Abstract
The glutamate–glutamine cycle faces a drain of glutamate by oxidation, which is balanced by the anaplerotic synthesis of glutamate and glutamine in astrocytes. De novo synthesis of glutamate by astrocytes requires an amino group whose origin is unknown. The deficiency in Aralar/AGC1, the main mitochondrial carrier for aspartate–glutamate expressed in brain, results in a drastic fall in brain glutamine production but a modest decrease in brain glutamate levels, which is not due to decreases in neuronal or synaptosomal glutamate content. In vivo 13C nuclear magnetic resonance labeling with 13C2acetate or (1-13C) glucose showed that the drop in brain glutamine is due to a failure in glial glutamate synthesis. Aralar deficiency induces a decrease in aspartate content, an increase in lactate production, and lactate-to-pyruvate ratio in cultured neurons but not in cultured astrocytes, indicating that Aralar is only functional in neurons. We find that aspartate, but not other amino acids, increases glutamate synthesis in both control and aralar-deficient astrocytes, mainly by serving as amino donor. These findings suggest the existence of a neuron-to-astrocyte aspartate transcellular pathway required for astrocyte glutamate synthesis and subsequent glutamine formation. This pathway may provide a mechanism to transfer neuronal-born redox equivalents to mitochondria in astrocytes.
Keywords: AGC1, Aralar, aspartate, glial glutamine, mitochondrial aspartate–glutamate carrier, OmniBank
Introduction
Glutamate is the main excitatory neurotransmitter in the central nervous system. The lack of pyruvate carboxylase in neurons makes them incapable of de novo synthesis of glutamate and γ-aminobutyric acid (GABA) from glucose (Shank et al, 1985). However, the release of glutamate is followed by uptake into astrocytes rather than neurons (Schousboe, 1981). This leads to a continuous drain of glutamate from neurons to astrocytes, which needs to be compensated through the supply of a glutamate precursor formed in astrocytes, glutamine. The glutamate–glutamine cycle for glutamatergic neurons and the GABA–glutamine cycle for GABAergic ones account for glutamate homeostasis in the corresponding terminals (Bak et al, 2006). In addition to transcellular cycling, some of the glutamate–glutamine is oxidized in the brain, about 10% to 30% under basal conditions (Rothman et al, 2003; Hertz and Kala, 2007) leading to a net loss of these compounds from the glutamate–glutamine cycle (McKenna, 2007; Schousboe et al, 1993; Sonnewald et al, 1993; Gamberino et al, 2007). This requires a continuous replenishment of glutamate and glutamine in astrocytes. Therefore, the small glial pool of glutamate, which is the precursor of the glutamine pool, is rapidly turning over (Cruz and Cerdán, 1999).
De novo glutamate and glutamine production in astrocytes requires the supply of one or two ammonia groups, respectively, and neurons are thought to supply one or both (reviewed in Bak et al (2006)). Two mechanisms have been proposed for the effective transfer of ammonia for amidation in the glutamine synthesis reaction: (1) ammonia (NH3) or ammonium (NH4+) transport (Bak et al, 2006) and (2) alanine-lactate nitrogen shuttle (Bak et al, 2006). The branched chain amino acid (BCAA)-branched chain keto acid nitrogen shuttle has been proposed to account for nitrogen donation to the glutamate amino group for net synthesis of glutamine involving pyruvate carboxylation (Lieth et al, 2001). The extent to which each of these processes actually supports glutamate and/or glutamine production in brain astrocytes, has not been established yet.
We now present evidence suggesting that aspartate is the neuron-born nitrogen donor for glial glutamate synthesis. The evidence was obtained in mice deficient in Aralar, the mitochondrial transporter of aspartate–glutamate present mainly in neurons (Ramos et al, 2003; Berkich et al, 2007; Xu et al, 2007). Aralar-KO mice have hypomyelination and a very drastic fall in brain aspartate and N-acetylaspartate (NAA) levels, because of lack of the only mitochondrial carrier that transports aspartate from the mitochondrial matrix to the cytosol in exchange for glutamate (Jalil et al, 2005). Similar features are present in a patient with Aralar deficiency (Wibom et al, 2009). We find that these mice have a marked decrease in the brain glutamine pool, but a more moderate decrease in the glutamate pool, consistent with a failure to produce glutamate and glutamine in the glial compartment. In vivo 13C-labeling experiments with (13C) acetate or glucose showed a dramatic loss of 13C-labeled glutamine in Aralar-KO mice, indicating a decrease in glutamine synthesis. Cultured astrocytes, from both wild-type (WT) and Aralar-KO mice, were able to use aspartate to produce glutamate and glutamine from glucose at concentrations in which GABA, alanine, or leucine were essentially ineffective, showing that brain aspartate, which is produced mainly in neurons is probably used as preferred nitrogen donor for glutamate and glutamine synthesis in astrocytes.
Materials and methods
Animals and Genotypes
Male SVJ129 × C57BL6 mice carrying a deficiency for ARALAR expression (Aralar−/−, Aralar+/−, and Aralar+/+) were obtained from Lexicon Pharmaceuticals, Inc (The Woodlands, TX, USA) (Jalil et al, 2005). The mice were housed in a humidity- and temperature-controlled room on a 12-hour light/dark cycle, receiving water and food ad libitum. Genotype was determined by PCR using genomic DNA obtained from tail or embryonic tissue samples (Nucleospin tissue kit, Macherey-Nagel, Dueren, Germany) as described previously (Jalil et al, 2005). All the experimental protocols used in this study were approved by the local Ethics Committees at the Center of Molecular Biology ‘Severo Ochoa,' Autonoma University (UAM), Madrid, and at the Institute of Biomedical Research ‘Alberto Sols' CSIC-UAM, Madrid, Spain.
Immunofluorescence
Animals were anesthetized by chloral hydrate (0.5 mg/g body weight; Sigma-Aldrich, St Louis, MO, USA) and perfused transcardially with saline buffer (0.9% NaCl in phosphate buffer) and then with 4% formaldehyde in phosphate buffer (formalin fixative). Brains were carefully removed and postfixed overnight in formalin (4°C) and transferred into sucrose (30% in phosphate buffer). Later, they were embedded in OCT compound (Tissue-Tek, Sakura Finetek Europe BV, Zoeterwoude, The Netherlands), frozen on dry ice and cut into 12 series of 30 μm coronal sections with a cryotome (free-floating sections). Sections were kept in cryoprotectant medium (25% glycerol, 25% ethylene glycol in 50 mmol/L phosphate buffer) and stored at −20°C until processing.
For immunofluorescence assay, cryoprotectant medium was washed with phosphate-buffered saline (PBS) before incubation in antigen retrieval medium (0.1% sodium dodecyl sulfate, 2 mmol/L EGTA (ethylene glycol-bis (2-amino-ethylether)-N,N,N′,N′-tetra-acetic acid), PBS) for 30 minutes at 37°C. Then sections were washed (PBS) and treated with NaBH4 (5 mg/mL) to quench endogenous autofluorescence. Afterwards, sections were preincubated in PBS with 10% horse serum and 0.5% Triton X-100, and then incubated overnight with anti-Aralar (1:100, polyclonal) and anti-Cox-I (1:100, Molecular Probes, Eugene, OR, USA; monoclonal) in PBS with 2% horse serum and 0.25% Triton X-100. Secondary antibodies Alexa 488 (Molecular Probes, 1:500) and Cy3 mAB (Jackson Inmunoresearch Laboratories Inc., West Grove, PA, USA, 1:500) were incubated for 1 to 2 hours before mounting with mowiol. TOPRO-3 ({1-(4-[3-methyl-2,3-dihydro-(benzo-1,3-thiazole)-2-propylidene]-quinolinium)-3-trimethylammonium propane diodide} (TP3)) (Invitrogen, Carlsbad, CA, USA; 1:750) was used as nuclear probe. Imaging was performed in a confocal Zeiss microscope.
Electron Microscopy
Electron microscopy of brain sections from wt and Aralar-KO mice, immunocytochemical detection of Aralar, and identification of profiles for different cell types is described in Supplementary Materials and methods.
Amino-Acid Analysis in Brain Tissue and Astroglial and Neuronal Cells
For analysis in brain, mice were anesthetized, and cerebral metabolism was arrested using high-power (5 kW) microwaves (Muramatsu, Osaka, Japan). The whole brain was immediately removed from the skull on dry ice and homogenized with four volumes of 3% sulfosalicylic or 5% perchloric acid as described earlier (Jalil et al, 2005). The sulfosalicylic acid supernatants were used for amino-acid analysis with a JEOL JLC-500 amino-acid analyzer (JEOL Ltd., Tokyo, Japan); and the neutralized perchloric acid extracts were quantified with a Biochrom 20 amino-acid analyzer (Pharmacia, Uppsala, Sweden) as described below.
For analysis in cell cultures, both media and cells were processed in 3% perchloric acid neutralized and centrifuged at 10,000g for 15 minutes. Samples were lyophilized and dissolved in lithium citrate loading buffer 0.2 mol/L pH 2.2 for quantification with an automatic amino-acid analyzer Biochrom 20 using a precolumn derivatization with ninhydrin and a cationic exchange column. Glutamine and glutamate content was also measured in astroglial cultures by end point enzymatic reactions as described in Supplementary Materials and methods.
Glutamate content in cerebellar neurons was also assayed in Triton-permeabilized cells grown on glass coverslips by online fluorimetry as described in Supplementary Materials and methods for synaptosomes.
Glucose, Lactate, and Pyruvate Determinations in Media from Glial and Neuronal Cell Cultures
The concentrations of glucose, lactate, and pyruvate in culture media were quantified by using enzymatic kits from Boehringer (Phoenix, AZ, USA), BioVision (Mountain View, CA, USA), and INstruchemie BV (Delfzijl, The Netherlands), respectively, following in each case the manufacturer's instructions. All essays were performed in 48-well microplates, with a FLUOstar OPTIMA reader in the absorbance mode.
In Vivo13C-Labeling Studies
The experiments were conducted at postnatal Day 20. Animals from each group (16 WT and 16 Aralar-KO mice) were weighed and deeply anesthetized with an intraperitoneal injection of a mixture of Ketolar (ketamine; 40 μg/g body weight) and Domtor (medetomidine; 1 μg/g body weight) just before the 13C substrate administration. Anesthesia was used in these experiments, as most of the mice were subjected to in vivo 1H nuclear magnetic resonance spectroscopy analysis (results not shown) before metabolic-labeling experiments. Some of these mice (10 WT and 9 Aralar-KO) were intraperitoneally injected with (1-13C) glucose (2 mmol/100 g body weight) and six animals of each genotype were intraperitoneally injected with (1,2-13C2) acetate (6 mmol/100 g body weight). Under these labeling conditions, the calculated initial plasma acetate concentration is several fold higher than the Km for its glial transporter (9 mmol/L; Waniewski and Martin, 1998). At this acetate concentration, neurons will also take up acetate by diffusion and metabolize it through acetate thiokinase, as the preference of astrocytes for acetate is due to transport (Waniewski and Martin, 1998). The physiological state of the animal was followed through all the experiment. Respiratory rate was monitored by a Biotrig system (Bruker Medical GmbH, Ettlingen, Germany), and body temperature was maintained at ∼37°C using a thermostatic blanket and a temperature-regulated circulating water bath. Fifteen minutes after 13C-label administration, the metabolism was arrested using a high-power (5 kW) focused microwave fixation system (Muromachi Kikai Co. Ltd., Tokyo, Japan). The brain was rapidly removed from the skull and immediately frozen in liquid N2. (1-13C) glucose (99.9% 13C) and (1,2-13C2) acetate were obtained from Cambridge Isotope Laboratories (Andover, MA, USA). 2H2O (99.9% 2H) was acquired from Apollo Scientific Ltd. (Stockport, Cheshire, CT, USA). The preparation of perchloric acid extracts of the individual biopsies (whole brain including cerebellum) and the procedures to carry out 13C nuclear magnetic resonance spectroscopy are described in Supplementary Materials and methods.
Statistical Analysis
Statistical analysis was performed using the SPSS package (SAS Institute Inc., Cary, NC, USA). The statistical significance of the differences was assessed by one-way analysis of variance followed by a post hoc Student's, Duncan/Tukey, or Student–Newman–Keuls t-test method, as indicated. The results are expressed as mean±standard error of the mean (s.e.m.).
Results
Brain Astrocytes have Limited Aralar/AGC1 Levels and Malate-Aspartate Shuttle Activity
We and others (Ramos et al, 2003; Berkich et al, 2007; Xu et al, 2007; Cahoy et al, 2008) have reported a preferential localization of Aralar mRNA and protein in neuron-rich areas in the postnatal mouse brain. In culture, neurons have much higher levels of Aralar protein than cultured glial cells (Ramos et al, 2003). Moreover, cultured astroglial cells express not only Aralar protein but also Citrin/AGC2 (Ramos et al, 2003; Supplementary Figure 1), an AGC isoform expressed in liver but hardly at all in brain, where it is expressed at very low levels only in a few neuronal clusters (Contreras et al, 2010), questioning the relevance of cultured astrocytes as reporters of brain astroglial cells. The brain from Aralar-KO mice has very low aspartate and NAA levels (Jalil et al, 2005), and because these two molecules are produced mainly by neurons and oligodendrocyte precursors (Urenjak et al, 1993), their fall in the brain from Aralar-KO mice was attributed to the lack of neuronal Aralar (Jalil et al, 2005). However, recent data from Lovatt et al (2007) suggested that Aralar mRNA is equally represented in brain astroglial cells and neurons, and it was concluded that malate-aspartate shuttle had low and similar levels in both cell types.
To clarify the role of Aralar in aspartate formation and the malate-aspartate shuttle activity in the two cell types, we have first studied cell aspartate levels and redox shuttle activity in astrocytes and neuronal cultures derived from wild-type and Aralar-KO mice. Cultured astrocytes from Aralar(+/+), Aralar(+/−), and Aralar(−/−) mice show a dose-dependent loss of Aralar, whereas citrin levels were unchanged (Supplementary Figure 1). However, the lack of Aralar resulted in a prominent decrease in aspartate levels in neurons, but not in astrocytes (Table 1). Aralar deficiency increased by almost twofold lactate production in neurons resulting in a rise in the lactate-to-pyruvate ratio (by about 13-fold); however, lactate production and the lactate-to-pyruvate ratio were not significantly increased in the culture medium of astrocytes (Table 2), even though astrocytes have much higher capacity to stimulate glycolysis than neurons (Herrero-Mendez et al, 2009). These results show that Aralar in astroglial cells is not significantly required for aspartate production and that these cells do not rely on the malate-aspartate shuttle to transfer redox equivalents to mitochondria. As the glucose concentration used in these studies was higher than physiological (25 to 30 mmol/L), glucose utilization and lactate production have also been measured at 5 mmol/L glucose in short-term experiments (2 hours). Aralar deficiency resulted in the same increase in lactate production in neuronal but not astrocyte cultures (results not shown).
Table 1. Cellular content of amino acids (nmol/mg protein) in extracts of cortical astroglial and cortical neuronal cultures from wild-type and Aralar-KO mice.
|
Astrocytes |
Neurons |
|||
|---|---|---|---|---|
| Wild type | Aralar-KO | Wild type | Aralar-KO | |
| Phosphoserine | 62.6±4.7 | 78.9±3.9 | 37.3±0.3 | 50.8±13.8 |
| Aspartate | 8.7±1.2 | 7.9±1.0 | 25.4±1.3 | 6.2±0.8*** |
| Threonine | 35.8±3.7 | 48.9±0.8 | 52.8±5.9 | 74.4±23.1 |
| Serine | 54.0±5.6 | 75.1±9.1 | 95.8±6.9 | 111.6±24.4 |
| Glutamate | 126.9±14.5 | 160.8±17.3 | 105.8±11.1 | 120.8±28.5 |
| Glutamine | 213.5±15.2 | 289.6±8.0* | 39.0±5.2 | 41.8±10.1 |
| Glycine | 64.1±9.4 | 108.8±33.7 | 160.6±19.3 | 309.5±97.3 |
| Alanine | 12.1±1.0 | 15.9±1.8 | 9.8±1.2 | 4.6±0.8* |
| Valine | 18.0±1.4 | 29.6±1.1 | 24.5±2.8 | 30.4±4.6 |
| Isoleucine | 16.2±0.8 | 25.3±1.3** | 21.7±2.5 | 25.7±3.0 |
| Leucine | 15.8±0.6 | 25.3±1.4** | 20.3±2.3 | 22.8±2.6 |
| Lysine | 19.9±1.2 | 29.9±3.4* | 35.9±3.4 | 64.5±14.9 |
| GABA | ND | ND | 13.1±0.7 | 17.3±2.6 |
DMEM, Dulbecco's modified Eagle medium; GABA, γ-aminobutyric acid; ND, not detectable.
Cortical astrocytes and neurons in culture were maintained in DMEM media containing 25 mmol/L glucose supplemented with 10% serum or NB-B27 containing 30 mmol/L glucose, respectively. All media were aspartate free and contained 0.5 mmol/L (neurons) or 4.5 mmol/L (astrocytes) glutamine. Media was renewed 24 hours before recollecting cellular extracts at 14 (for astrocytes) or 10 DIV (for neurons). Amino acids were quantified with an automatic amino-acid analyzer Biochrom 20 using a cationic exchange column and precolumn derivatization with ninhydrin. Results are presented as mean±s.e.m. (n=4). Data were statistically evaluated by one-way analysis of variance followed by Student's t-test. Comparisons between control and Aralar-deficient cultures were significant where indicated ***P⩽0.001; **P⩽0.01; *P⩽0.05.
Table 2. Glucose and pyruvate consumption, and lactate net formation measured in culture media of astroglial and neuronal cultures from wild-type (WT) and Aralar-KO (KO) mice.
| Cell type | Genotype | Glucose consumed (μmol/mg prot/24 h) | Lactate net formation (μmol/mg prot/24 h) | Pyruvate consumed (μmol/mg prot/24 h) | Lact/Pyr ratio |
|---|---|---|---|---|---|
| Astrocytes | WT | 278.1±2.7 | 152.14±18.8 | 5.8±0.1 | 348.7±30.5 |
| KO | 295.1±22.2 | 176.3±4.4 | 5.8±0.0 | 446.6±27.5 | |
| Neurons | WT | 259.0±3.5 | 10.3±0.9 | 3.6±0.0 | 49.8±3.0 |
| KO | 174.0±9.8*** | 18.5±0.7*** | 3.8±0.0*** | 638.7±196.6* |
DMEM, Dulbecco's modified Eagle medium; Ext Gluc, extracellular glucose; GABA, γ-aminobutyric acid; Lac, Lactate; Pyr, Pyruvate.
Cortical astrocytes and neurons were cultured for 14 and 10 DIV, respectively, and subsequently incubated for 24 hours in DMEM containing 25 mmol/L glucose (astrocytes) and NB-B27 containing 30 mmol/L glucose (neurons). Fetal calf serum present in glial cultures contains 240 μmol/L lactate and 367 μmol/L pyruvate. To reproduce the conditions, the same concentration of lactate and pyruvate were added in neuronal cultures. At the end of the experiments, the glucose, lactate, and pyruvate concentrations in the media were determined by using kits from Boehringer (glucose), BioVision (lactate), and INstruchemie BV (pyruvate). The consumption of glucose, pyruvate, and net formation of lactate by the cells was calculated on the basis of cellular protein. Results are mean±s.e.m. (n=6) of three independent experiments. Data were statistically evaluated by one-way analysis of variance followed by Student's t-test. Comparisons between control and Aralar-deficient cultures were significant where indicated ***P⩽0.001; *P⩽0.05.
Taken together, the results show that neurons depend on Aralar for aspartate production, malate-aspartate shuttle activity and glucose oxidation, and cultured astrocytes, even though expressing Aralar, do not.
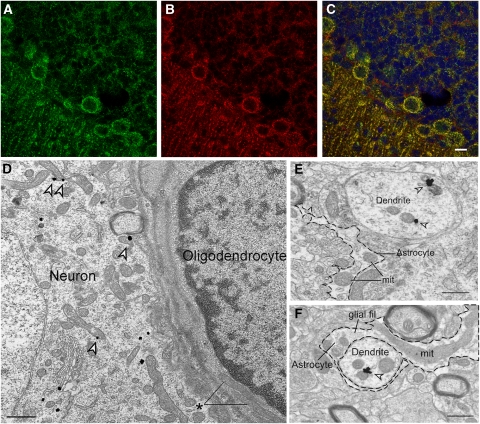
We have next studied the distribution of Aralar in the brain with the use of Aralar immunofluorescence. Colabeling with cytochrome oxidase antibodies was clearly observed in well-characterized neuronal regions (Figures 1A–1C), but not in the brain of Aralar-KO mice (Supplementary Figure 2). To further clarify the lack of Aralar in glial mitochondria, electron microscopy analysis in vibratome sections was performed (Figures 1D–1F). A very high proportion of Aralar-immunogold labeling was localized to neurons (94.1%±18.0%) in comparison to glial profiles (7.2%±0.5% P=0.0001). In neurons (Figures 1D–1F), Aralar showed a prominent localization in mitochondria (77.7% P=0.0001) and some labeling of membranes of smooth endoplasmic reticulum and intracytoplasmic tubulovesicles. The proportion of Aralar-immunogold particles localized in mitochondria in glial cells (40.3%) was statistically much lower than in neurons (two-way analysis of variance in the interaction for the two factors; type of profile and subcellular localization; P=0.0001). These results indicate that, at the protein level, Aralar is localized preferentially, if not exclusively, in neurons.
Figure 1.
Aralar immunolabeling in brain neurons and astrocytes. (A–C) Brain sections of Aralar+/+ mice (A–C) were probed against Aralar (green) (A) and Cox-I (red) (B) antibodies. Merged images are shown in (C), with nucleus marked with Topro-3 (blue). Scale bar, 10 μm. Electron microscopy analysis of Aralar immunolabeling in brain sections (D–F). (D) Prominent mitochondrial Aralar-immunogold (arrowheads) labeling in a neuronal somata, showing also some cytoplasmic gold particles. The neuron is adjacent to a glial cell (oligodendrocyte), showing the typical heterocromatic nucleus and electrodense cytoplasm, in which no Aralar immunolabeling is observed. Note the lack of labeling in the two large glial mitochondria (asterisk). Scale bar, 0.5 μm. (E) Arrowheads indicate Aralar-immunogold labeling on mitochondria within a dendrite. Where indicated (mit) two large unlabeled mitochondria appear in an astrocytic process (with an irregular contour marked with a dashed line), in the neuropil close to the Aralar-immunolabeled dendrite. Scale bar, 0.5 μm. (F) An astrocyte process showing several unlabeled mitochondria ensheathes a neuronal dendrite with one labeled mitochondria (arrowhead). The astrocyte shows a typical irregular sinuous contour and glial filaments (glial fil). Scale bar, 0.5 μm. Immunogold particles were counted in 220 electron micrographs at × 50,000 from 22 vibratome sections from five different mice. The number of particles counted in mitochondria or other regions was 731 or 210 in neurons, and 43 or 29 in glia, respectively.
Changes in Amino-Acid Levels in Brain from Aralar-KO Mice
As previously observed (Jalil et al, 2005), there is a dramatic fall in aspartate levels in brain from Aralar-KO mice at 20 days of age (Table 3). Other amino acids, particularly glutamate, undergo only a modest decrease (of about 25%). Essential amino acids, such as isoleucine, leucine, and lysine do not decrease. However, there is a striking decrease in serine (to 25%) and alanine (to 32%) levels and also a prominent fall in glutamine levels to about 23% in the brain of Aralar-KO mice.
Table 3. Amino-acid content in brain extracts from wild-type and Aralar-KO mice at 20 days of postnatal age.
| Brain amino acids | Wild type (nmol/g tissue) | Aralar-KO (nmol/g tissue) (% versus WT) |
|---|---|---|
| Phosphoserine | 431±20 | 540±39* |
| Aspartate | 3006±279 | 429±108*** (14%) |
| Threonine | 388±20 | 340±9 |
| Serine | 1242±69 | 314±33*** (25%) |
| Glutamate | 9335±815 | 7024±455* (75%) |
| Glutamine | 3806±340 | 890±167*** (23%) |
| Glycine | 1863±219 | 1563±174 |
| Alanine | 755±100 | 244±22*** (32%) |
| Valine | 123±5 | 126±24 |
| Isoleucine | 50±2 | 45±9 |
| Leucine | 76±3 | 86±13.1 |
| Lysine | 501±51 | 413±33 |
| GABA | 1358±141 | 1324±207 |
GABA, γ-aminobutyric acid; WT, wild type.
Data are expressed as nmol/g tissue (mean±s.e.m.; n=6–10). Statistical comparisons were performed using one-way analysis of variance followed by post hoc Duncan/Tukey t-test. ***P⩽0.001; *P⩽0.05.
The decrease in serine may be due to its increased utilization for pyruvate synthesis, as the pyruvate-to-lactate ratio falls drastically in neuronal cultures from Aralar(−/−) mice (Table 2). The drop in pyruvate levels may also explain the decrease in alanine, which is observed both in brain and in cultured neurons from Aralar-KO mice (Tables 1 and 3; Supplementary Discussion).
No Changes in Neuronal Glutamate Levels or Synaptosomal Glutamate Release in Aralar-KO Mice
Cultured neurons from Aralar-KO mice did not show any difference in glutamate content, either when derived from cortical (Table 1) or cerebellar cultures (5.17±0.21 and 5.34±0.11 nmol/106 cells in Aralar(+/+) and Aralar(−/−) neurons, respectively (mean±s.e.m.; n=4 to 7).
We next evaluated the glutamate release and content in synaptosomes from Aralar-KO mice. There were no significant differences between genotypes (Supplementary Figure 3) in terms of rate of release, either in basal conditions (low K+, with EGTA or Ca2+), or after KCl stimulation (high K+, with EGTA or Ca2+). In addition, there were no differences in total glutamate content between genotypes (241.3±31 and 230±43 nmol glutamate/mg protein, mean±s.e.m. of three experiments performed in triplicate, in synaptosomes from Aralar(+/+) and Aralar(−/−) mice, respectively). These results contrast with previous proposals (Palaiologos et al, 1988) involving the malate-aspartate shuttle in the formation of neurotransmitter glutamate (see Supplementary Discussion).
No Changes in Glutamine Utilization by Neurons from Aralar-KO Mice
Having shown that neuronal glutamate content and synaptosomal glutamate levels or release do not change in Aralar-KO, we studied whether glutamine metabolism was changed in neurons from Aralar-KO mice. Glutamine is deamidated to glutamate by phosphate-activated glutaminase and glutamate may be oxidized (glutamate dehydrogenase (GDH)) or transaminated (mitochondrial aspartate aminotransferase) into α-ketoglutarate (α-KG) which is subsequently decarboxylated by α-KG dehydrogenase (α-KGDH). None of these activities (phosphate-activated glutaminase, GDH, and mitochondrial aspartate aminotransferase) changed in the brain of the Aralar-KO mice except for an increase in α-KGDH activity (Supplementary Table 1). Cortical or cerebellar neurons from Aralar(−/−) mice did not show any changes in CO2 production from glutamine (glutamine-derived CO2 production 6.8±0.8 and 16.3±0.8 nmol Gln/mg protein/h (Aralar+/+ neurons) and 8.4±0.1 and 14.2±01 nmol Gln/mg/h (Aralar−/− neurons) from cortex and cerebellum, respectively (mean±s.e.m., n=6)). This is consistent with the lack of effect of AST inhibitor aminooxyacetate on CO2 production from glutamine in synaptosomes (McKenna et al, 1993). Thus, decreased glutamine levels in brain from Aralar-KO mice are not due to a change in neuronal metabolism of glutamine.
In Vivo Labeling of Cerebral Glutamine and Glutamate with (13C2) Acetate or (1-13C) Glucose
To analyze other possible causes for the reduced glutamine levels in Aralar-KO brain, we investigated glutamate–glutamine synthesis in vivo from (13C2) acetate or (1-13C) glucose. Figure 2 (and Supplementary Figure 4) shows that 13C-labeled glutamine in the brain from Aralar-KO mice is below detection limit, regardless of whether 13C-acetate or 13C-glucose are used as cerebral substrates. The 13C enrichments found in glutamate or glutamine in the control mice are similar to those observed previously (Rodrigues et al, 2007). However, the absence of 13C-labeled glutamine in the Aralar −/− mice is unique to these animals, revealing that neuronal deprivation of Aralar results in a net decrease in astroglial synthesis of glutamine.
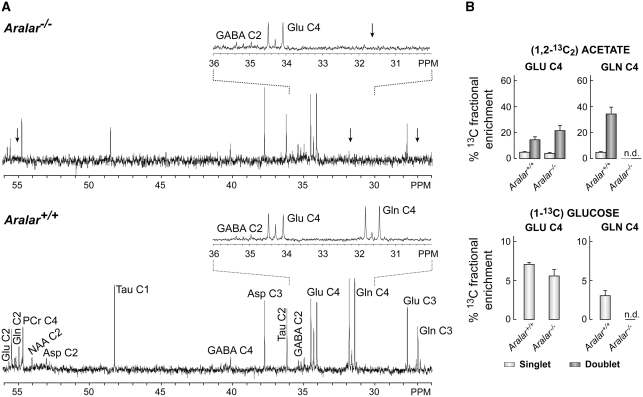
Figure 2.
Lack of 13C-labeled glutamine in Aralar−/− mice. (A) Expansions of representative proton-decoupled 13C nuclear magnetic resonance spectra (125.13 MHz, 25°C, pH 7.2) of neutralized perchloric acid extracts from the cerebral tissue of control (Aralar+/+) and Aralar-deficient (Aralar−/−) mice intraperitoneally injected with (1,2-13C2) acetate. Only the 26 to 56 p.p.m. region is shown, with insets highlighting the 30 to 36 p.p.m. region. Asp, aspartate (C2: 53.0 p.p.m.; C3: 37.8 p.p.m.); GABA, γ-aminobutyric acid (C2: 35.4 p.p.m., C4: 40.4 p.p.m.); Gln, glutamine (C2: 55.0 p.p.m.; C3: 27.0 p.p.m.; C4: 31.6 p.p.m.); Glu, glutamate (C2: 55.4 p.p.m.; C3: 27.7 p.p.m.; C4: 34.2 p.p.m.); NAA, N-acetylaspartic acid (C2: 54.2 p.p.m.); PCr, phosphocreatine (C4′: 54.4 p.p.m.); p.p.m., parts per million; Tau, taurine (C1: 48.4 p.p.m.; C2: 36.1 p.p.m.). (B) Combined fractional 13C enrichment of glutamate C4 and glutamine C4, of extracts from the cerebral tissue of control (Aralar+/+) and Aralar-deficient (Aralar−/−) mice injected with (1,2-13C2) acetate or with (1-13C) glucose, determined as indicated in the text. See Cerdán et al (1990) for assignments of singlets and doublets. Arrows indicate the absence of Gln labeling in the KO mice extracts. Results are expressed as the mean±s.e.m.
Decreased labeling of cerebral glutamine in the face of a very modest decrease in the cerebral glutamate pool strongly suggests that it is the astroglial synthesis of glutamine and the small astroglial glutamate pool from which it derives, which are affected. The extremely small size of the glial glutamate pool does not allow to detect changes in the labeling from 13C-glucose of this pool, which is masked by the large total cerebral glutamate pool (Chapa et al, 2000). The absence of changes in glutamate labeling from 13C-acetate can be explained by residual acetate metabolism in neurons, as the plasma acetate concentrations used in this study were well above the limit for the glial acetate transporter (Waniewski and Martin, 1998) and agrees with the lack of changes in glutamate levels in Aralar-deficient neurons or synaptosomes (Table 1; Supplementary Figure 3) and with the absence of changes in glutamine metabolism in Aralar-deficient neurons. As glutamine synthetase activity is unchanged in Aralar-KO mouse brain (Supplementary Table 1), the failure to synthesize glutamine in astrocytes must be due to a metabolic limitation in the synthesis of glutamate in brain astrocytes.
Astrocytes use Preferentially Aspartate as Nitrogen Donor for Glutamate and Glutamine Synthesis
There is no decrease in the levels of glutamate and glutamine in cultured astrocytes from Aralar-KO mice (Table 1). As the culture medium provides some essential nutrients such as glutamine, this may compensate for deficits in vivo and complicates the comparisons of cultured cells to in vivo.
The failure to synthesize glutamate and glutamine in brain astroglia could arise from a defective production of α-KG in these cells. The decrease in pyruvate levels in Aralar-KO neuronal cultures (final pyruvate concentrations in the culture medium drop from 19.7±1.1 μmol/L in control neurons to 3.2±0.6 μmol/L in Aralar-KO neurons) possibly also occurs in vivo and may result in a drain of pyruvate in astrocytes jeopardizing oxaloacetate (OAA) production by pyruvate carboxylase and subsequent α-KG formation. However, α-KG levels in cultured astrocytes from WT and Aralar-KO mice were the same (1.75±0.4 and 1.67±0.2 nmol/mg protein, respectively, n=4), whereas a clear increase in α-KG levels was detected in the brain from Aralar-KO mice (mean±s.e.m. of four mice per group: 4.96±0.16 and 13.07±1.3 nmol/g weight, in WT and Aralar-KO mice, respectively, P<0.001). These results indicate that the drop in glial glutamate synthesis is not due to a lack of α-KG but most probably to a shortage of the amino-group donor.
The absence of Aralar leads to a prominent fall in brain aspartate levels (Table 3) and aspartate content of neurons in culture (Table 1) and to decreases in alanine content in brain (Table 3) and neuronal cultures (Table 1). Aspartate production requires the aspartate aminotransferase reaction, which is thought to proceed in the direction of aspartate synthesis in mitochondria and in that of OAA synthesis in the cytosol, as both enzymes are components of the malate-aspartate shuttle that drives reduction equivalents to mitochondria in an irreversible way in normally polarized mitochondria (Berkich et al, 2007). The lack of Aralar removes the main pathway whereby neuronal mitochondria take up glutamate from the cytosol and this entails a fall in mitochondrial synthesis of aspartate through OAA transamination. In addition, mitochondrial aspartate cannot egress to the cytosol. On the other hand, decreases in alanine content in neurons and brain of the Aralar-KO mouse are likely related through alanine aminotransferase to their low pyruvate levels. We reasoned that exogenous, neuron-born aspartate or alanine may be the limiting factor for glutamate synthesis in astroglia.
To explore the role of aspartate or alanine as amino source for glutamate formation in astrocytes, we investigated glutamate and glutamine formation in primary astrocyte cultures exposed to glucose, to provide the carbon skeleton of glutamate, and different amino acids added either alone, to test for glutamate formation by astrocytes (by transamination), or in combination with glutamate, to test their role in supplying the amido nitrogen of glutamine (recycling).
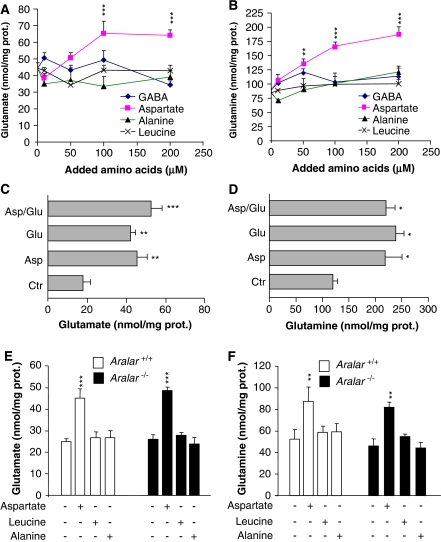
The supply of 10 to 200 μmol/L aspartate to astroglial cultures resulted in glutamate (Figure 3A) and glutamine (Figure 3B) levels significantly higher than those obtained with all the other amino acids tested including alanine, BCAAs (leucine), and GABA. In fact, only aspartate, but not alanine, GABA, or leucine, was able to induce a significant increase in glutamine and glutamate synthesis. However, aspartate did not enhance glutamate or glutamine synthesis when added together with glutamate (Figures 3C and 3D). Taken together, these findings show unambiguously that aspartate is the main nitrogen source for de novo glutamate synthesis, but it is not used for glutamine synthesis from glutamate.
Figure 3.
Aspartate promotes glutamate synthesis in astroglial cell cultures. (A, B) Cortical astrocytes cultures from wild-type (WT) mice (DIV14) were incubated for 1 hour in KRBH (140 mmol/L NaCl, 3.6 mmol/L KCl, 0.5 mmol/L NaH2PO4, 0.5 mmol/L MgSO4, 1.5 mmol/L CaCl2, 2 mmol/L NaHCO3, 10 mmol/L Hepes, pH 7.4) containing 2 mmol/L glucose in the absence or presence of supplemented amino acids (γ-aminobutyric acid (GABA), aspartate, alanine, or leucine; 10 to 200 μmol/L). Cellular extracts and media were separately recovered to measure glutamate (A) and glutamine (B) content, respectively, by an enzymatic end point method. (C, D) Cortical astrocytes from WT mice (DIV14) were incubated for 1 hour in KRBH-2 mmol/L glucose (Ctr) and in the presence of added aspartate (Asp; 50 μmol/L), glutamate (Glu; 50 μmol/L) or both together (Asp/Glu). Cellular extracts and media were separately recovered to measure glutamate (C) and glutamine (D) content, respectively, as described above. Under those conditions, astroglial cultures were viable as detected by using the calcein-acetoxy methylester/propidium iodide essay. (E, F) Cortical astrocytes from WT and Aralar-KO mice (DIV14) were incubated for 2 hours in KRBH containing 15 mmol/L glucose in the absence or presence of 100 μmol/L aspartate, 100 μmol/L alanine, or 100 μmol/L leucine. Cellular extracts and media were separately recovered to measure glutamate (E) and glutamine (F) content, respectively, as described. Intracellular glutamate (A, C, E) and extracellular glutamine content (B, D, F) are expressed as nmol/mg protein. Results are mean±s.e.m. (n=6) of three independent experiments. Data were statistically evaluated by one-way analysis of variance followed by Student–Newman–Keuls's t-test method (***P⩽0.001; **P⩽0.01; *P⩽0.05). KRBH, Krebs-Ringer bicarbonate-HEPES buffer.
Brain glial cells have the capacity to label aspartate C3 derived from (1-13C) glucose or from (1,2-13C2) acetate (Aureli et al, 1997; Preece and Cerdán, 1996). The prominent in vivo labeling of aspartate C3 from (1-13C) glucose is due to the conversion of 13C-labeled pyruvate into 13C-labeled OAA and aspartate through the activity of glial pyruvate carboxylase. Surprisingly, aspartate C3 labeling from (1-13C) glucose is maintained in Aralar-KO mice (Supplementary Figure 4). In vivo labeling with (1,2-13C) acetate results in a prominent singlet in aspartate C3, which arises from the loss of one of the two labeled carbons in OAA formed in the first turn of the tricarboxylic acid cycle after incorporation of unlabeled acetyl-CoA moities. The prominent aspartate C3 singlet observed under these conditions is then due to unlabeled acetyl-CoA dilution occurring in the rapidly turning over glial tricarboxylic acid cycle. Remarkably, the aspartate C3 singlet formed by labeling with (1,2-13C) acetate is the same in the brain from wild-type and Aralar-KO mice (Figure 2A). These findings clearly indicate that the glial pathway of aspartate labeling is independent of the activity of the mitochondrial aspartate–glutamate carrier and that this labeled aspartate is not used in the synthesis of their own glutamate. In contrast, labeling of aspartate C2 with (1-13C) glucose, which reflects the neuronal aspartate pool (Navarro et al, 2008) is severely reduced in the Aralar-KO mouse brain (Supplementary Figure 4). Thus, even when glial cells have the capacity to label aspartate, this aspartate is unavailable for their own synthesis of glutamate, which requires an exogenous source of this amino acid. In agreement with this, the addition of aspartate results in the same stimulation of glutamate and glutamine synthesis in cultured astroglial cells from Aralar-WT and Aralar-KO mice (Figures 3E and 3F). These results provide strong support to the notion that the drastic fall in glutamine synthesis observed in the brain from Aralar-KO mice is not due to a constitutive failure in its synthesis by brain astrocytes but to a limitation or lack in the supply of exogenous aspartate arising from the neighboring neurons.
Discussion
The role of aspartate as glutamate and glutamine precursor in astrocytes was investigated long ago by labeling with 15N (Yudkoff et al, 1986, 1987; Erecinska et al, 1993). Aspartate was rapidly taken up from the medium (Yudkoff et al, 1986, 1987; Erecinska et al, 1993), and its amino group was transferred to glutamate in the aspartate aminotransferase reaction. The newly made glutamate was the precursor of glutamine through glutamine synthetase (Yudkoff et al, 1987; Erecinska et al, 1993), a reaction taking place exclusively in the cytosol of astrocytes (Martínez-Hernandez et al, 1977). However, the importance of this pathway for de novo synthesis of glutamate in brain astroglial cells remained unknown. The results reported herein suggest that aspartate is the main nitrogen donor for glial glutamate synthesis. On the other hand, the second amino group required for glutamine synthesis, the amido group, does not directly require aspartate. This may arise from ammonia itself (released by neurons), or from glial GDH, which operates mainly in the direction of glutamate oxidation in astrocytic mitochondria, particularly in response to high external glutamate levels (McKenna, 2007; McKenna et al, 1996).
Although alanine levels fall in neurons (Table 1) and brain (Table 3) of the Aralar-KO mouse, it is unlikely that the fall in alanine causes the failure to synthesize glial glutamate. The marked preference for aspartate over alanine in glial glutamate production (Figure 3) stands against this possibility. The BCAAs do not compensate for the lack of aspartate in glial glutamate synthesis, as the fall in glutamine levels in the Aralar-KO mouse brain takes place in face of unchanged levels of BCAAs (Table 3) and a knockout mouse for mitochondrial-branched chain aminotransferase, the enzyme required for glial glutamate synthesis from BCAAs, has high circulating BCAAs but has not been shown to have defects in brain glutamate or glutamine synthesis (She et al, 2007). Taken together, the results suggest that the fall in aspartate levels causes the decrease in astroglial glutamate and glutamine synthesis in the brain from Aralar-KO mice. Our results strongly support that any defect in aspartate supply may immediately have consequences on glial glutamate and glutamine synthesis. As NAA is a donor of aspartate, which is known to be transported out of neurons and its levels decrease in Aralar-deficient brain (Jalil et al, 2005; Wibom et al, 2009), we cannot exclude a role of NAA as aspartate donor for glial glutamate synthesis, although the absence of the NAA cleavage enzyme, aspartoacylase, in astrocytes (Madhavarao et al, 2004) makes it unlikely.
With the development of the blood–brain barrier, brain cells must rely on their own aspartate production from the first week onwards (Price et al, 1984). The lack of Aralar explains the progressive decline in brain aspartate that is closely followed by that of NAA, glutamine, and myelin lipids, including the major myelin lipid galactocerebroside, at a time where the central nervous system pathology of the Aralar-KO mouse develops (Jalil et al, 2005). This pathology, and that of the patient with Aralar deficiency (Wibom et al, 2009), is similar to the deficiency in UDP-galactose:ceramidegalactosyl transferase (Coetzee et al, 1996), the enzyme required for the synthesis of myelin galactocerebrosides. However, ceramidegalactosyl transferase deficiency is milder than Aralar deficiency even though there is a total lack of myelin galactocerebrosides in the ceramidegalactosyl transferase-KO mouse and only a partial loss in the Aralar-KO mouse. This suggests that the progressive failure to synthesize astroglial glutamate and glutamine is likely to contribute to the central nervous system pathology in Aralar deficiency.
Aspartate is released by neurons to the extracellular space and a vesicular transporter of aspartate is found in synaptic microvesicles (Miyaji et al, 2008). The preference for aspartate as nitrogen donor for glutamate synthesis may be due to: (1) the large activity of aspartate aminotransferase in astrocytes and (2) the active aspartate transport systems in the glial membrane. Brain aspartate aminotransferase has about 6 to 10 times higher activity than that of alanine or branched chain amino-acid aminotransferases (Goldlust et al, 1995). In addition, astrocytes have high-affinity sodium-dependent aspartate/glutamate transport systems, GLT1/EAAT2/Slc1a2, and the GLAST/EAAT1/Slc1a3 transporter (Kanner, 2006) that allows a rapid scavenging of low-micromolar concentrations of aspartate in astrocytes (Yudkoff et al, 1986; Erecinska et al, 1993). This is not so for leucine and possibly alanine transport systems LAT2 (Bröer et al, 2007), which have much lower affinity (the Km for leucine transport in astrocytes is 400 μmol/L (Yudkoff et al, 1996) and being Na+ independent, do not allow the accumulation of the transported amino acid against a concentration gradient.
Redox shuttles in astrocytes have much lower activity than those in neurons (Ramos et al, 2003; Berkich et al, 2007; Xu et al, 2007; Cahoy et al, 2008), and the relative effects of Aralar deficiency in lactate production and glucose utilization in neurons and astrocytes in culture found in this study (Table 2) strongly support a much more prominent role of the malate-aspartate shuttle in neurons than in astrocytes. This is consistent with a higher redox state of cytosolic NADH/NAD+ in astrocytes (Kasischke et al, 2004), the higher standing lactate-to-pyruvate ratios in astroglial cultures (Table 2) and the ability to upregulate glycolysis because of a high 6-phosphofructo-2-kinase/fructo-2,6-bisphosphatase-3 activity in astrocytes but not in neurons (Herrero-Mendez et al, 2009). However, the neuron-to-astrocyte aspartate efflux pathway described herein (Figure 4) may provide a means to transfer NADH/NAD+ redox potential to astrocyte mitochondria, as an alternative transcellular shuttle system. Indeed, aspartate uptake coupled to OAA production provides a substrate for cytosolic malate dehydrogenase resulting in NADH consumption in the cytosol and malate formation. As the α-ketoglutarate–malate carrier is equally represented in neuronal and glial mitochondria (Berkich et al, 2007), aspartate utilization in glutamate formation in astrocytes will be stoichiometrically related to reducing equivalent transfer to mitochondria. In this way, transcellular aspartate traffic would result in malate oxidation by astrocytic mitochondria. Whether this pathway of carbon flow from neuronal aspartate to glial OAA utilization and reducing equivalent transfer in mitochondria could contribute to the preference for aspartate in glial glutamate production remains to be established. Alternatively, malate formed in astroglial cytosol may be transferred back to neurons, as malate is released to a higher extent from cultured astrocytes than from cultured neurons (Westergaard et al, 1994).
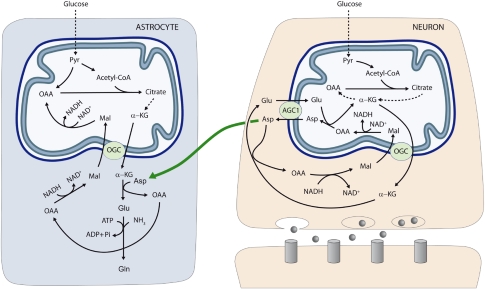
Figure 4.
Neuron-to-glia transcelullar aspartate efflux pathway for glial glutamate synthesis. Neuronal mitochondria are provided with Aralar/AGC1/Slc25a12 and the oxoglutarate carrier/OGC/Slc25a11 and carry out the malate-aspartate shuttle to transfer NADH reducing equivalents to the mitochondrial matrix. AGC1 is irreversible in polarized mitochondria and the main pathway of glutamate supply to the mitochondrial matrix. As cAST functions in the direction of glutamate formation in cells with an active malate-aspartate shuttle, mitochondria are the only site where aspartate is produced (in the mitochondrial aspartate aminotransferase reaction), and aspartate leaves the matrix through AGC1 to reach the cytosol. De novo glutamate synthesis in astroglial cells takes place in the cytosol in the cAST reaction with aspartate as amino-nitrogen donor to α-KG. A second amino group (possibly arising from ammonia itself formed in neurons in the phosphate-activated glutaminase reaction) is acquired in the glutamine synthetase reaction and glial glutamine is now transferred to neurons along the glutamate–glutamine cycle (not shown). Oxaloacetate (OAA) arising from the cAST reaction is converted to malate, and malate entry in glial mitochondria along the OGC provides an alternative pathway for redox transfer to mitochondria, which partly compensates for the lack of a malate-aspartate shuttle in brain astrocytes. In this way, equivalent transfer to astroglial mitochondria is stoichiometrically related to de novo glutamate production. Alternatively, malate formed in astroglial cytosol may be transferred back to neurons, as malate is released to a higher extent from cultured astrocytes than from cultured neurons (Westergaard et al, 1994) (not shown). The presence of mitochondria in the fine peridentritic processes of astrocytes (Figures 1E and 1F) indicates astrocytic oxidative capability near synapses. This confirms the reports by the Nedergaard group (Lovatt et al, 2007) and suggests that astrocytes need not be predominately glycolytic to supply their energy during brain activation. Indeed, the labeling of Asp C3 (Supplementary Figure 4) by [1-13C]glucose and its dilution by unlabeled AcCoA in the [1,2-13C]acetate study indicates that astrocytes oxidize glucose and must have some redox carrier system. AGC, aspartate–glutamate carrier; Asp, aspartate; Gln, glutamine; Glu, glutamate; α-KG, α-ketoglutarate; Mal, malate; OAA, oxalacetic acid; OGC, α-ketoglutarate–malate carrier; Pyr, pyruvate.
Acknowledgments
The authors thank Isabel Manso and Bárbara Sesé for technical support. TBR held a fellowship from the Fundação para a Ciência e Tecnologia/Ministério da Ciência e Ensino Superior, Portugal (SFRH/BPD/26881/2006). IL-F held a fellowship from the Comunidad de Madrid, Spain.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported in part by Ministerio de Educación y Ciencia Grants BFU2008-04084/BMC (to JS) and SAF2008-01327 (to SC), Comunidad de Madrid Grants S-GEN-0269-2006 MITOLAB-CM (to JS) and S-BIO-2006-0170 MULTIMAG (to SC), European Union Grant LSHM-CT-2006-518153 (to JS), and Fundación Médica Mutua Madrileña (to BP). The CIBER de Enfermedades Raras is an initiative of the ISCIII.
Supplementary Material
References
- Aureli T, Di Cocco ME, Calvani M, Conti F. The entry of [1-13C]glucose into biochemical pathways reveals a complex compartimentation and metabolite trafficking between glia and neurons: a study by 13C-NMR spectroscopy. Brain Res. 1997;765:218–227. doi: 10.1016/s0006-8993(97)00514-3. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Berkich DA, Ola MS, Cole J, Sweatt AJ, Hutson SM, LaNoue KF. Mitochondrial transport proteins of the brain. J Neurosci Res. 2007;85:3367–3377. doi: 10.1002/jnr.21500. [DOI] [PubMed] [Google Scholar]
- Bröer S, Bröer A, Hansen JT, Bubb WA, Balcar VJ, Nasrallah FA, Garner B, Rae C. Alanine metabolism, transport, and cycling in the brain. J Neurochem. 2007;102:1758–1770. doi: 10.1111/j.1471-4159.2007.04654.x. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdán S, Künnecke B, Seelig J. Cerebral metabolism of [1,2-13C2] acetate as detected by in vivo and in vitro13C NMR. J Biol Chem. 1990;265:12916–12926. [PubMed] [Google Scholar]
- Chapa F, García-Martín ML, García-Espinosa MA, Cerdan S. Metabolism of (1-13C) glucose and (2-13C,2-2H3) acetate in the neuronal and glial compartments of the adult rat brain as detected by [13C, 2H] NMR spectroscopy. Neurochem Int. 2000;37:217–228. doi: 10.1016/s0197-0186(00)00025-5. [DOI] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Contreras L, Urbieta A, Kobayashi K, Saheki T, Satrústegui J. Low levels of citrin (SLC25A13) expression in adult mouse brain restricted to neuronal clusters. J Neurosci Res. 2010;88:1009–1016. doi: 10.1002/jnr.22283. [DOI] [PubMed] [Google Scholar]
- Cruz F, Cerdán S. Quantitative 13C NMR studies of metabolic compartmentation in the adult mammalian brain. NMR Biomed. 1999;12:451–462. doi: 10.1002/(sici)1099-1492(199911)12:7<451::aid-nbm571>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Pleasure D, Nelson D, Nissim I, Yudkoff M. Cerebral aspartate utilization: near-equilibrium relationships in aspartate aminotransferase reaction. J Neurochem. 1993;60:1696–1706. doi: 10.1111/j.1471-4159.1993.tb13393.x. [DOI] [PubMed] [Google Scholar]
- Gamberino WC, Berkich DA, Lynch CJ, Xu B, LaNoue KF. Role of pyruvate carboxylase in facilitation of synthesis of glutamate and glutamine in cultured astrocytes. J Neurochem. 2007;69:2312–2325. doi: 10.1046/j.1471-4159.1997.69062312.x. [DOI] [PubMed] [Google Scholar]
- Goldlust A, Su TZ, Welty DF, Taylor CP, Oxender DL. Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy Res. 1995;22:1–11. doi: 10.1016/0920-1211(95)00028-9. [DOI] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis. 2007;22:199–218. doi: 10.1007/s11011-007-9068-z. [DOI] [PubMed] [Google Scholar]
- Jalil MA, Begun L, Contreras L, Pardo B, Iijima M, Li MX, Ramos M, Marmol P, Horiuchi M, Shimotsu K, Nakagawa S, Okubo A, Sameshima M, Isashiki Y, del Arco A, Kobayashi K, Satrústegui J, Saheki T. Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J Biol Chem. 2005;280:31333–31339. doi: 10.1074/jbc.M505286200. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Lieth E, LaNoue KF, Berkich DA, Xu B, Ratz M, Taylor C, Hutson SM. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J Neurochem. 2001;76:1712–1713. doi: 10.1046/j.1471-4159.2001.00156.x. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membrane Biol. 2006;213:89–100. doi: 10.1007/s00232-006-0877-5. [DOI] [PubMed] [Google Scholar]
- Madhavarao CN, Moffett JR, Moore RA, Viola RE, Namboodiri MA, Jacobowitz DM. Immunohistochemical localization of aspartoacylase in the rat central nervous system. J Comp Neurol. 2004;472:318–329. doi: 10.1002/cne.20080. [DOI] [PubMed] [Google Scholar]
- Martínez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S. Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci. 1993;15:320–329. doi: 10.1159/000111351. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in the brain. J Neurosci Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Miyaji T, Echigo N, Senoh S, Omote H, Moriyama Y. Identification of a vesicular aspartate transporter. Proc Natl Acad Sci USA. 2008;105:11720–11724. doi: 10.1073/pnas.0804015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro D, Zwingmann C, Butterworth RF. Region selective alterations of glucose oxidation and amino acid synthesis in the thiamine-deficient rat brain: re-evaluation using 1H/13C nuclear magnetic resonance spectroscopy. J Neurochem. 2008;106:603–612. doi: 10.1111/j.1471-4159.2008.05410.x. [DOI] [PubMed] [Google Scholar]
- Palaiologos G, Hertz L, Schousboe A. Evidence that aspartate aminotransferase activity and ketodicarboxylate carrier function are essential for biosynthesis of transmitter glutamate. J Neurochem. 1988;51:317–320. doi: 10.1111/j.1471-4159.1988.tb04872.x. [DOI] [PubMed] [Google Scholar]
- Preece NE, Cerdán S. Metabolic precursors and compartimentation of cerebral GABA in vigabatrin-treated rats. J Neurochem. 1996;67:1718–1725. doi: 10.1046/j.1471-4159.1996.67041718.x. [DOI] [PubMed] [Google Scholar]
- Price MT, Pusateri ME, Crow SE, Buchsbaum S, Olney JW, Lowry OH. Uptake of exogenous aspartate into circumventricular organs but not other regions of adult mouse brain. J Neurochem. 1984;42:740–744. doi: 10.1111/j.1471-4159.1984.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Ramos M, del Arco A, Pardo B, Martínez-Serrano A, Martínez-Morales JR, Kobayashi K, Yasuda T, Bogónez E, Bovolenta P, Saheki T, Satrústegui J. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Dev Brain Res. 2003;143:33–46. doi: 10.1016/s0165-3806(03)00097-x. [DOI] [PubMed] [Google Scholar]
- Rodrigues TB, Granado N, Ortiz O, Cerdán S, Moratalla R. Metabolic interactions between glutamatergic and dopaminergic neurotransmitter systems are mediated through D1 dopamine receptors. J Neurosci Res. 2007;85:3284–3293. doi: 10.1002/jnr.21302. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hyder F, Shulman RG. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Ann Rev Physiol. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons and glial cells. Int Rev Neurobiol. 1981;22:1–45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Huang R, Peng L, Hertz L. Glutamate and glutamine metabolism and compartmentation in astrocytes. Dev Neurosci. 1993;15:359–366. doi: 10.1159/000111356. [DOI] [PubMed] [Google Scholar]
- Shank RP, Bennet GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Petersen SB, Unsgard G, Schousboe A. Metabolism of [U-13C] glutamate in astrocytes studied by 13C NMR spectroscopy: incorporation of more label into lactate than into glutamine demonstrates the importance of the tricarboxylic acid cycle. J Neurochem. 1993;61:1179–1182. doi: 10.1111/j.1471-4159.1993.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Release of alpha-ketoglutarate, malate and succinate from cultured astrocytes: possible role in amino acid neurotransmitter homeostasis. Neurosci Lett. 1994;176:105–109. doi: 10.1016/0304-3940(94)90882-6. [DOI] [PubMed] [Google Scholar]
- Wibom R, Lasorsa FM, Töhönen V, Barbaro M, Sterky FH, Kucinski T, Naess K, Jonsson M, Pierri CL, Palieri F, Wedell A. AGC1 deficiency associated with global cerebral hypomyelination. New Engl J Med. 2009;361:489–495. doi: 10.1056/NEJMoa0900591. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ola MS, Berkich DA, Gardner TW, Barber AJ, Palmieri F, Hutson SM, LaNoue KF. Energy sources for glutamate neurotransmission in the retina: absence of the aspartate/glutamate carrier produces reliance on glycolysis in glia. J Neurochem. 2007;101:120–131. doi: 10.1111/j.1471-4159.2006.04349.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nissim I, Hummeler K, Medow M, Pleasure D. Utilization of [15N] glutamate by cultured astrocytes. Biochem J. 1986;234:185–192. doi: 10.1042/bj2340185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Nissim I, Pleasure D. [15N]aspartate metabolism in cultured astrocytes. Studies with gas chromatography-mass spectrometry. Biochem J. 1987;241:193–201. doi: 10.1042/bj2410193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Grunstein L, Nissim I, Stern J, Pleasure D, Nissim I. Astrocyte leucine metabolism: significance of branched-chain amino acid transamination. J Neurochem. 1996;66:378–385. doi: 10.1046/j.1471-4159.1996.66010378.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.