Abstract
Intracellular Ca2+ release events (‘Ca2+ sparks') and transient activation of large-conductance Ca2+-activated potassium (BK) channels represent an important vasodilator pathway in the cerebral vasculature. Considering the frequent occurrence of cerebral artery constriction after subarachnoid hemorrhage (SAH), our objective was to determine whether Ca2+ spark and BK channel activity were reduced in cerebral artery myocytes from SAH model rabbits. Using laser scanning confocal microscopy, we observed ∼50% reduction in Ca2+ spark activity, reflecting a decrease in the number of functional Ca2+ spark discharge sites. Patch-clamp electrophysiology showed a similar reduction in Ca2+ spark-induced transient BK currents, without change in BK channel density or single-channel properties. Consistent with a reduction in active Ca2+ spark sites, quantitative real-time PCR and western blotting revealed decreased expression of ryanodine receptor type 2 (RyR-2) and increased expression of the RyR-2-stabilizing protein, FKBP12.6, in the cerebral arteries from SAH animals. Furthermore, inhibitors of Ca2+ sparks (ryanodine) or BK channels (paxilline) constricted arteries from control, but not from SAH animals. This study shows that SAH-induced decreased subcellular Ca2+ signaling events disable BK channel activity, leading to cerebral artery constriction. This phenomenon may contribute to decreased cerebral blood flow and poor outcome after aneurysmal SAH.
Keywords: cerebral aneurysm, FKBP12.6, potassium channels, ryanodine receptors, vascular smooth muscle, vasospasm
Introduction
Cerebral aneurysm rupture and the ensuing subarachnoid hemorrhage (SAH) has an enormous impact on individuals and society, with 30-day mortality rates approaching 50% and the majority of survivors left with moderate-to-severe disability (Hop et al, 1997). For decades, ‘angiographically defined' cerebral vasospasm of conduit arteries (>1 mm in diameter) has been considered to be the major contributor to death and disability in SAH patients surviving the initial intracranial bleed. However, recent evidence indicates that factors other than large-artery vasospasm contribute to SAH-induced pathologies (Macdonald et al, 2007). Additional factors contributing to the deleterious consequences of aneurysmal SAH may include global transient ischemia, early brain injury, disruption of the blood–brain barrier, and activation of inflammatory pathways (Ostrowski et al, 2006; Prunell et al, 2005). It has now been realized that SAH may also impact small-diameter arteries and arterioles, i.e., those involved in the autoregulation of cerebral blood flow (Hattingen et al, 2008; Ishiguro et al, 2002; Ohkuma et al, 2000).
In resistance arteries from healthy animals, vasoconstrictor stimuli such as physiologic increases in intravascular pressure lead to smooth muscle membrane potential depolarization, increased voltage-dependent Ca2+ channel (VDCC) activity, and elevated global cytosolic calcium (Knot and Nelson, 1998). Global cytosolic Ca2+ represents averaged Ca2+ levels throughout the cytoplasm and is a key regulator of smooth muscle contraction. One important dynamic negative feedback mechanism to limit vasoconstriction is the activation of large-conductance Ca2+- and voltage-sensitive potassium (BK) channels by Ca2+ sparks. Ca2+ sparks are localized Ca2+ release events occurring through ryanodine receptors (RyRs) in the sarcoplasmic reticulum (SR) abutting the plasma membrane. Ca2+ sparks oppose the contractile actions of global cytosolic Ca2+ by promoting smooth muscle relaxation by activation of plasmalemmal BK channels, leading to membrane potential hyperpolarization and decreased Ca2+ influx through VDCCs (Nelson et al, 1995; Wellman and Nelson, 2003). In the vasculature, functional BK channels are composed of pore-forming α1 subunits, encoded by the gene KCNMA1 and regulatory β1-subunits, encoded by the gene KCNMB1 (Tanaka et al, 1997). Loss-of- and gain-of-function polymorphisms of KCNMA1 and KCNMB1 have been linked to asthma and blood pressure regulation in humans (Kelley-Hedgepeth et al, 2009; Tomas et al, 2008). Furthermore, decreased KCNMB1 expression causes reduced BK channel Ca2+ and voltage sensitivity, and is linked to enhanced vasoconstriction, hypertension, and diabetes (Amberg and Santana, 2003; Brenner et al, 2000; Dong et al, 2008). These vascular pathologies have all been associated with a decrease in BK channel activity in response to Ca2+ sparks, rather than with a reduction in Ca2+ spark activity.
Decreased BK channel activity following SAH could lead to vasoconstriction and compromise cerebral autoregulation. However, BK channel properties and expression seem to be unaffected in the basilar arteries obtained from a canine SAH model (Jahromi et al, 2008b). In this study, we show that decreased BK channel activity does contribute to enhanced pressure-dependent constriction of resistance-sized cerebral arteries from SAH model rabbits. However, rather than reduced BK channel activity resulting from decreased KCNMA1 or KCNMB1 expression, we provide evidence that impaired BK channel activity results from a decrease in subcellular Ca2+ signaling from the SR to BK channels, i.e., reduced Ca2+ spark frequency. To our knowledge, these findings represent the first demonstration of a vascular pathology caused by a decrease in Ca2+ spark activity. This SAH-induced reduction in Ca2+ spark frequency reflects a decrease in the number of functional Ca2+ spark discharge sites caused by a decrease in the expression of SR RyR-2 Ca2+-release channels and an increase in the expression of the RyR-2-stabilizing protein, FKBP12.6. This novel pathway of decreased vascular BK channel activity may contribute to impaired autoregulation, reduced cerebral blood flow, and the development of neurologic deficits frequently observed in patients after aneurysmal SAH.
Materials and methods
Rabbit Subarachnoid Hemorrhage Model
New Zealand White rabbits (males, weighing 3.0 to 3.5 kg; Charles River Laboratories, Saint Constant, Quebec, Canada) were used for a double injection SAH model using surgical procedures described previously (Ishiguro et al, 2002; Ishiguro et al, 2005). In brief, under isoflurane anesthesia, a small midline suboccipital incision was centered over the foramen magnum and neck muscles dissected until the dura was visualized. Unheparinized autologous blood (2.5 mL) was then injected into the subarachnoid space through the cisterna magna. The animal was then positioned on an incline board at a 45° angle with the head down in neutral position for 30 minutes. To minimize increases in intracranial pressure, a similar volume of cerebral spinal fluid was removed before the injection of blood. Forty-eight hours later, the above procedures were repeated. Buprenophine (0.01 mg/kg) was administered every 12 hours for 36 hours after each surgery as an analgesic. Five days after the initial surgery, rabbits were killed by exsanguination under deep pentobarbital anesthesia (60 mg/kg, intravenous), and the posterior cerebral and cerebellar arteries (100 to 250 μm diameter) were dissected for in vitro studies in cold (4°C), oxygenated (20% O2/5% CO2/75%N2) physiologic saline solution (PSS) of the following composition (in mmol/L): 118.5 NaCl, 4.7 KCl, 24 NaHCO3, 1.18 KH2PO4, 2.5 CaCl2, 1.2 MgCl2, 0.023 EDTA, 11 glucose. Age-matched animals that did not undergo surgical procedures were used as controls. All protocols were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health (NIH) publication No. 85-23) and followed protocols approved by the Institutional Animal Care and Use Committee of the University of Vermont.
Electrophysiology
Transient BK Currents
Arteries were enzymatically dissociated to obtain individual smooth muscle cells as described previously (Ishiguro et al, 2005). Transient BK currents were measured using the perforated whole-cell patch-clamp technique at room temperature (RT) (Wellman et al, 2002). The composition of the HEPES-PSS bath solution was (in mmol/L): 134 NaCl, 6 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, and 10 HEPES (pH 7.4). Patch pipettes (8 to 10 MΩ) were filled with an internal solution that contained (in mmol/L) 110 K+ aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, 0.05 EGTA, and 0.2 amphotericin B (pH 7.2). Transient outward currents were recorded over a range of holding potentials from −40 to 0 mV (∼5 minutes at each membrane potential). Recordings were analyzed using the Mini Analysis 6.0.3 program (Synaptosoft, Inc., Decatur, GA, USA) to determine transient BK current amplitude, frequency, rise time (30% to 70%), and decay time (90% to 10%). The threshold for current peak detection was set at two and half times the single-channel amplitude of BK channel (e.g., 5.0 pA at −40 mV) (Perez et al, 2001).
Single-Channel BK Current Recordings
The BK single-channel currents were recorded in excised inside-out membrane patches at RT (Perez et al, 2001). The bath and pipette solution contained (in mmol/L): 140 KCl, 1 MgCl2, 5 EGTA, 1.9 CaCl2, and 10 HEPES (pH 7.2), with a calculated free Ca2+ concentration of 100 nmol/L (WinMax C software, Stanford University, Stanford, CA, USA; http://www.maxchelator.stanford.edu/). When CaCl2 was omitted from the bath solution (‘zero' Ca2+), free Ca2+ concentration was calculated to be <1 nmol/L. The number of channels per patch was determined at +80 mV in 100 nmol/L free Ca2+. Ca2+ and voltage sensitivity of BK channels were determined from Boltzman's fit of data.
Whole-Cell Voltage-Dependent K+ Current Recordings
Whole-cell K+ currents were measured using the conventional whole-cell configuration of the patch-clamp technique (Koide et al, 2007). The composition of bath solution was the same as the 6 mmol/L K+ HEPES-PSS described above. The internal solution contained (in mmol/L): 87 K+ aspartate, 20 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 EGTA, and 25 KOH (pH 7.2). From a holding potential of −70 mV, outward K+ currents were elicited by a series of 500 milliseconds depolarizing voltage steps, followed by a step to −40 mV for 300 milliseconds. Voltage steps were made at 10 mV increments to +50 mV at intervals of 10 seconds from a holding potential of −70 mV. All electrophysiologic studies were performed using Axopatch 200B, pCLAMP 9.2 and Clampfit 9.2 software (Axon Instruments Inc., Foster City, CA, USA).
Ca2+ Spark Measurements
Freshly isolated cerebral artery myocytes were incubated with the fluorescent Ca2+ indicator fluo-4 AM (10 μmol/L; Ex 488 nm, Em 520 nm; Invitrogen, Carlsbad, CA, USA) and 0.036% pluronic acid for 30 minutes at RT, followed by a brief wash with HEPES-PSS. Ca2+ sparks were detected as described previously using a Noran Oz laser scanning confocal system (Noran, Middleton, WI, USA) coupled to an inverted Nikon TMD microscope (Nikon, Melville, NY, USA) equipped with a × 60 water-immersion lens (N.A.1.2) (Perez et al, 2001). Images were acquired at a frequency of 58.3 Hz (approximately every 17.2 milliseconds) for 20 seconds at RT. Fractional fluorescent changes (F/F0) were analyzed in 2.1 × 2.1 μm2 analysis areas using custom software, and F/F0 changes >1.3 defined as Ca2+ sparks. F0 was obtained by averaging 30 images containing no Ca2+ events.
RNA Isolation and Quantitative Real-Time Reverse Transcription-PCR
Total RNA was extracted from the posterior cerebral arteries (100 to 250 μm in diameter, ∼1 mg wet weight) using RNA STAT-60 (Tel-Test Inc., Friendswoods, TX, USA) and cDNA was synthesized by SuperScript First-Strand Synthesis System (Invitrogen). The primer sets were designed using Primer-BLAST (NIH, Bethesda, MD, USA) for a unique region of targeted mRNA sequence and are detailed in the Online Supplementary information (Table 1). The expression of mRNA was quantified by real-time PCR using SYBR Green JumpStart Taq ReadyMix and a real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). Quantification was performed using standard curves constructed by amplification of serially diluted plasmids containing target genes, and the threshold cycle value for each sample was used to calculate the initial quantity of the cDNA template. The results of quantitative real-time PCR were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression.
Western Blotting
Freshly isolated cerebral arteries were homogenized in buffer containing (in mmol/L) 100 NaCl, 20 Tris-HCl (pH 7.4), 1 EDTA, 1 EGTA, 5 dithiothreitol, 1 phenylmethylsulfonyl fluoride, 0.1 leupeptin, and 1% Triton-X using glass microgrinders. After sonication for 10 minutes on iced water, tissue debris was removed by centrifugation (8000 × g, 5 minutes). The protein concentration of lysate was measured by modified Bradford assay (Coomassie Plus; Pierce, Rockford, IL, USA) using bovine serum albumin as a standard. The lysate (20 μg of protein) was mixed with 5 × loading buffer (625 mmol/L Tris-HCl; pH 6.8, 20% SDS, and 25% glycerol), and incubated at 37°C for 15 minutes. Proteins were separated using 4% to 20% acrylamide gradient gels, and electrophoretically transferred onto a nitrocellulose membrane. After blocking the membrane with 5% nonfat milk (1 hour at RT), the membrane was incubated overnight with primary antibody at 4°C. After incubation with the appropriate secondary antibody for 1 hour at RT, signals were detected by chemiluminescence for RYR-2 or by an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA) for FKBP12.6 and α-smooth muscle actin. Bands depicting each protein were analyzed using ImageJ software (NIH). Antibodies were used as follows: anti-RyR-2 mouse monoclonal antibody (clone C3-33, 1:200; ABR, Golden, CO, USA), anti-FKBP12.6 goat polyclonal antibody (1:200, Santa Cruz, Santa Cruz, CA, USA), anti-α smooth muscle actin mouse monoclonal antibody (1:100,000, Sigma, St Louis, MO, USA), peroxidase-conjugated sheep anti-mouse IgG (1:5,000, GE Healthcare, Piscataway, NJ, USA), IRdye700-conjugated donkey anti-goat IgG and IRdye800-conjugated goat anti-mouse IgG (1:10,000, Rockland, Gilbertsville, PA, USA).
Diameter Measurements in Isolated Arteries
Freshly isolated arteries from control and SAH rabbits were cannulated in a 5 mL myograph chamber (Living Systems Instrumentation, Inc., Burlington, VT, USA) and perfused with PSS (pH 7.4) aerated with 20% O2/5% CO2/75% N2 at 37°C, as described previously (Ishiguro et al, 2002). The arterial diameter was measured using video edge detection equipment and recorded using data acquisition software (Dataq Instruments, Inc., Akron, OH, USA). Arteries were discarded if an initial constriction representing <50% decrease in diameter was observed when arteries were exposed to elevated extracellular K+ (60 mmol/L). Arterial constrictions to paxilline and ryanodine are expressed as a percentage decrease in diameter from the basal level of pressure-induced myogenic tone at an intravascular pressure of 80 mm Hg. Vasodilation to cumulative additions of acetylcholine and sodium nitroprusside are expressed as percentage dilation of pressure-induced (myogenic) tone. The fully dilated (passive) diameter was determined at the end of each experiment by exposing the arteries to Ca2+-free PSS containing diltiazem (100 μmol/L) and forskolin (1 μmol/L).
Measurement of Cytosolic Ca2+ in Pressurized Cerebral Arteries
Freshly isolated cerebral arteries were cannulated and loaded with the ratiometric Ca2+-sensitive fluorescent dye, fura-2 AM (5 μmol/L; Invitrogen), with 0.1% pluronic acid for 45 minutes at RT. To allow for equilibration and deesterification of fura-2 AM, the arteries were superfused with PSS at 37°C for 30 minutes. After the equilibration period, fluorescent ratio (R) was obtained from background corrected 510 nm emission from arterioles alternately excited at 340 and 380 nm using software developed by IonOptix Inc. (Milton, MA, USA). Cytosolic Ca2+ concentration was estimated using the following equation (Grynkiewicz et al, 1985): [Ca2+]=Kd × β × (R−Rmin)/(Rmax−R). Calibration values were not significantly different between the arteries from control and SAH animals and were pooled. Rmin (0.46±0.02) and Rmax (10.80±0.66) represent the ratios of emission signal under Ca2+-free and Ca2+-saturated conditions, respectively, and were obtained from separate sets of arteries in the presence of ionomycin (10 μmol/L) and nigericin (5 μmol/L). The ratio of Ca2+-free over Ca2+-bound fluorescence intensities at F380, β (11.5±0.70), was obtained from Rmin and Rmax measurements. An apparent dissociation constant (Kd) of 282 nmol/L of fura-2 for Ca2+ was used (Knot and Nelson, 1998).
Statistical Analysis
Data are expressed as mean±s.e.m. and analyzed by Student's unpaired t-test or one-way ANOVA (analysis of variance), followed by Tukey's multiple comparison test. Statistical significance was considered at the level of P<0.05 (*) or P<0.01 (**).
Results
Transient BK Channel Currents are Decreased after Subarachnoid Hemorrhage
At physiologic membrane potentials (e.g., −40 mV), micromolar increases in cytosolic Ca2+ are required to induce significant BK channel activation (Perez et al, 2001). In cerebral artery myocytes, subcellular Ca2+ signaling events (Ca2+ sparks) lead to localized elevations of Ca2+ sufficient to cause the transient activation of nearby BK channels. Ca2+ spark-induced transient BK currents were recorded using the perforated patch whole-cell configuration of the patch-clamp technique (Figure 1). At −40 mV, transient BK current frequency, but not amplitude, was decreased ∼60% in cerebral artery myocytes freshly isolated from SAH model rabbits. Membrane potential depolarization increased the frequency and amplitude of transient BK currents to a similar extent in myocytes from both control and SAH animals, i.e., frequency was ∼60% lower at all voltages in cells from the SAH group. Temporal characteristics of these events (rise time and decay time) were similar between groups, as was cell size as indexed by cell capacitance (Online Supplementary information (Table 2)). These data show a dramatic decrease in the frequency of Ca2+ spark-induced BK channel activity after experimental SAH.
Figure 1.
Decreased frequency of transient outward BK channel currents in cerebral artery myocytes from SAH model rabbits. (A) Representative traces of transient BK currents in cerebral artery myocytes from control and SAH rabbits. Holding potential was changed in 10 mV increments approximately every 5 minutes. (B) Expanded traces are from the region denoted by black bars in panel A. (C) Average traces were obtained from all events at −40 mV shown in panel A (control: 52 events, SAH: 18 events). (D, E) Summarized data of transient BK current frequency and amplitude, respectively. Control: n=9 cells from 6 animals; SAH: n=8 from 6 animals. *P<0.05, **P<0.01. BK, large-conductance Ca2+-activated potassium; SAH, subarachnoid hemorrhage.
Maintained Expression and Properties of BK Channels in Cerebral Artery Myocytes after Subarachnoid Hemorrhage
The apparent SAH-induced decrease in transient BK current frequency could conceivably reflect a fundamental change in voltage or Ca2+ sensitivity or in the expression of the BK channel after SAH. To address this issue, single BK channel currents were measured in excised ‘inside-out' membrane patches. The BK channel open-state probability (PO) and voltage dependence with 100 nmol/L intracellular free Ca2+ were not altered after SAH and a similar right-ward shift in voltage-dependent activation was observed in BK channels from both groups exposed to intracellular solution containing <1 nmol/L free Ca2+ (Figures 2A and 2B). Single-channel slope conductance was similar for channels from control (231.7±3.6 pS, n=9) and SAH (231.6±3.5 pS, n=9) animals, consistent with previous reports for BK channels (Perez et al, 2001). Furthermore, the density of functional BK channels detected in membrane patches was not different between groups (Figure 2C). To view the ensemble behavior of BK channels across the membrane of the entire cell, voltage-dependent K+ currents were measured during depolarizing voltage steps using conventional whole-cell patch-clamp electrophysiology. In this study, [Ca2+]i was maintained below 25 nmol/L by cell dialysis with 10 mmol/L of the Ca2+ chelator, EGTA, included in the intracellular (pipette) solution. Ca2+ spark or RyR activation of BK currents does not occur using this approach and the amplitude of conventional whole-cell BK currents reflects plasma membrane BK channel density and their open-state probability at the applied voltage and [Ca2+]i. Steady-state outward currents sensitive to the BK channel blocker paxilline (1 μmol/L, 10 minutes) were similar in myocytes from control and SAH animals (Figure 2D). To confirm that expression of BK channel pore-forming α-subunits and regulatory β1-subunits are maintained in our SAH model, quantitative real-time PCR was performed on RNA extracted from intact cerebral arteries. Both BK channel α- and β1-subunit mRNA, expressed as a ratio to GAPDH mRNA, were not significantly different between control and SAH animals (Figure 2E, n=6 for each group). These data suggest that the SAH-induced decrease in the frequency of transient BK currents is not caused by a decrease in the number or functionality of BK channels.
Figure 2.
Maintained expression and properties of BK channel currents in cerebral artery myocytes after SAH. (A) Representative BK single-channel recordings from inside-out membrane patches in the presence of 100 nmol/L free Ca2+. Closed state (C) and individual channel open states (O1 to O5) are included in each record. (B) Ca2+ and voltage sensitivity of BK channels in control (n=6 to 16 patches from 4 animals) and SAH myocytes (n=12 to 19 patches from 4 animals). Free Ca2+ concentration calculated for the ‘Zero' Ca2+ solution was <1 nmol/L. Open-state probability curves were obtained from Boltzman's fit of data. (C) BK channel densities shown as channel number per patch at +80 mV in control (n=25) and SAH cerebral myocytes (n=27). NS: P>0.05. (D) Current–voltage relationship of paxilline-sensitive currents in myocytes from control (open circles, n=5) and SAH rabbits (closed circles, n=6). (E) Summary of quantitative real-time PCR for α- and β1-subunits of the BK channel, normalized to GAPDH. BK, large-conductance Ca2+-activated potassium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SAH, subarachnoid hemorrhage.
Ca2+ Spark Frequency is Decreased in Subarachnoid Hemorrhage Model Animals
The BK channel properties and expression appear unaltered in smooth muscle cells from the cerebral arteries of SAH animals. Therefore, we explored the possibility that the lower transient BK current frequency reflects a fundamental decrease in Ca2+ spark activity. Ca2+ sparks were optically measured in freshly isolated cerebral artery myocytes from control and SAH rabbits using laser scanning confocal microscopy and the fluorescent Ca2+ indicator, fluo-4. Ca2+ sparks were observed in cells from both control and SAH animals (Figure 3A, also see movies included within the Online Supplementary data). However, as with the frequency of transient BK currents, whole-cell Ca2+ spark frequency was dramatically lower (∼50%) in myocytes isolated from SAH animals (SAH: 0.38±0.04 Hz; control: 0.76±0.05 Hz, Figure 3B). Although frequency was decreased, Ca2+ spark amplitude, expressed as a fractional change in fluorescence intensity (F/F0), was similar in myocytes isolated from control and SAH animals (Figure 3C). Other spatio-temporal characteristics such as rise time, duration, size, and decay were similar for Ca2+ sparks recorded from myocytes of control and SAH animals (Online Supplementary information (Table 2)).
Figure 3.
Decreased Ca2+ spark frequency, but not amplitude, in cerebral artery myocytes from SAH animals. (A) Representative Ca2+ spark images and fractional fluorescent traces from cerebral artery myocytes isolated from control and SAH rabbits. Gray scale images are an average of 30 images without Ca2+ spark activity (F0 image). Red crosses depict where individual Ca2+ sparks occurred during the 20-second recordings. Pseudo-color images illustrate Ca2+ sparks in control and SAH myocytes. Fractional fluorescent (F/F0) records are from 2.1 × 2.1 μm2 analysis areas (white boxes in first enlarged images) centered over Ca2+ sparks. White bars represent 10 μm. (B, C) Summarized data of whole-cell frequency and amplitude, respectively, of Ca2+ sparks in cerebral artery myocytes (control: n=45 cells from 5 animals, SAH: n=48 cells from 6 animals). **P<0.01. SAH, subarachnoid hemorrhage.
A Reduction in Functional Ca2+ Spark Sites Underlies Decreased Ca2+ Spark Frequency in Subarachnoid Hemorrhage Model Animals
In the smooth muscle, Ca2+ sparks tend to occur repeatedly within a limited number of distinct areas or spark sites (Janiak et al, 2001; Pucovsky and Bolton, 2006). The SAH-induced decrease in Ca2+ spark frequency could reflect either a reduction in the number of functional spark sites or a decrease in the frequency of events occurring at all spark sites throughout the cell. Spark sites were defined as regions where one or more Ca2+ sparks were observed during a 20-second imaging period (Figures 4A–4C). Two or more Ca2+ sparks were considered to originate from the same spark site if these events exhibited a >50% overlap of their spatial spread (determined at 50% peak amplitude). In 45 cerebral artery myocytes from 5 control animals, a total of 1082 Ca2+ sparks were observed with an average of 7.9±0.6 spark sites per cell. However, in myocytes from SAH animals, the number of active spark sites was decreased by ∼43% to 4.5±0.4 (n=594 sparks in 48 cells from 6 animals, Figure 4D). Although the number of functional spark sites was decreased, Ca2+ spark frequency at individual sites was similar in cells isolated from control and SAH animals (Figure 4E). Furthermore, the correlation between the number of functional spark sites and whole-cell Ca2+ spark frequency was similar between groups (Figure 4F). These data suggest SAH-induced decreased Ca2+ spark frequency reflects a reduction in the number of functional spark sites.
Figure 4.
Decreased functional Ca2+ spark sites in cerebral artery myocytes from SAH animals. (A) Gray scale images are an average of 30 images without Ca2+ spark activity (F0 image) with red crosses depicting the location of individual Ca2+ sparks during 20-second recordings. White boxes on pseudo-color images depict distinct spark sites within each cell. (B) Numbered images depict individual spark sites within each cell. (C) Corresponding fractional fluorescent (F/F0) traces from spark sites shown in panel B. Arrows indicate Ca2+ sparks accounted to each site. (D) Summarized data of the number of spark sites observed in each cell. (E) Average Ca2+ spark frequency in individual spark sites. (F) Relationship between the number of spark sites in individual cells and whole-cell Ca2+ spark frequency. Solid lines with symbol color (control: gray, SAH: black) represent linear regression analysis of data. Data were obtained from 1,082 sparks from 355 spark sites in 45 cells from 5 control animals and 594 sparks from 217 spark sites in 48 cells from 6 SAH animals. **P<0.01. SAH, subarachnoid hemorrhage.
Ryanodine Receptor Type 2 Expression is Decreased and FKBP12.6 Expression is Increased in the Cerebral Arteries from Subarachnoid Hemorrhage Animals
It is conceivable that the SAH-induced decrease in functional spark sites could reflect either an increase in the activation threshold of RyRs or decreased SR Ca2+ load. To assess RyR activity, Ca2+ sparks were examined in the presence of a relatively low concentration (10 μmol/L) of the RyR activator, caffeine. Caffeine lowers the luminal Ca2+ threshold for RyR activation (Kong et al, 2008), and at micromolar concentrations causes an increase in both Ca2+ spark frequency and the number of active spark sites in the smooth muscle (Janiak et al, 2001; Wellman et al, 2001). In absolute terms, the number of active Ca2+ spark sites and whole-cell Ca2+ spark frequency in the presence of caffeine (10 μmol/L) remained decreased in myocytes isolated from SAH animals (Figures 5A–5C; control: n=7 cells from 3 animals, SAH: n=14 cells from 3 animals). However, on a relative basis, i.e., when expressed as a percentage of basal activity, treatment with caffeine (10 μmol/L) increased the number of active spark sites by ∼50% in cerebral artery myocytes from both control and SAH animals. Corresponding to the increase in the number of active spark sites, caffeine (10 μmol/L) also caused a similar percentage increase in whole-cell Ca2+ spark frequency in cerebral artery myocytes from both groups. The SR Ca2+ content was also examined in the cerebral arteries isolated from control and SAH animals using the ratiometric fluorescent Ca2+-sensitive dye, fura-2. Decreased SR Ca2+ content has been reported to decrease whole-cell Ca2+ spark frequency and reduce the amplitude of individual Ca2+ sparks (ZhuGe et al, 1999). Rapid application of high concentrations (millimolar) of caffeine leads to a significant release of Ca2+ from the SR through activation of RyRs. The relative amplitude of these caffeine-induced global Ca2+ transients has been used as an index of SR Ca2+ content (Santana et al, 1997; Wellman et al, 2001). The amplitudes of caffeine-induced global Ca2+ transients were not significantly different in the cerebral arteries from control and SAH model animals (Figure 5D). Furthermore, quantitative real-time PCR showed that mRNA levels of the SR Ca2+-binding proteins calsequestrin-2 and calreticulin and the smooth muscle sarco/endoplasmic reticulum Ca2+-ATPase, SERCA-2 were not significantly different in the arteries from control and SAH animals (Figure 5E; n=6 for each group). These data suggest that RyR activation properties and SR Ca2+ load in myocytes from SAH animals are unaltered, and that increasing RyR activity does not restore the number of functional spark sites to control levels.
Figure 5.
RyR activation properties and SR Ca2+ load in the cerebral arteries from SAH animals. (A to C): The RyR activator, caffeine (10 μmol/L), did not restore the number of functional spark sites or whole-cell Ca2+ spark frequency in cerebral artery myocytes from SAH animals to control levels. (Panel A) Average of 30 consecutive images without Ca2+ spark activity, with white crosses depicting the location of Ca2+ sparks observed during 20-second recordings in the absence and presence (+Caffeine) of caffeine. White bars represent 10 μm. (Panels B and C) Summary of the effect of caffeine on (panel B) the number of functional spark sites per cell and (panel C) whole-cell Ca2+ spark frequency. Control: n=7 cells from 3 animals, SAH: n=14 cells from 3 animals. *P<0.05, **P<0.01 control versus SAH. (D) Estimated Ca2+ content in the sarcoplasmic reticulum (SR) of the cerebral arteries from control and SAH animals. The amplitude of global cytosolic Ca2+ transients induced by rapid application of a high concentration of caffeine (10 mmol/L) was not significantly different between groups (control: n=4, SAH: n=3). (E) Summarized data of quantitative real-time PCR for smooth muscle sarco-endoplasmic reticulum Ca2+-ATPase (SERCA-2), calsequestrin-2, and calreticulin expressed as a ratio to GAPDH (n=6 for each group). **P<0.01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RyR, ryanodine receptor; SAH, subarachnoid hemorrhage.
The RyR-2 subtype is the dominantly expressed RyR isoform in the arterial smooth muscle, and is believed to underlie Ca2+ spark activity in these cells (Ji et al, 2004). The activity of RyR-2 is modulated by the FK506-binding protein, FKBP12.6, which stabilizes RyR-2 channels in the closed state (Zalk et al, 2007). Conditional overexpression of FKBP12.6 has been reported to decrease Ca2+ spark frequency in cardiac myocytes (Gellen et al, 2008). Thus, decreased expression of RyR-2 or increased expression of FKBP12.6 may contribute to a reduction in Ca2+ spark activity after SAH. To examine whether RyR-2 expression was decreased after SAH, quantitative real-time reverse transcription-PCR was performed on RNA extracted from intact cerebral arteries (Figure 6A). When normalized to GAPDH, RyR-2 expression was decreased by ∼65% in the cerebral arteries from SAH animals compared with controls (n=6 for each group). Protein levels of RyR-2, detected by western blot, were also decreased by ∼50% in cerebral artery homogenates from SAH rabbits (Figures 6B and 6C; n=4 for each group). Interestingly, we also observed that FKBP12.6 mRNA expression was increased twofold (n=5) and protein levels were over fourfold greater (n=4) in cerebral artery homogenates from SAH compared with control animals (Figure 6). These data are consistent with decreased RyR-2 expression and increased FKBP12.6 expression contributing to a reduction in functional spark sites and decreased Ca2+ spark frequency in myocytes from SAH animals.
Figure 6.
Decreased expression of ryanodine receptor-2 (RyR-2) and increased expression of FKBP12.6 in the cerebral arteries from SAH animals. (A) Summarized quantitative real-time PCR data for RyR-2 (n=6) and FKBP12.6 (n=5). **P<0.01 (B) Immunoreactive bands corresponding to RyR-2, FKBP12.6, and α-smooth muscle actin in the cerebral arteries detected by western blot. (C) Summarized RyR-2 and FKBP12.6 protein levels expressed as a ratio to α-smooth muscle actin (n=4 for each groups). *P<0.05. SAH, subarachnoid hemorrhage.
Decreased Ryanodine Receptor and BK Channel Activity Contribute to Enhanced Cerebral Artery Constriction after Subarachnoid Hemorrhage
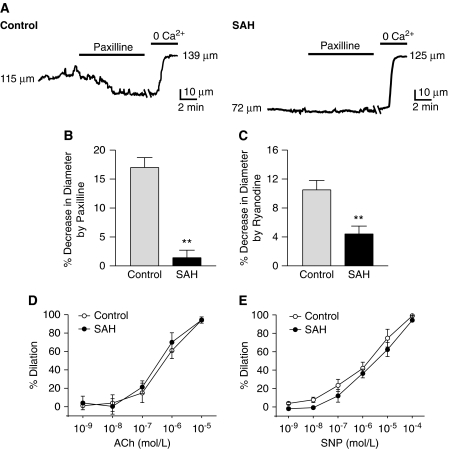
At physiologic intravascular pressures (e.g., 80 mm Hg), constriction was greater in the cerebral arteries isolated from SAH (34.7%±2.7% decrease in diameter, n=9) compared with control (18.5%±2.6% decrease in diameter, n=9) animals. To examine whether SAH-induced decreased Ca2+ spark and transient BK current activity contribute to enhanced cerebral artery constriction, arterial diameter measurements were made in the presence and absence of paxilline, a blocker of BK channels and ryanodine, a blocker of RyRs (Figures 7A–7C and Online Supplementary information (Table 3)). At an intravascular pressure of 80 mm Hg, paxilline (1 μmol/L) caused a robust constriction in the arteries from control animals, decreasing the diameter from 94.6±10.2 to 78.4±8.4 μm, or by 17.1% (Figure 7B). Thus, with the arteries from control animals, the BK channel block mimicked SAH to enhance constriction. In contrast, paxilline did not significantly constrict the arteries from SAH animals (diameter before paxilline: 96.6±8.1 μm; diameter in the presence of paxilline 95.5±8.6 μm), consistent with SAH causing a decrease in Ca2+ spark-induced BK channel activity. In a similar manner, the inhibitor of Ca2+ sparks, ryanodine (10 μmol/L), caused a significantly greater constriction in the arteries from control compared with SAH animals (Figure 7C). Also consistent with SAH-induced decreased BK channel activity and enhanced vasoconstriction, global cytosolic Ca2+, measured using fura-2, was elevated in the cerebral arteries from SAH animals (F340/F380: 1.18±0.04; estimated Ca2+: 222±12 nmol/L, n=4) compared with control animals (F340/F380: 1.00±0.07; estimated Ca2+: 175±12 nmol/L, n=4). In contrast to the SAH-induced disruption of the Ca2+ spark/BK channel dilator pathway, dilations to the endothelial-dependent agonist, acetylcholine, and the endothelial-independent dilator, sodium nitroprusside, were similar in the arteries from control and SAH animals (Figures 7D and E). These data are consistent with decreased Ca2+ spark-induced BK channel activity contributing to enhanced constriction of the cerebral arteries from SAH animals.
Figure 7.
Inhibitors of Ca2+ sparks and BK channels constrict the cerebral arteries from control, but not SAH model animals. (A) Diameter recordings of pressurized (80 mm Hg) cerebral arteries from control (left) and SAH (right) animals treated with the BK channel blocker paxilline (1 μmol/L). Maximum dilation was obtained using a combination of diltiazem (100 μmol/L) and forskolin (1 μmol/L) in Ca2+-free physiologic saline solution. (B, C) Summarized data of constriction caused by paxilline (panel B, n=5) or ryanodine (panel C, n=4), expressed as a percentage decrease in diameter, of pressurized arteries isolated from control and SAH animals. (D) Vasodilation to cumulative additions of acetylcholine (ACh) is expressed as percentage dilation of pressure-induced (myogenic) tone. Data expressed as mean±s.e.m.; n=7 control, n=5 SAH. (E) Vasodilation to cumulative additions of sodium nitroprusside (SNP) is expressed as percentage dilation of pressure-induced (myogenic) tone. Data are expressed as mean±s.e.m.; n=4 control, n=4 SAH. BK, large-conductance Ca2+-activated potassium; SAH, subarachnoid hemorrhage.
Discussion
In this study, we provide evidence that a reduction in subcellular Ca2+ release events (i.e., Ca2+ sparks) underlies decreased BK channel activity and enhanced cerebral artery constriction after SAH. The following observations support this concept: (1) cell-wide Ca2+ spark frequency is decreased in cerebral artery myocytes from SAH animals, reflecting a decrease in active Ca2+ spark discharge sites; (2) mRNA and protein levels of RyR-2, the SR Ca2+ release channel responsible for Ca2+ sparks in the smooth muscle, is reduced in cerebral artery myocytes after SAH; (3) mRNA and protein levels of the RyR-2-stabilizing protein, FKBP12.6, is increased in the cerebral arteries from SAH animals; (4) the frequency of Ca2+ spark-induced transient BK currents is decreased in cerebral artery myocytes from SAH animals, with no change in BK channel properties, whole-cell BK currents, or BK subunit expression; (5) constriction caused by inhibitors of Ca2+ sparks (ryanodine) or BK channels (paxilline) is decreased in the cerebral arteries from SAH animals. Taken together, these data suggest that SAH-induced decreased Ca2+ spark frequency and the resulting decrease in transient BK channel activity promotes membrane potential depolarization, enhanced Ca2+ influx by VDCCs, and cerebral artery constriction.
To our knowledge, these findings represent the first demonstration of a vascular pathology caused by a decrease in Ca2+ spark frequency. Although altered frequency of Ca2+ spark-induced transient BK currents has been reported in other cardiovascular diseases such as hypertension and hypovolemic shock, these phenomena have been attributed to altered BK channel function/expression. Enhanced constriction associated with hypertension and diabetes has been linked to decreased expression of the BK channel β1-subunit (Amberg and Santana, 2003; Brenner et al, 2000; Dong et al, 2008), whereas an acute upregulation of BK channel β1-subunit contributes to vasodilation and the decrease in peripheral resistance associated with hemorrhagic shock (Zhao et al, 2007). In the above-mentioned pathologies, Ca2+ spark activity was unaltered. In marked contrast, we observed a parallel decrease in the frequency of Ca2+ sparks and Ca2+ spark-induced transient BK currents after SAH. Notably, experimental SAH did not directly alter BK channel properties or expression. Our observed lack of direct effect of SAH on BK channels in the pial arteries obtained from SAH model rabbits is consistent with observations using basilar artery myocytes obtained from a dog SAH model (Jahromi et al, 2008b).
We found that the overall decrease in Ca2+ spark frequency in myocytes from SAH animals reflected a decrease in the number of functional spark sites. Both the number of functional spark sites and the whole-cell Ca2+ spark frequency were reduced by ∼50% in myocytes after SAH. Although the RyR activator, caffeine (10 μmol/L), caused proportionately the same increase in the number of functional spark sites and Ca2+ spark frequency in myocytes from control and SAH animals, both parameters remained ∼50% lower in SAH animals. Spark sites within arterial myocytes comprise a variable number of RyRs, and functional spark sites require the activity of a critical number of RyRs for Ca2+-induced Ca2+ release and for Ca2+ sparks to occur (Janiak et al, 2001; Pucovsky and Bolton, 2006). Using quantitative real-time PCR and western blot, we observed a significant reduction in mRNA and protein levels of RyR-2 in the cerebral arteries after SAH. A decrease in RyR-2 expression has been previously reported in cardiac myocytes following heart failure (Go et al, 1995) and in the vascular smooth muscle undergoing phenotypic changes during cell culture (Berra-Romani et al, 2008). It is conceivable that the SAH-induced decreased RyR-2 expression caused some, but not all, spark sites to no longer have the threshold number of RyRs required for Ca2+ spark initiation.
In addition to decreased RyR-2 expression, increases in the relative expression of proteins that reduce RyR-2 activity may also contribute to the observed decrease in the number of functional Ca2+ spark sites. For example, we observed increased mRNA and protein levels of the RyR-2 regulatory protein, FKBP12.6. Binding of FKBP12.6 stabilizes RyR-2 in the closed state (Zalk et al, 2007). Ablation of the gene encoding FKBP12.6 has been reported to increase Ca2+ spark frequency in the smooth muscle (Ji et al, 2004) and conversely, conditional overexpression of FKBP12.6 has been linked to decreased Ca2+ spark frequency in cardiac myocytes (Gellen et al, 2008). Our current data suggest that increased FKBP12.6 expression acting in concert with decreased RyR-2 expression underlie the decrease in active Ca2+ sparks in cerebral artery myocytes from SAH model animals. On the basis of this study, the relative contribution of decreased RyR-2 expression versus increased FKBP12.6 expression to the observed SAH-induced reduction in Ca2+ spark activity is unclear.
Decreased SR Ca2+ content has also been linked to decreased Ca2+ spark frequency and amplitude in the smooth muscle (ZhuGe et al, 1999); thus, reduced SR Ca2+ could potentially contribute to decreased Ca2+ spark frequency after SAH. However, our data argue against this possibility. First, we observed no difference in the amplitude of global Ca2+ transients induced by rapid application of a high concentration (10 mmol/L) of caffeine after SAH. Second, we observed a reduction in the number of functional Ca2+ spark sites, rather than a uniform reduction in Ca2+ spark frequency, and Ca2+ spark amplitude was similar in myocytes from control and SAH animals. Furthermore, mRNA levels of SR-Ca2+-ATPase (SERCA-2) and the SR Ca2+-binding proteins, calsequestrin-2 and calreticulin, were similar in the cerebral arteries from control and SAH animals. It is possible that additional factors such as RyR-2 phosphorylation (Zalk et al, 2007) could contribute to a reduction in functional Ca2+ spark sites in cerebral artery myocytes after SAH.
This study may help to explain the impact of SAH leading to enhanced constriction of small-diameter cerebral arteries and decreased cerebral blood flow. We have reported an increase in VDCC current density, in part owing to the emergence of R-type VDCCs encoded by the gene CaV2.3, in cerebral artery myocytes from SAH model rabbits (Ishiguro et al, 2005). Membrane potential depolarization caused by a decrease in Ca2+ spark-induced transient BK currents would be predicted to cause an increase in the open-state probability of VDCCs and combined with enhanced VDCC expression would increase global cytosolic Ca2+ leading to enhanced contraction of cerebral artery myocytes. Additional mechanisms such as decreased voltage-dependent potassium (KV) channel expression (Jahromi et al, 2008a) and activity (Ishiguro et al, 2006; Koide et al, 2007; Quan and Sobey, 2000) and increased protein kinase C activity (Nishizawa et al, 2000) are also likely to contribute to enhanced cerebral artery constriction after SAH.
In conclusion, we report that the frequency of Ca2+ sparks and associated transient BK currents were significantly decreased in cerebral artery myocytes after SAH, contributing to enhanced vasoconstriction. Our findings suggest this SAH-induced decrease in Ca2+ spark frequency results from a reduction in RyR-2 expression and increased FKBP12.6 expression, leading to a decrease in the number of functional spark sites within these myocytes. This mechanism may contribute to a reduction in cerebral blood flow and the development of neurologic deficits experienced by patients after cerebral aneurysm rupture. Furthermore, this mechanism of decreased vascular BK channel activity may also contribute to other types of brain pathologies, including traumatic brain injury.
Acknowledgments
The authors thank Ms Sheila Russell for her assistance and acknowledge the University of Vermont Neuroscience COBRE molecular biology and imaging core facilities.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the Totman Medical Research Trust Fund, the Peter Martin Brain Aneurysm Endowment, the NIH (R01 HL078983, R01 HL078983-05S1, R01 HL44455, R37 DK053832, R01 DK065947, R01 HL098243, R01 HL077378, NCRR P20 RR16435, and P01 HL095488), and the American Heart Association (0725837T, 0725841T, 0815736D).
Supplementary Material
References
- Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–C790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Dong L, Zheng YM, Van Riper D, Rathore R, Liu QH, Singer HA, Wang YX. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. J Cereb Blood Flow Metab. 2008;28:377–386. doi: 10.1038/sj.jcbfm.9600536. [DOI] [PubMed] [Google Scholar]
- Gellen B, Fernandez-Velasco M, Briec F, Vinet L, LeQuang K, Rouet-Benzineb P, Benitah JP, Pezet M, Palais G, Pellegrin N, Zhang A, Perrier R, Escoubet B, Marniquet X, Richard S, Jaisser F, Gomez AM, Charpentier F, Mercadier JJ. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation. 2008;117:1778–1786. doi: 10.1161/CIRCULATIONAHA.107.731893. [DOI] [PubMed] [Google Scholar]
- Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hattingen E, Blasel S, Dettmann E, Vatter H, Pilatus U, Seifert V, Zanella FE, Weidauer S. Perfusion-weighted MRI to evaluate cerebral autoregulation in aneurysmal subarachnoid haemorrhage. Neuroradiology. 2008;50:929–938. doi: 10.1007/s00234-008-0424-4. [DOI] [PubMed] [Google Scholar]
- Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–664. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Morielli AD, Zvarova K, Tranmer BI, Penar PL, Wellman GC. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Puryear CB, Bisson E, Saundry CM, Nathan DJ, Russell SR, Tranmer BI, Wellman GC. Enhanced myogenic tone in cerebral arteries from a rabbit model of subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol. 2002;283:H2217–H2225. doi: 10.1152/ajpheart.00629.2002. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Wellman TL, Honda A, Russell SR, Tranmer BI, Wellman GC. Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ Res. 2005;96:419–426. doi: 10.1161/01.RES.0000157670.49936.da. [DOI] [PubMed] [Google Scholar]
- Jahromi BS, Aihara Y, Ai J, Zhang ZD, Nikitina E, Macdonald RL. Voltage-gated K+ channel dysfunction in myocytes from a dog model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2008a;28:797–811. doi: 10.1038/sj.jcbfm.9600577. [DOI] [PubMed] [Google Scholar]
- Jahromi BS, Aihara Y, Ai J, Zhang ZD, Weyer G, Nikitina E, Yassari R, Houamed KM, Macdonald RL. Preserved BK channel function in vasospastic myocytes from a dog model of subarachnoid hemorrhage. J Vasc Res. 2008b;45:402–415. doi: 10.1159/000124864. [DOI] [PubMed] [Google Scholar]
- Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C22–C33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- Ji G, Feldman ME, Greene KS, Sorrentino V, Xin HB, Kotlikoff MI. RYR2 proteins contribute to the formation of Ca2+ sparks in smooth muscle. J Gen Physiol. 2004;123:377–386. doi: 10.1085/jgp.200308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley-Hedgepeth A, Peter I, Montefusco MC, Levy D, Benjamin EJ, Vasan RS, Mendelsohn ME, Housman D, Huggins GS, Mitchell GF. The KCNMB1 E65K variant is associated with reduced central pulse pressure in the community-based Framingham Offspring Cohort. J Hypertens. 2009;27:55–60. doi: 10.1097/HJH.0b013e328317c8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol (Lond) 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Penar PL, Tranmer BI, Wellman GC. Heparin-binding EGF-like growth factor mediates oxyhemoglobin-induced suppression of voltage-dependent potassium channels in rabbit cerebral artery myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1750–H1759. doi: 10.1152/ajpheart.00443.2007. [DOI] [PubMed] [Google Scholar]
- Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SR. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Obara K, Nakayama1 K, Koide M, Yokoyama T, Yokota N, Ohta S. Protein kinase C delta and alpha are involved in the development of vasospasm after subarachnoid hemorrhage. Eur J Pharmacol. 2000;398:113–119. doi: 10.1016/s0014-2999(00)00311-3. [DOI] [PubMed] [Google Scholar]
- Ohkuma H, Manabe H, Tanaka M, Suzuki S. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2000;31:1621–1627. doi: 10.1161/01.str.31.7.1621. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- Perez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–C1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56:1082–1092. [PubMed] [Google Scholar]
- Pucovsky V, Bolton TB. Localisation, function and composition of primary Ca2+ spark discharge region in isolated smooth muscle cells from guinea-pig mesenteric arteries. Cell Calcium. 2006;39:113–129. doi: 10.1016/j.ceca.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Quan L, Sobey CG. Selective effects of subarachnoid hemorrhage on cerebral vascular responses to 4-aminopyridine in rats. Stroke. 2000;31:2460–2465. doi: 10.1161/01.str.31.10.2460. [DOI] [PubMed] [Google Scholar]
- Santana LF, Kranias EG, Lederer WJ. Calcium sparks and excitation-contraction coupling in phospholamban-deficient mouse ventricular myocytes. J Physiol. 1997;503:21–29. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas M, Vazquez E, Fernandez-Fernandez JM, Subirana I, Plata C, Heras M, Vila J, Marrugat J, Valverde MA, Senti M. Genetic variation in the KCNMA1 potassium channel alpha subunit as risk factor for severe essential hypertension and myocardial infarction. J Hypertens. 2008;26:2147–2153. doi: 10.1097/HJH.0b013e32831103d8. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-senstive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am J Physiol Cell Physiol. 2001;281:C1029–C1037. doi: 10.1152/ajpcell.2001.281.3.C1029. [DOI] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zhao Y, Pan B, Liu J, Huang X, Zhang X, Cao C, Hou N, Wu C, Zhao KS, Cheng H. Hypersensitivity of BKCa to Ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res. 2007;101:493–502. doi: 10.1161/CIRCRESAHA.107.157271. [DOI] [PubMed] [Google Scholar]
- ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol. 1999;113:215–228. doi: 10.1085/jgp.113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.