Abstract
Previous studies have indicated that the primary targets for vasopressin actions on the injured brain are the cerebrovascular endothelium and astrocytes, and that vasopressin amplifies the posttraumatic production of proinflammatory mediators. Here, the controlled cortical impact model of traumatic brain injury in rats was used to identify the sources of vasopressin in the injured brain. Injury increased vasopressin synthesis in the hypothalamus and cerebral cortex adjacent to the posttraumatic lesion. In the cortex, vasopressin was predominantly produced by activated microglia/macrophages, and, to a lesser extent, by the cerebrovascular endothelium. These data further support the pathophysiological role of vasopressin in brain injury.
Keywords: activated microglia, arginine vasopressin, cerebrovascular endothelium, macrophages, traumatic brain injury
Introduction
It has been well documented that pathophysiological stimuli, such as hyperosmolality or hemorrhage, which cause a substantial increase in the levels of arginine vasopressin (AVP) in peripheral circulation, also bring about a significant increase in the concentration of AVP in the cerebrospinal fluid (CSF). Although the saturable transport of AVP across the blood–brain barrier (BBB) and blood–CSF barrier has been described previously (Zlokovic et al, 1990, 1991), the Km values for this transport are high compared with the concentrations of AVP observed in the plasma, suggesting that AVP found in the CSF does not originate from peripheral circulation, but is synthesized centrally and released into the CSF. The paraventricular and supraoptic hypothalamic nuclei, which send long axonal processes to the posterior pituitary, are the major sources of circulating AVP (Sofroniew, 1983). However, AVP-producing neurons have also been found in other brain areas located both within the hypothalamus and in a number of extrahypothalamic brain regions. Arginine vasopressin-containing axonal processes have been traced from the paraventricular hypothalamic nucleus to the lateral cerebral ventricle (Buijs et al, 1978), suggesting that they may release AVP into the CSF. Our previous studies have shown that the epithelium of the choroid plexus, the CSF-secreting tissue located in all four cerebral ventricles, can also produce AVP (Chodobski et al, 1997). More recently, we have shown that during chronic hyperosmotic stress, the choroidal synthesis of AVP is upregulated in a manner similar to that observed in the hypothalamic paraventricular and supraoptic nuclei (Szmydynger-Chodobska et al, 2006).
Increased levels of AVP in the plasma and CSF have been reported in patients with ischemic stroke and traumatic brain injury (TBI) (Sørensen et al, 1985; Barreca et al, 2001; Xu et al, 2007). These clinical observations are consistent with the results obtained from animal studies, in which AVP was shown to promote disruption of the BBB, exacerbate cerebral edema, and augment the loss of neural tissue in various forms of brain injury (e.g., Vakili et al, 2005; Szmydynger-Chodobska et al, 2010). Brain injury has also been shown to result in a substantial increase in the expression of the V1a receptor (AVPR1A) on cortical astrocytes and on the cerebrovascular endothelium (Szmydynger-Chodobska et al, 2004), suggesting that these two types of parenchymal cells are the primary targets for AVP in the injured brain. Whereas it is presently not known whether the AVPR1A is expressed on the luminal and/or abluminal surface of the brain endothelium, the astrocytic AVPR1As are clearly located behind the BBB. Brain injury is associated with disruption of the BBB, but an increase in the permeability of the BBB observed after TBI is transient and, therefore, the extent to which blood-borne AVP would be able to access its receptors expressed on brain parenchymal cells is uncertain. This raises the important question as to whether AVP could be produced in the injured brain parenchyma. Accordingly, the aim of this study was to test the hypothesis that, in the controlled cortical impact model of TBI, injury is not only accompanied by an increased production of AVP in the hypothalamus but also results in the upregulation of AVP synthesis locally in the traumatized cortex.
Materials and methods
The Rat Model of Traumatic Brain Injury
Adult male Long-Evans rats weighing 250 to 350 g (Harlan, Indianapolis, IN, USA) were used. The controlled cortical impact model of TBI was used as described previously (Szmydynger-Chodobska et al, 2010). The surgical procedures were in accordance with the guidelines of the Animal Care and Use Committee of Rhode Island Hospital.
Real-Time Reverse-Transcriptase PCR
Real-time reverse-transcriptase-PCR was performed as described previously (Szmydynger-Chodobska et al, 2010). Cyclophilin A was used for the normalization of the data obtained. The following primers and TaqMan probes were used: 5′-CCGAGTGTCGAGAGGGTTT-3′ (forward primer for AVP), 5′-CAGAATCCACGGACTCTTGTG-3′ (reverse primer for AVP), 5′-CGGGAGCAGAGCAACGCCA-3′ (probe for AVP), 5′-GGTGAAAGAAGGCATGAGCA-3′ (forward primer for cyclophilin A), 5′-GCTACAGAAGGAATGGTTTGATG-3′ (reverse primer for cyclophilin A), and 5′-TTTGGGTCCAGGAATGGCAAGAC-3′ (probe for cyclophilin A). The predicted sizes of PCR products were 135 and 152 bp for AVP and cyclophilin A, respectively. The 50-μL PCR reaction mixtures contained 0.2 mmol/L mixed dNTPs, 0.2 μmol/L each primer, 0.1 μmol/L TaqMan probe, 5 mmol/L MgCl2, 2 Units (AVP) or 1 Unit (cyclophilin A) of HotStart Taq DNA polymerase (Qiagen, Valencia, CA, USA), and 1/20 (cerebral cortex) or 1/200 (hypothalamus) of the reverse transcription reaction product. For cyclophilin A, 1/2,000 of the reverse transcription reaction product was used. The reaction mixtures were heated to 95°C for 15 minutes and were then subjected to 45 cycles of denaturation (15 seconds) at 96°C (AVP) or 94°C (cyclophilin A), and annealing/extension (60°C, 45 seconds).
Immunohistochemistry
Immunohistochemical procedures were performed as described previously (Szmydynger-Chodobska et al, 2010). The following primary antibodies were used: rabbit polyclonal anti-AVP antibody (diluted 1:2,000) from ImmunoStar (Hudson, WI, USA) and mouse monoclonal anti-rat CD11b (clone MRC OX-42; 1 μg/mL) and anti-RECA-1 (clone HIS52; 5 μg/mL) antibodies from Serotec (Oxford, UK).
Statistical Analysis
For statistical evaluation of data, ANOVA (analysis of variance) was used, followed by the Newman–Keuls test for multiple comparisons among means. The results are presented as mean values±s.e.m. P<0.05 was considered statistically significant.
Results
Posttraumatic Increase in Arginine Vasopressin mRNA in the Injured Cortex and Hypothalamus
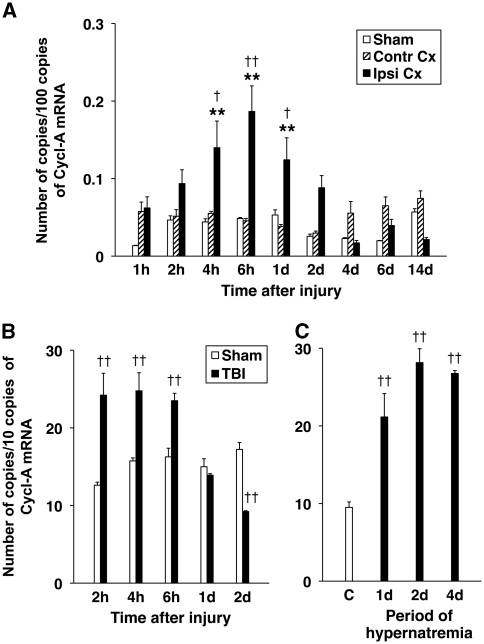
Changes in AVP expression in the cerebral cortex adjacent to the posttraumatic lesion were analyzed using real-time reverse transcriptase-PCR. This analysis showed a gradual increase in AVP mRNA, which attained statistical significance at 4 hours after TBI and peaked at 6 hours after injury (Figure 1A). This increase in cortical synthesis of AVP was transient, and at 2 days after TBI, the level of AVP mRNA in the ipsilateral cortex was not significantly different from that found in the contralateral cortex or the cortex from sham-injured rats. No changes in AVP synthesis in the contralateral cortex or the cortex from sham-injured animals were observed after injury.
Figure 1.
Real-time RT-PCR analysis of posttraumatic changes in AVP expression. The number of copies of transcripts for AVP relative to the message for cyclophilin A (Cycl-A) is shown. (A) Temporal changes in AVP mRNA in the cerebral cortex adjacent to the posttraumatic lesion (Ipsi Cx) compared with the contralateral cortex from the corresponding region (Contr Cx) and the cortex from sham-injured rats (Sham) (n=6 per group). (B) Temporal change in AVP mRNA in the hypothalamus from injured (TBI) versus sham-injured rats (Sham) (n=6 per group). (C) Induction of hypothalamic synthesis of AVP in response to chronic hypernatremia (n=4 per group). Chronic hypernatremia was produced using the standard salt-loading protocol in which the rats were given 2% NaCl solution as the only fluid to drink. The control group obtained water. It must be noted that the magnitude of increase in hypothalamic synthesis of AVP in response to injury was similar to that observed in chronic hypernatremia. **P<0.01 for the ipsilateral versus contralateral cortex. †P<0.05, ††P<0.01 for the ipsilateral cortex/hypothalamus versus sham or control. AVP, arginine vasopressin; d, day; h, hour; RT-PCR, reverse transcriptase PCR; TBI, traumatic brain injury.
In addition, we observed a rapid (within 2 hours after TBI) upregulation of hypothalamic synthesis of AVP in response to injury (Figure 1B). The magnitude of this increase in AVP mRNA was similar to that observed in chronic hypernatremia (Figure 1C). The level of hypothalamic AVP mRNA decreased abruptly at 1 day after injury, and at 2 days after TBI, it was significantly lower than that found in sham-injured rats. No changes in hypothalamic AVP synthesis in sham-injured animals were observed after TBI.
Immunohistochemical Identification of Parenchymal Cells Producing Arginine Vasopressin in the Injured Cortex
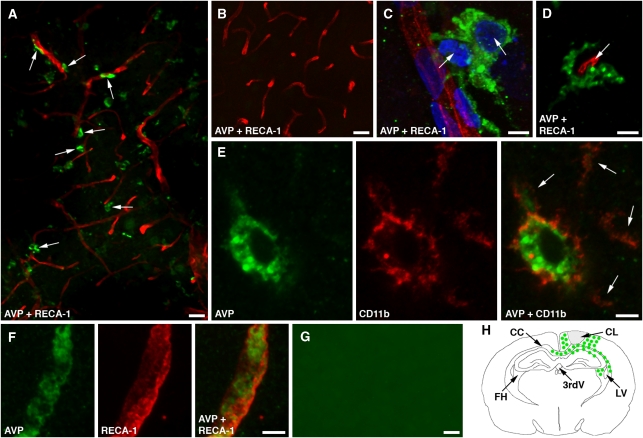
The immunohistochemical analysis showed that in the injured cortex, AVP is predominantly synthesized by activated microglia/macrophages, with many of these AVP-immunopositive cells being intimately associated with cortical microvessels (Figure 2A). The AVP-producing microglia were characterized by rounded cell bodies and thick processes typical of activated microglia. The processes of these microglial cells were frequently found to make a close contact with cortical microvessels (Figure 2C). Vasopressin was also expressed by amoeboid microglia and/or perivascular macrophages (Figure 2D). The identity of these AVP-producing cells was confirmed by double immunostaining for AVP and CD11b, a marker for microglia/macrophages (Figure 2E). In addition, AVP was found to be synthesized by the cerebrovascular endothelium (Figure 2F), but the number of cortical microvessels that were immunoreactive for AVP was smaller than that of AVP-expressing microglia/macrophages. In the injured cortex, the AVP-positive microglia/macrophages were already detectable at 6 hours after TBI. The maximum level of AVP expression was observed at 1 day after TBI and the number of AVP-positive cells decreased substantially at 2 days after TBI. Vasopressin was not expressed in the contralateral cortex (Figure 2B). In addition to the injured cortex, the AVP-immunoreactive microglia/macrophages were localized to the corpus callosum, hippocampal CA1 region, and fimbria hippocampi (Figure 2H).
Figure 2.
Immunohistochemical localization of AVP-expressing cells in the injured cortex at 1 day after TBI. Confocal microscopy images are shown. (A) Double immunostaining of the ipsilateral cortex with anti-AVP antibody and an antibody to RECA-1, a marker for the vascular endothelium. It must be noted that many AVP-positive microglia/macrophages are located close to cortical microvessels (arrows). (B) Double immunostaining of the contralateral cortex with anti-AVP and anti-RECA-1 antibodies. (C) A high-magnification microphotograph of two AVP-positive microglia (arrows) closely associated with a cortical microvessel. The nuclei are stained with DAPI. (D) An AVP-positive perivascular macrophage surrounding a cortical microvessel (arrow). (E) Double immunostaining for AVP and CD11b, a marker for microglia/macrophages, confirms that these parenchymal cells produce AVP. The microglial processes are marked with arrows. (F) The AVP-immunoreactive product associated with the cerebrovascular endothelium. (G) Negative control staining for AVP. In these experiments, the brain sections were incubated with anti-AVP antibody that had been preabsorbed overnight with synthetic AVP at 100 μg/mL. The ipsilateral cortex is shown. Bars: panels A, B, and G, 25 μm; panels C–F, 5 μm. (H) Schematic representation of localization of AVP-positive cells in the injured brain.; AVP, arginine vasopressin; CC, corpus callosum; CL, cortical lesion; FH, fimbria hippocampi; LV, lateral ventricle; RT-PCR, reverse transcriptase PCR; TBI, traumatic brain injury; 3rdV, 3rd ventricle.
Discussion
Traumatic or ischemic brain injury has previously been reported to be accompanied by elevated concentrations of AVP in the plasma (Barreca et al, 2001; Xu et al, 2007). Consistent with these clinical findings, we showed that TBI results in a significant upregulation of hypothalamic synthesis of AVP. Similarly, the increased expression of AVP in the hypothalamic paraventricular and supraoptic nuclei was found in a rodent model of cerebral ischemia (Liu et al, 2000). An increase in the hypothalamic expression of AVP observed after TBI was comparable with that found in response to chronic hypernatremia, a strong pathophysiological stimulus upregulating the hypothalamic synthesis of AVP (Szmydynger-Chodobska et al, 2006).
A new and important finding of this study was an increase in AVP production observed in the cerebral cortex adjacent to the posttraumatic lesion and in other brain areas affected by impact, including the ipsilateral corpus callosum and hippocampus. In the cerebral cortex, only single isolated axonal processes containing AVP have been identified (Sofroniew, 1983), and our immunohistochemical analysis indicated that the major source of AVP in the injured cortex are activated microglia/macrophages. Morphologically, microglia respond to injury by retracting their processes and rounding their cell bodies, but the differentiation of activated microglia from macrophages may be difficult because these two types of cells tend to express similar markers. Interestingly, many of these AVP-expressing microglia/macrophages were found to be intimately associated with cortical microvessels. This anatomic localization of AVP-producing cells may have an important role in AVP-dependent amplification of posttraumatic synthesis of proinflammatory mediators by the brain endothelium and astrocytes (Szmydynger-Chodobska et al, 2010).
In the injured cortex, AVP was also found to be produced by the cerebrovascular endothelium, but the number of cortical microvessels that positively stained for AVP was noticeably smaller than that of microglia/macrophages which expressed AVP. These observations are in line with previous electron microscopy studies, in which AVP was shown to be produced by the endothelium of large cerebral arteries (Loesch et al, 1993).
The molecular mechanisms underlying the upregulation of AVP expression in the injured brain are presently unclear, but it is possible that the presence of activating protein 1-responsive element in the promoter region of the AVP gene (Yoshida et al, 2006) has a role in this increase in AVP synthesis. Consistent with this idea, we have previously shown that TBI results in the activation (phosphorylation) of the transcription factors, c-Jun and ATF2, the components of activating protein-1, and their upstream kinase, c-Jun N-terminal kinase (Szmydynger-Chodobska et al, 2010).
In summary, we showed that TBI results in a significant increase in AVP synthesis in the hypothalamus and cerebral cortex adjacent to the posttraumatic lesion. In the injured cortex, AVP was predominantly produced by activated microglia/macrophages, many of which were closely associated with cortical microvessels. Vasopressin was also produced by the cerebrovascular endothelium, but the number of cortical microvessels found to be immunoreactive for AVP was smaller than that of AVP-expressing microglia/macrophages. These results provide further supporting evidence for the pathophysiological role of vasopressin in brain injury.
Acknowledgments
The authors thank Ms Julie Sarri for her technical assistance and Ms Virginia Hovanesian for her help in acquiring and processing confocal microscopy images.
The authors declare no conflict of interest.
Footnotes
This work was supported by grant NS49479 from the NIH (to AC) and by funds from the Department of Emergency Medicine at the Alpert Medical School of Brown University.
References
- Barreca T, Gandolfo C, Corsini G, Del Sette M, Cataldi A, Rolandi E, Franceschini R. Evaluation of the secretory pattern of plasma arginine vasopressin in stroke patients. Cerebrovasc Dis. 2001;11:113–118. doi: 10.1159/000047622. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Swaab DF, Dogterom J, van Leeuwen FW. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 1978;186:423–433. doi: 10.1007/BF00224932. [DOI] [PubMed] [Google Scholar]
- Chodobski A, Loh YP, Corsetti S, Szmydynger-Chodobska J, Johanson CE, Lim YP, Monfils PR. The presence of arginine vasopressin and its mRNA in rat choroid plexus epithelium. Mol Brain Res. 1997;48:67–72. doi: 10.1016/s0169-328x(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Jin Y, Zheng H, Chen G, Tan B, Wu B. Arginine vasopressin gene expression in supraoptic nucleus and paraventricular nucleus of hypothalamus following cerebral ischemia and reperfusion. Chin Med Sci J. 2000;15:157–161. [PubMed] [Google Scholar]
- Loesch A, Domer FR, Alexander B, Burnstock G. Electron-immunocytochemistry of peptides in endothelial cells of rabbit cerebral vessels following perfusion with a perfluorocarbon emulsion. Brain Res. 1993;611:333–337. doi: 10.1016/0006-8993(93)90522-o. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Sørensen PS, Gjerris A, Hammer M. Cerebrospinal fluid vasopressin in neurological and psychiatric disorders. J Neurol Neurosurg Psychiatry. 1985;48:50–57. doi: 10.1136/jnnp.48.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Chung I, Chodobski A. Chronic hypernatremia increases the expression of vasopressin and voltage-gated Na channels in the rat choroid plexus. Neuroendocrinology. 2006;84:339–345. doi: 10.1159/000097989. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Chung I, Koźniewska E, Tran B, Harrington FJ, Duncan JA, Chodobski A. Increased expression of vasopressin V1a receptors after traumatic brain injury. J Neurotrauma. 2004;21:1090–1102. doi: 10.1089/0897715041651033. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Fox LM, Lynch KM, Zink BJ, Chodobski A. Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. J Neurotrauma. 2010;27:1449–1461. doi: 10.1089/neu.2010.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakili A, Kataoka H, Plesnila N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:1012–1019. doi: 10.1038/sj.jcbfm.9600097. [DOI] [PubMed] [Google Scholar]
- Xu M, Su W, Huang WD, Lu YQ, Xu QP, Chen ZJ. Effect of AVP on brain edema following traumatic brain injury. Chin J Traumatol. 2007;10:90–93. [PubMed] [Google Scholar]
- Yoshida M, Iwasaki Y, Asai M, Takayasu S, Taguchi T, Itoi K, Hashimoto K, Oiso Y. Identification of a functional AP1 element in the rat vasopressin gene promoter. Endocrinology. 2006;147:2850–2863. doi: 10.1210/en.2005-1222. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Hyman S, McComb JG, Lipovac MN, Tang G, Davson H. Kinetics of arginine-vasopressin uptake at the blood-brain barrier. Biochim Biophys Acta. 1990;1025:191–198. doi: 10.1016/0005-2736(90)90097-8. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Segal MB, McComb JG, Hyman S, Weiss MH, Davson H. Kinetics of circulating vasopressin uptake by choroid plexus. Am J Physiol. 1991;260:F216–F224. doi: 10.1152/ajprenal.1991.260.2.F216. [DOI] [PubMed] [Google Scholar]