering RNA (siRNA), partially reverted the postconditioning-induced neuroprotection. The results of this study support the idea that the enhancement of NCX3 expression and activity might represent a reasonable strategy to reduce the infarct extension after stroke.
Keywords: AKT, NCX, neuroprotection, postconditioning, sodium/calcium exchanger
Introduction
Owing to the failure of clinical trials for pharmacological neuroprotective strategies in stroke (Gladstone et al, 2002), attention of researchers has been recently focused to the identification of additional transductional and transcriptional pathways eventually activated by endogenous neuroprotective mechanisms. Interestingly, it has been recently reported that ischemic preconditioning, a sublethal ischemic episode applied before a longer harmful ischemia (Dirnagl et al, 2003; Gidday, 2006; Kirino, 2002), and ischemic postconditioning, a sublethal ischemia subsequent to a prolonged harmful ischemic episode (Burda et al, 2006; Pignataro et al, 2006, 2008; Scartabelli et al, 2008; Yellon and Hausenloy, 2005; Zhao, 2007; Zhao et al, 2006a), are both able to exert a remarkable neuroprotection. Therefore, ischemic preconditioning and postconditioning represent useful tools to identify the transductional and transcriptional factors activated during these two experimental conditions and that can be targeted in the attempt to identify new neuroprotective molecular targets. However, ischemic preconditioning, though scientifically fascinating, is not clinically applicable, as the harmful anoxic event is not predictable. Therefore, the molecular characterization of a postischemic neuroprotective approach seems to be more realizable to identify new drugable molecular targets. The molecular mechanisms contributing to postconditioning-mediated tissue protection have been classified as: (1) triggers, such as adenosine, opioids, erythropoietin, nitric-oxide, reactive oxygen species, cytokines and bradykinin; (2) transductors, such as reperfusion injury salvage kinase pathways and other protein kinases; and finally (3) effectors, such as mitochondrial permeability transition pore and mitochondrial potassium ATP channels (Pignataro et al, 2009; Zhao, 2007, 2009).
Among the several transductors, the well-known families of mitogen-activated protein kinases and phosphatidylinositol 3-kinase have been proposed as important factors of the ischemic postconditioning neuroprotection (Pignataro et al, 2008; Zhao et al, 2006b). Interestingly, we showed earlier that NCX1 and NCX3, two of the three brain isoforms of the plasma membrane Na+/Ca2+ exchanger, are novel additional targets for the survival action of the (phosphatidylinositol 3-kinase)/Akt pathway (Formisano et al, 2008). In fact, AKT functions as a major downstream target of phosphatidylinositol 3-kinase, and after phosphorylation, it phosphorylates some substrates on the serine or threonine residues, including glycogen synthase kinase-3, Caenorhabditis elegans DAF-16 transcription factor, Bad, phosphodiesterase 3B, ATP-citrate lysase, and the tuberous sclerosis complex-2 tumor suppressor gene product tuberin (Chan, 2004). In particular, it has been proposed that phosphatidylinositol 3-kinase/Akt signaling pathway by phosphorylating-specific substrates is determinant for the control of cell death in ischemic neurons during stroke (Chan, 2004).
In addition, these Na+/Ca2+ exchanger (NCX) isoforms have a fundamental role in regulating and maintaining cellular calcium and sodium homeostasis (Annunziato et al, 2004) and are involved in the pathophysiology of stroke damage. In particular, it has been shown that NCX gene expression after permanent middle cerebral artery occlusion (MCAO) in rats is regulated in a differential manner, depending on the exchanger isoform (NCX1, -2, or -3) and on the region involved in the insult (Boscia et al, 2006; Pignataro et al, 2004). Furthermore, NCX1 and NCX3 downregulation or genetic ablation worsens the experimentally induced ischemic damage in mice and rats (Molinaro et al, 2008; Pignataro et al, 2004).
The aim of this study was to elucidate whether the NCX isoforms, NCX1, NCX2, and NCX3, might take part as effectors in the neuroprotection evoked by postconditioning. For this purpose, we investigated the effect of ischemic postconditioning on:
NCX1, NCX2, and NCX3 expression in the temporoparietal cortex at different time intervals from postconditioning;
the effect of NCX1 and NCX3 silencing induced by small interfering RNA (siRNA) on neuroprotection induced by ischemic postconditioning;
the effect of p-AKT inhibition on NCX3 expression during ischemic postconditioning.
Materials and methods
Chemicals
The siRNA against rat NCX1 (GenBank accession number NM_019268) and rat NCX3 (GenBank accession number U53420) were from Qiagen (Milan, Italy), whereas the negative control was an siCONTROL nontargeting (Qiagen).
The siRNA corresponding to rat NCX1 (GenBank accession number NM_019268) contained a 19-nucleotide sequence corresponding to the nucleotides 2000 to 2018 downstream of the transcription start site of rat NCX1.
The siRNA against NCX3 contains a 19-nucleotide sequence corresponding to coding region +124 to 142 relative to the first nucleotide of the start codon of rat NCX3 (GenBank accession number U53420), whose specificity was verified by BLAST.
All common reagents were of the highest quality and were purchased from Sigma (Milan, Italy).
Experimental Groups
Male Sprague–Dawley rats (Charles River, Varese, Italy) weighting 250 to 300 g were housed under diurnal lighting conditions (12 hours darkness/light). Experiments were performed according to the international guidelines for animal research. Experiments were performed according to the international guidelines for animal research and approved by the Animal Care Committee of ‘Federico II', University of Naples, Italy.
Focal Ischemia
Transient focal ischemia was induced, as described earlier (Pignataro et al, 2008), by suture occlusion of the MCA in male rats anesthetized using 1.5% sevofluorane, 70% N2O, and 28.5% O2. Ischemia was induced by introducing a 5-O surgical monofilament nylon suture (Doccol, Redlands, CA, USA) from the external carotid artery into the internal carotid artery and advancing it into the circle of Willis to the branching point of the MCA, thereby occluding the MCA (Longa et al, 1989). Achievement of ischemia was confirmed by monitoring regional cerebral blood flow in the area of the right MCA. Cerebral blood flow was monitored through a disposable microtip fiber optic probe (diameter 0.5 mm) connected through a Master Probe to a laser Doppler computerized main unit (PF5001; Perimed, Jarfalla, Sweden) and analyzed using PSW Perisoft 2.5 (Pignataro et al, 2008). Animals that did not show a cerebral blood flow reduction of at least 70% were excluded from the experimental group, as well as animals that died after ischemia induction. Rectal temperature was maintained at 37°C±0.5°C with a thermostatically controlled heating pad and lamp. All surgical procedures were performed under an operating stereomicroscope.
Postconditioning Experimental Protocol
Ischemic postconditioning was induced as described earlier (Pignataro et al, 2008). Briefly, after 100 minutes of MCAO, reperfusion was established for 10 minutes after which the MCA was reoccluded for 10 minutes. Animals were then recovered for 24 hours. The success of the experimental procedures was confirmed by measuring CBF in all the experimental steps.
Evaluation of the Infarct Volume
Rats were decapitated 24 hours after ischemia. The ischemic volume was evaluated by 2,3,5-triphenyl tetrazolium chloride staining. The brains were cut into 1 mm coronal slices with a vibratome (Campden Instrument, Lafayette, IN, USA; 752M). Sections were incubated in 2% 2,3,5-triphenyl tetrazolium chloride for 20 minutes and in 10% formalin overnight. The infarction area was calculated with image analysis software (Image-Pro Plus) (Bederson et al, 1986).
The total infarct volume, corrected for edema, was expressed as percentage of the volume of the hemisphere ipsilateral to the lesion.
Use of p-AKT Inhibitor and Small Interfering RNA for NCX3 and NCX1
To assess the effect of p-AKT on NCX3 expression, 10 rats were treated 15 minutes before postconditioning induction with the inhibitor of phosphatidylinositol-3 kinase LY294002 (5 μL, 10 μmol/L, in 3% dimethyl sulfoxide) or vehicle alone (3% dimethyl sulfoxide).
The role of NCX1 and NCX3 in the neuroprotection exerted by ischemic postconditioning was evaluated by treating postconditioned rats with siRNA for NCX1 or NCX3 (1 μL, 0.5 μmol/L in artificial cerebral spinal fluid (aCSF). The siRNA for NCX3 or control siRNA were injected four times, every 12 hours starting 2 days before ischemia induction and lasting 12 hours before postconditioning induction.
All chemicals were intracerebroventricularly injected at the following coordinates from the bregma: anteroposterior, −0.4 mm; laterolateral, −2.0 mm; depth, −2.5 mm (Paxinos and Watson, 1997).
Western Blotting Analysis
Cortical samples were harvested from ischemic brains of rats subjected to 100 minutes of MCAO or from brains of postconditioned rats. In both experimental conditions, two groups of ipsilateral and contralateral temporoparietal cortex were obtained at different reperfusion times after the last occlusion: (1) 0.5 hours; (2) 5 hours; and (3) 24 hours. Two further groups of samples were obtained from brains of sham-operated animals.
Rat brain samples were homogenized using an 18-gauge needle in a lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 1% Triton X-100) containing protease inhibitors (aprotinin, leupeptin, phenylmethylsulfonyl fluoride, and pepstatin), and phosphatase inhibitor (Sigma cocktail). After centrifugation at 12,000g at 4°C for 20 minutes, the supernatants were collected. Protein concentration was estimated using the Bradford reagent (Biorad, Segrate, Milan, Italy). Then, 100 μg of protein was mixed with a Laemmli sample buffer. The samples were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. Blots were probed with antibodies to NCX1 (1:1000, Swant, Bellinzona, Switzerland), NCX2 (1:1000, Alpha Diagnostic, San Antonio, TX, USA), NCX3 (1:2000, kind gift from Professor Philipson and Nicoll), p-AKT (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), AKT (1:1000, Santa Cruz Biotechnology), and β-actin (1:2000; Sigma, St Louis, MO, USA) diluted in tris-buffered saline 5% non-fat milk overnight (4°C), and detected using horseradish peroxidase-conjugated secondary antibody (1:2000; Amersham Pharmacia Biotech, Piscataway, NJ, USA; 60 minutes at room temperature in 5% nonfat milk) and an enhanced luminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
mRNA Expression Analysis by Real-Time Polymerase Chain Reaction
Rat cortex dissected from the ipsilesional hemisphere of sham-operated and postconditioned rats 5 and 24 hours after surgery were frozen on dry ice. Brain samples were ground into powered dry ice, then Trizol Reagent solution (Invitrogen, Milan, Italy) was added. Total RNA was extracted and purified in accordance with the manufacturer's protocol. For reverse transcription, 2.0 μg of each extracted RNA was digested with DNase and reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Segrate, Milan, Italy). Amplification was performed using Power Sybr Green PCR Master Mix (Applied Biosystem, Milan, Italy) according to the manufacturer's protocol. All data were normalized to hypoxanthine phosphoribosyltransferase 1 mRNA levels and expressed as percentage of the mRNA levels detected in sham-operated animals. Sequences of the primers used were the following:
- Hypoxanthine phosphoribosyltransferase:
F (444 to 467) TGGAAAGAACGTCTTGATTGTTGA R (528 to 507) GCTGTACTGCTTGACCAAGGAA
- NCX3:
F (3229 to 3250) CCTCTGTGCCAGATACATTTGC R (3311 to 3292) GCCGGTGACATTGCCAATAG
Confocal Double Immunofluorescence
Confocal immunofluorescence procedures were performed as described earlier (Boscia et al, 2009). The animals were euthanized 5 hours after sham surgery, tMCAO or tMCAO+postconditioning onset. The rats were anesthetized intraperitoneally with chloral hydrate (300 mg/kg) and perfused transcardially with 4% w/v paraformaldehyde in phosphate buffer. The brains were sectioned coronally, and, after blocking, sections were incubated with the following primary antisera: rabbit polyclonal anti-NCX3 (1:4000, kindly provided by Dr Nicoll and Professor Philipson) and mouse monoclonal antiphospho-AKT Ser473 (1:500, USBiological, Swampscott, MA, USA). Subsequently, sections were incubated in a mixture of the fluorescent-labeled secondary antibodies (Alexa 488/Alexa 594-conjugated antimouse/antirabbit IgG). Images were observed using a Zeiss LSM510 META/laser scanning confocal microscope. Single images were taken with an optical thickness of 0.7 m and a resolution of 1024 × 1024.
Statistical Analysis
Values are expressed as means±s.e.m. Statistical analysis was performed with two-way analysis of variance, followed by Newman–Keuel's test. Statistical significance was accepted at the 95% confidence level (P<0.05).
Results
Ischemic Postconditioning Induces an NCX3 Overexpression in the Periischemic Temporoparietal Cortex
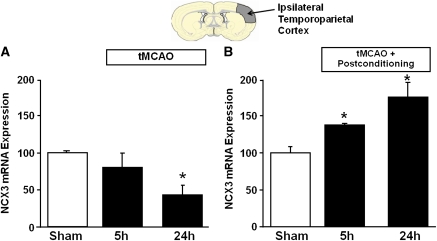
To assess the role played by the three different NCX proteins present in the postconditioned brain, their expression was evaluated in the ipsilesional temporoparietal cortex of rats subjected to ischemic postconditioning at different reperfusion time intervals: 0.5, 5, and 24 hours, and compared with the expression of the three proteins in the same brain region after tMCAO alone (Figure 1). The reduction in NCX1 expression induced by the ischemic insult alone was completely prevented if the animals were exposed to ischemia followed by a sublethal postconditioning ischemia evaluated at 0.5, 5, and 24 hours (Figure 1A). By contrast, NCX2 expression did not show any change in the two experimental groups (Figure 1B). The effect of postconditioning on NCX3 expression was still more dramatic. In fact, the reduced expression of NCX3 induced by cerebral ischemia alone was completely counteracted after ischemic postconditioning and more interestingly, 24 hours after ischemic postconditioning NCX3 expression was further increased (almost 90% increment) (Figure 1C). To assess whether NCX3 protein increase was due to an increased transcription, NCX3 mRNA detected through real-time polymerase chain reaction was performed in the ipsilesional temporoparietal cortex of rats subjected to ischemic postconditioning at different reperfusion time intervals and compared with the expression of the NCX3 mRNA in the same brain region after ischemia alone (Figure 2). After ischemia, NCX3 mRNA expression was reduced by almost 50% 24 hours after reperfusion (Figure 2A), whereas after postconditioning, NCX3 mRNA expression measured at 5 and 24 hours after reperfusion progressively increased by 40.2%±5.0% and 78.2%±14.8%, respectively, as compared with sham-operated animals (Figure 2B).
Figure 1.
Na+/Ca2+ exchanger (NCX) protein expression in rat ipsilateral cortex after ischemia and postconditioning. (A) Time course of NCX1 protein expression after tMCAO and tMCAO+postconditioning in the ipsilateral temporoparietal cortex. (B) Time course of NCX2 protein expression after tMCAO and tMCAO+postconditioning in the ipsilateral temporoparietal cortex. (C) Time course of NCX3 protein expression after tMCAO and tMCAO+postconditioning in the ipsilateral temporoparietal cortex. A representative brain slice cartoon, indicating the area of interest is on the top of the figure. Data were normalized on the basis of β-actin levels and expressed as percentage of sham-operated controls (sham). Values are mean±s.e.m. *P<0.05, compared with sham. n=6 to 8 animals for each column of the figure. MCAO, middle cerebral artery occlusion.
Figure 2.
NCX3 mRNA expression in rat ipsilateral cortex after ischemia and postconditioning. Time course of NCX3 protein expression after tMCAO (A) and tMCAO+postconditioning (B) in the ipsilateral temporoparietal cortex. A representative brain slice cartoon, indicating the area of interest is on the top of the figure. Data were normalized on the basis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and expressed as percentage of sham-operated controls (sham). Values are mean±s.e.m. *P<0.05, compared with sham. n=6 to 8 animals for each column of the panel. MCAO, middle cerebral artery occlusion; NCX, Na+/Ca2+ exchanger.
The p-AKT Inhibitor LY294002 Reverts NCX3 Overexpression Induced by Ischemic Postconditioning
As it has been previously shown that the neuroprotective effect induced by ischemic postconditioning is in part mediated by p-AKT activation (Pignataro et al, 2008), we confirmed that the increase in AKT phosphorylation was less marked after ischemia alone (Figure 3A) than after ischemic postconditioning (Figure 3B) and showed that the NCX3 overexpression induced by ischemic postconditioning was p-AKT mediated. In fact, the p-AKT inhibitor LY294002 reverted NCX3 protein upregulation induced by postconditioning (188±11.2 versus 123.1±3.3) (Figure 3C), thus suggesting that NCX3 overexpression induced by postconditioning may occur through p-AKT.
Figure 3.
(A) p-AKT expression in rat ipsilateral cortex after ischemia. (B) p-AKT expression in rat ipsilateral cortex after ischemic postconditioning. (C) The p-AKT inhibitor LY294002 reverts NCX3 increased expression induced by postconditioning treatment. (A) Time course of p-AKT protein expression after 100′ tMCAO in the ipsilateral temporoparietal cortex. On the x axis are indicated reperfusion hours. Data were normalized on the basis of β-actin levels and expressed as percentage of sham-operated controls. (B) Time course of p-AKT protein expression after tMCAO+postconditioning in the ipsilateral temporoparietal cortex. On the x axis are indicated reperfusion hours. The effect of the p-AKT inhibitor LY294002 on AKT phosphorylation is illustrated in the last column of the histogram. Data were normalized on the basis of β-actin levels and expressed as percentage of sham-operated controls. (C) Representative Western blots of NCX3 protein levels in the temporoparietal cortex of sham-operated animals (control), rats subjected to tMCAO+postC administered with the vehicle, and rats subjected to tMCAO+postC administered with LY294002. Values are mean±s.e.m. *P<0.05, compared with control group. n=6 to 8 animals for each column of the panels (A–C). MCAO, middle cerebral artery occlusion; NCX, Na+/Ca2+ exchanger.
NCX3 and Phospho-AKT Colocalize After Ischemic Postconditioning
To further explore the relationship between the increased NCX3 expression and AKT activation observed during ischemic postconditioning, we performed colocalization experiments with both NCX3 and p-AKT antibodies in the rat cerebral temporoparietal cortex of sham-operated, ischemic and ischemic postconditioned rats (Figure 4). Confocal double immunofluorescence experiments revealed that after tMCAO plus postconditioning both NCX3 and p-AKT immunoreactivities strongly increased in the ipsilateral temporoparietal cortex, with most of the cells displaying intense coexpression (Figure 4, panels G–I). By contrast, in the ipsilateral hemisphere of sham-operated (panels A–C) or ischemic animals (panels D–F), no significant colocalization was found.
Figure 4.
Colocalization of NCX3 and p-AKT in the temporoparietal cortex of sham-operated, ischemic, and ischemic postconditioned rats 5 hours after sham surgery, ischemia, or postconditioning. (A–C) Confocal microscopic images displaying both NCX3 (red) and p-AKT (green) immunoreactivity in IV and V cortical layers of sham animals. (D–F) Confocal microscopic images displaying both NCX3 (red) and p-AKT (green) immunoreactivity in IV and V cortical layers of ischemic animals. (G–I) Confocal microscopic images displaying both NCX3 (red) and p-AKT (green) immunoreactivity in IV and V cortical layers of ischemic postconditioned rats. A representative brain slice cartoon indicating the area of interest is on the right bottom of panels A, D, G. Scale bars in (A–I): 50 μm. NCX, Na+/Ca2+ exchanger.
NCX3 Silencing Prevents the Neuroprotective Effect Induced by Ischemic Postconditioning
To assess whether the upregulation of NCX3 induced by ischemic postconditioning had a pathophysiological meaning, rats subjected to ischemic postconditioning were intracerebroventricular treated with siRNA for NCX3 and infarct volume was then evaluated. The results showed that NCX3 siRNA intracerebroventricular injected before postconditioning onset was able to produce a marked reduction in NCX3 expression in the region where the tMCAO induced an ischemic damage (Figure 5A). The NCX3 silencing was able to revert the neuroprotective effect induced by ischemic postconditioning as percentage of infarct volume was 19.7±0.8 in rats subjected to 100′tMCAO+postconditioning silencing control (siCTL) and 40.2±2.1 in rats subjected to 100′tMCAO+postconditioning+siNCX3 (Figure 5B). By contrast, the downregulation of NCX1 protein induced by siNCX1 (Figure 5A) was not able to counteract the neuroprotective effect induced by ischemic postconditioning (19.7±0.8 in rats subjected to postconditioning+siCTL; 21.3±4.7 in rats subjected to postconditioning+siNCX1) (Figure 5B).
Figure 5.
(A) NCX3 protein expression in rat brain after NCX3 siRNA treatment. (B) Effect of the p-AKT inhibitor LY294002, siNCX1 and siNCX3 on neuroprotective effect mediated by ischemic postconditioning. (A) Representative Western blots of NCX1 and NCX3 protein levels in the temporoparietal cortex of rats intracerebroventricular treated with siNCX1 or siNCX3, respectively. (B) Infarct volume in rats subjected to tMCAO, tMCAO+postC+siCTL, tMCAO+postC+siNCX1, tMCAO+postC+siNCX3, tMCAO+postC+LY294002. Rats were euthanized 24 hours after tMCAO. *P<0.05 versus tMCAO; ^P<0.05 versus all experimental groups. Each column represents the mean±s.e.m. (n=5 to 7) of the percentage of the infarct volume compared with the ipsilateral hemisphere. MCAO, middle cerebral artery occlusion; NCX, Na+/Ca2+ exchanger.
Interestingly, the neuroprotective effect induced by ischemic postconditioning was counteracted also by intracerebroventricular infusion of LY294002, a selective inhibitor of p-AKT pathway (infarct volume 42.1%±4.9%). The silencing of NCX3 does not affect p-AKT expression (data not shown).
Discussion
In this study we showed that among the three NCX isoforms, expressed in the CNS, NCX3 represents an additional new molecular effector involved in the neuroprotection exerted by ischemic postconditioning. In particular, we showed that p-AKT is the mediator of this action as (1) p-AKT expression after postconditioning increased and timely mirrored that of NCX3; (2) NCX3 downregulation, induced by siRNA, reverted the neuroprotection induced by ischemic postconditioning; and (3) the selective p-AKT inhibition prevented NCX3 upregulation thus reverting the postconditioning-induced neuroprotection.
That NCX3 is overexpressed during postconditioning may be related to its ability to counteract the dysregulation of intracellular Na+ ([Na+]i) and Ca2+ ([Ca2+]i) homeostasis occurring in the brain under anoxic conditions. This peculiar capability of NCX3 isoform to maintain [Ca2+]i and [Na+]i homeostasis in anoxic conditions might be correlated to its ability to operate, unlike the other two NCX isoforms, NCX1 and NCX2, even when ATP levels are reduced (Secondo et al, 2007). As a matter of fact, the three NCX isoforms display a different sensitivity to ATP levels (Linck et al, 1998; Secondo et al, 2007). In particular, during ATP depletion, NCX1 and NCX2 isoform activity is reduced, whereas NCX3 is still operative (Linck et al, 1998; Secondo et al, 2007). On the other hand, the NCX3 neuroprotective role in ischemic postconditioning is in accord with the results showing that in homozygous ncx3−/− mice subjected to MCAO, an increased brain damage occurs (Molinaro et al, 2008). In addition, the silencing of NCX3 expression by RNA interference increases cerebellar granule neurons vulnerability to Ca2+ overload and excitotoxicity and renders BHK cells transfected with NCX3 extremely vulnerable to chemical hypoxia (Bano et al, 2005; Secondo et al, 2007). Furthermore, ischemic rats treated with NCX3 antisense displayed a remarkable broadening of the infarct volume (Pignataro et al, 2004).
It is important to underline that NCX1 downregulation induced by siRNA was not able to revert the postconditioning-induced neuroprotection, thus showing that NCX1 does not have a relevant role in this phenomenon. This result can be explained taking into account the above-mentioned different sensitivity of NCX1 and NCX3 to ATP levels (Secondo et al, 2007). In addition, NCX1 and NCX3 promoters show structural differences that render NCX3 a better target for the prosurvival kinase CREB, an AKT downstream player (Gabellini et al, 2003). In fact, earlier results showed that the stimulation of NCX3 promoter is mediated by the CRE sequence, which binds transcription factors of the ATF/CREB family, an AKT downstream pathway (Gabellini et al, 2003).
In an earlier study we showed that during ischemia, Akt is transiently phosphorylated, and consequently activated, only for a short interval of time after reperfusion (Pignataro et al, 2008), whereas after postconditioning, the phosphorylation of Akt persists longer, being still present in the phosphorylated form even 24 hours later (Pignataro et al, 2008). In this study, we confirmed these data and in addition we showed that after ischemic postconditioning AKT phosphorylation is greater than that observed after ischemia alone. The time course of the increase of AKT phosphorylation after postconditioning parallels the same time interval at which an NCX3 upregulation occurs. Furthermore, double staining experiments in temporoparietal cortex of postconditioned rats further confirm the greater increase in p-AKT and NCX3 expression if compared with ischemic rats. The tight relationship existing between NCX3 and p-AKT was further demonstrated by confocal microscopy results showing that the increased expression of these two proteins occurs in the same cells. As the downregulation of NCX3 expression induced by siRNA did not modify p-AKT expression, the effect of p-AKT on NCX3 can be considered unidirectional. Consistent with the present results, we previously showed that NCX3 represents a novel additional target for the survival action of the p-Akt pathway (Formisano et al, 2008).
Altogether, our data support the importance of p-AKT in mediating postconditioning neuroprotection and suggest NCX3 as one of the additional signals downstream to p-AKT and involved in the neuroprotective effect of ischemic postconditioning. The results of this study support the idea that the enhancement of NCX3 expression and activity might be a reasonable strategy to reduce the infarct extension after a harmful ischemic insult. However, at the present time, compounds able to selectively enhance NCX3 expression or activity are not available.
Acknowledgments
The authors thank Mr Vincenzo Grillo and Mr Carmine Capitale for technical support. The authors express their gratitude to Dr Kenneth D Philipson and Dr Deborah A Nicoll (Department of Physiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA) for allowing them to use their invaluable rabbit polyclonal NCX3 antibody.
The authors declare no conflict of interest.
Footnotes
This study was supported by COFIN 2008; by ‘Ministero Affari Esteri, Direzione Generale per la Promozione e la Cooperazione Culturale Fondi Italia-Cina Legge 401/1990 2008' Ministero della Salute, Progetto ordinario 2008; Ministero della Salute, Ricerca Oncologica 2006; Ministero della Salute, Progetto Strategico 2007; Ministero della Salute, Progetto Ordinario 2007 (all to L Annunziato).
References
- Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Boscia F, Gala R, Pannaccione A, Secondo A, Scorziello A, Di Renzo G, Annunziato L. NCX1 expression and functional activity increase in microglia invading the infarct core. Stroke. 2009;40:3608–3617. doi: 10.1161/STROKEAHA.109.557439. [DOI] [PubMed] [Google Scholar]
- Boscia F, Gala R, Pignataro G, de Bartolomeis A, Cicale M, Ambesi-Impiombato A, Di Renzo G, Annunziato L. Permanent focal brain ischemia induces isoform-dependent changes in the pattern of Na+/Ca2+ exchanger gene expression in the ischemic core, periinfarct area, and intact brain regions. J Cereb Blood Flow Metab. 2006;26:502–517. doi: 10.1038/sj.jcbfm.9600207. [DOI] [PubMed] [Google Scholar]
- Burda J, Danielisova V, Nemethova M, Gottlieb M, Matiasova M, Domorakova I, Mechirova E, Ferikova M, Salinas M, Burda R. Delayed postconditionig initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006;26:1141–1151. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Future targets and cascades for neuroprotective strategies. Stroke. 2004;35:2748–2750. doi: 10.1161/01.STR.0000143325.25610.ac. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Formisano L, Saggese M, Secondo A, Sirabella R, Vito P, Valsecchi V, Molinaro P, Di Renzo G, Annunziato L. The two isoforms of the Na+/Ca2+ exchanger, NCX1 and NCX3, constitute novel additional targets for the prosurvival action of Akt/protein kinase B pathway. Mol Pharmacol. 2008;73:727–737. doi: 10.1124/mol.107.042549. [DOI] [PubMed] [Google Scholar]
- Gabellini N, Bortoluzzi S, Danieli GA, Carafoli E. Control of the Na+/Ca2+ exchanger 3 promoter by cyclic adenosine monophosphate and Ca2+ in differentiating neurons. J Neurochem. 2003;84:282–293. doi: 10.1046/j.1471-4159.2003.01511.x. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Linck B, Qiu Z, He Z, Tong Q, Hilgemann DW, Philipson KD. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3) Am J Physiol. 1998;274:C415–C423. doi: 10.1152/ajpcell.1998.274.2.C415. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Molinaro P, Cuomo O, Pignataro G, Boscia F, Sirabella R, Pannaccione A, Secondo A, Scorziello A, Adornetto A, Gala R, Viggiano D, Sokolow S, Herchuelz A, Schurmans S, Di Renzo G, Annunziato L. Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J Neurosci. 2008;28:1179–1184. doi: 10.1523/JNEUROSCI.4671-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Pignataro G, Gala R, Cuomo O, Tortiglione A, Giaccio L, Castaldo P, Sirabella R, Matrone C, Canitano A, Amoroso S, Di Renzo G, Annunziato L. Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke. 2004;35:2566–2570. doi: 10.1161/01.STR.0000143730.29964.93. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Scorziello A, Di Renzo G, Annunziato L. Post-ischemic brain damage: effect of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. FEBS J. 2009;276:46–57. doi: 10.1111/j.1742-4658.2008.06769.x. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Xiong Z, Simon RP.2006Ischemic post-conditioning: a new neuroprotective strategyProgram no. 760.3 2006 Neuroscience Meeting Planner. Atlanta, GA, USA: Society for Neuroscience, 2006 (online) [Google Scholar]
- Scartabelli T, Gerace E, Landucci E, Moroni F, Pellegrini-Giampietro DE. Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K-Akt signaling pathway: a novel postconditioning strategy. Neuropharmacology. 2008;55:509–516. doi: 10.1016/j.neuropharm.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Secondo A, Staiano RI, Scorziello A, Sirabella R, Boscia F, Adornetto A, Valsecchi V, Molinaro P, Canzoniero LM, Di Renzo G, Annunziato L. BHK cells transfected with NCX3 are more resistant to hypoxia followed by reoxygenation than those transfected with NCX1 and NCX2: possible relationship with mitochondrial membrane potential. Cell Calcium. 2007;42:521–535. doi: 10.1016/j.ceca.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Realizing the clinical potential of ischemic preconditioning and postconditioning. Nat Clin Pract Cardiovasc Med. 2005;2:568–575. doi: 10.1038/ncpcardio0346. [DOI] [PubMed] [Google Scholar]
- Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. J Neuroimmune Pharmacol. 2007;2:313–318. doi: 10.1007/s11481-007-9089-8. [DOI] [PubMed] [Google Scholar]
- Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–885. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006a;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006b;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]