Abstract
Hemodynamic and cerebrovascular factors are crucially involved in secondary damage after traumatic brain injury (TBI). With magnetic resonance imaging, this study aimed to quantify regional cerebral blood flow (CBF) by arterial spin labeling and cerebral blood volume by using an intravascular contrast agent, during 14 days after lateral fluid-percussion injury (LFPI) in rats. Immunohistochemical analysis of vessel density was used to evaluate the contribution of vascular damage. Results show widespread ipsilateral and contralateral hypoperfusion, including both the cortex and the hippocampus bilaterally, as well as the ipsilateral thalamus. Hemodynamic unrest may partly be explained by an increase in blood vessel density over a period of 2 weeks in the ipsilateral hippocampus and perilesional cortex. Furthermore, three phases of perilesional alterations in CBF, progressing from hypoperfusion to normal and back to hypoperfusion within 2 weeks were shown for the first time in a rat TBI model. These three phases were similar to hemodynamic fluctuations reported in TBI patients. This makes it feasible to use LFPI in rats to study mechanisms behind hemodynamic changes and to explore novel therapeutic approaches for secondary brain damage after TBI.
Keywords: animal models, arterial spin labeling, cerebral hemodynamics, magnetic resonance imaging, traumatic brain injury, vascular density
Introduction
Traumatic brain injury (TBI) is a major cause of mortality and morbidity worldwide. It is estimated to be responsible for 1.5% to 2% of deaths and leads to permanent disability in ∼2% to 3% of individuals in the Western world (Thurman et al, 1999; Pasco et al, 2007). The complex pathophysiology of TBI stems from primary, mechanical damage due to impact forces and from subsequent secondary damage caused by the interplay between neuronal, glial, and vascular events (DeWitt and Prough, 2003; Kharatishvili et al, 2006).
Hemodynamic and cerebrovascular factors are crucially involved in secondary damage after TBI. However, very few animal studies have investigated these changes and these studies have typically focused on the acute phase, within 2 days after TBI. By using radiolabeled microspheres, cerebral blood flow (CBF) throughout the brain was shown to be significantly decreased for at least 2 hours after lateral fluid-percussion injury (LFPI) (Yuan et al, 1988; Yamakami and McIntosh, 1989; McIntosh et al, 1989), and the extent of acute hypoperfusion correlated with delayed necrosis (McIntosh et al, 1989). Acute posttraumatic hypoperfusion was later confirmed by laser Doppler flowmetry (Muir et al, 1992; Bryan et al, 1995; Nilsson et al, 1996; DeWitt et al, 1997). More recently, laser Doppler flowmetry was used to show pericontusional hypoperfusion at 4 hours after controlled cortical impact (CCI) injury, and orthogonal polarization spectral imaging showed that this acute hypoperfusion was due to vasoconstriction (Thomale et al, 2002). This study also found hyperemia occurring between 24 and 48 hours after TBI, but this is yet to be shown after LFPI. Acute perilesional hemodynamic changes after TBI in rats are therefore well described, but postacute perfusion changes and cerebrovascular remodeling remain unexplored.
Recent developments in magnetic resonance imaging (MRI) have made it possible to perform long-term follow-up studies of hemodynamics in individual animals after TBI. For example, Pasco et al (2007) used dynamic susceptibility contrast MRI to visualize and quantify regional hypoperfusion and reduced regional cerebral blood volume (CBV) up to 5 hours after LFPI in rats. Sequential imaging showed that CBF and CBV normalized by day 3 (Pasco et al, 2007). Shen et al (2007) used susceptibility weighted imaging to measure blood oxygenation changes attributed to CBF in the injured cortex and sagittal sinus of weight-drop-injured rats (Shen et al, 2007). They validated susceptibility weighted imaging measurements by absolute quantification of regional CBF using arterial spin labeling (ASL). As susceptibility weighted imaging and ASL are noninvasive, such MRI techniques can be used to research the longitudinal changes in hemodynamics after TBI in animals and patients.
Although there is variability between TBI patients (Bonne et al, 2003), a general three-phase temporal profile of their cortical CBF variations is clinically established. Initial hypoperfusion recovers and may be superseded by hyperemia at 1 to 3 days after TBI, yet another hypoperfusion phase may occur later during days 4 to 15 (Marion et al, 1991; Bouma et al, 1991; Kelly et al, 1997; Martin et al, 1997). We hypothesized that postacute hemodynamic responses to TBI in rodents match those of patients. This study aims to quantify absolute regional CBF using ASL MRI and intravascular contrast agent to measure regional CBV over a period of 14 days after LFPI. Furthermore, an immunohistochemical study of corresponding regional blood vessel density is used to evaluate the contribution of vascular damage to CBF and CBV disruption after TBI.
Materials and methods
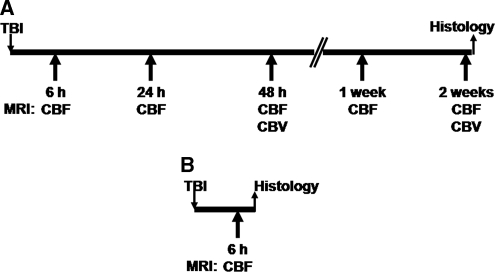
The study design is shown in Figure 1.
Figure 1.
Study design. (A) Group A (nine TBI rats, four sham controls) was followed up for 2 weeks with magnetic resonance imaging (MRI) and perfused for histology thereafter. (B) Group B (six TBI rats, two sham controls) was prepared to provide histologic data for the 6-hour time point. CBF, cerebral blood flow; CBV, cerebral blood volume; TBI, traumatic brain injury.
Animals
Adult male Sprague–Dawley rats (n=31, Harlan Netherlands B.V., Horst, The Netherlands) weighing 280 to 380 g were used. Rats were housed individually under controlled conditions (12-hour light/dark cycle, temperature 22°C±1°C, humidity 50% to 60%, with ad libitum access to food and water). All animal procedures were approved by the Animal Ethics Committee of the Provincial Government of Southern Finland (ESLH-2009-07538/Ym-23), and conducted in accordance with the guidelines set by the European Community Council Directives 86/609/EEC.
Lateral Fluid-Percussion Brain Injury
Traumatic brain injury was induced by LFPI as described previously (Kharatishvili et al, 2006; McIntosh et al, 1989). In brief, rats were anesthetized by an injection cocktail (6 mL/kg, intraperitoneal) containing sodium pentobarbital (58 mg/kg), chloral hydrate (60 mg/kg), magnesium sulfate (127.2 mg/kg), propylene glycol (42.8%), and absolute ethanol (11.6%). A 5-mm diameter hole was drilled between the bregma and lambda on the left convexity (anterior edge 2.0 mm posterior to the bregma; lateral edge adjacent to the left lateral ridge). Severe LFPI was induced by a transient (21 to 23 mseconds) fluid pulse impact against the exposed dura by using a fluid-percussion device (AmScien Instruments, Richmond, VA, USA). The impact pressure was measured by an extracranial transducer and controlled to 3.2 to 3.4 atmospheres (atm). After impact, the dura was checked to ensure it had remained intact. Sham-operated control animals underwent all surgical procedures, except the fluid-percussion impact. Animals that did not survive the first 72 hours after injury were predetermined to be excluded from the study (Pitkanen and McIntosh, 2006).
Magnetic Resonance Imaging
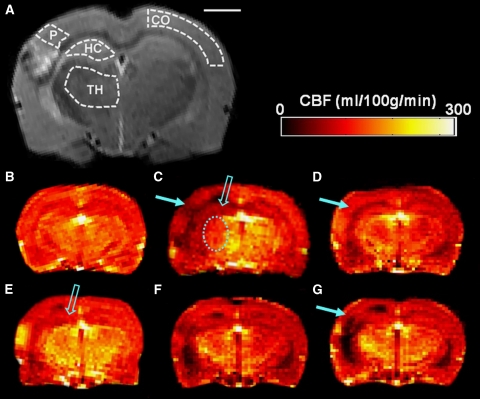
All MRI were performed using a 4.7 T scanner interfaced with a Varian Inova console (Varian, Palo Alto, CA, USA), with an actively decoupled linear volume transmission coil (length=80 mm) and quadrature surface receiver coil pair (Rapid Biomedical, Rimpar, Germany), which provided imaging coverage of the whole brain. Imaging was performed at 6 hours, 24 hours, 2 days, 7 days, and 14 days after TBI or sham operation. Regions of interest (ROIs) included the ipsilateral perilesional cortex, contralateral cortex, ipsilateral and contralateral hippocampus, and ipsilateral and contralateral thalamus. Outlines of each ROI are shown in Figure 2A. Results from rats surviving their entire follow-up period are presented.
Figure 2.
Coronal magnetic resonance imaging (MRI) slices of the rat brain showing anatomic data and cerebral blood flow (CBF) data. (A) Regions of interest (ROIs) drawn on a T2-weighted image taken from a representative trauma rat 2 weeks after lateral fluid-percussion injury (LFPI). CO, contralateral cortex; HC, hippocampus; P, perilesional cortex; TH, thalamus. Scale bar= 2 mm. CBF maps from (B) a control rat and the representative trauma rat acquired at (C) 6 hours, (D) 24 hours, (E) 48 hours, (F) 1 week, and (G) 2 weeks after traumatic brain injury (TBI). The solid arrow highlights CBF changes in the perilesional cortex. An open arrow highlights ipsilateral hippocampal CBF changes at 6 hours and 2 days after trauma. The dashed oval indicates acute hypoperfusion in the ipsilateral thalamus at 6 hours after injury.
Anesthesia
Rats were anesthetized under isoflurane in a carrier gas mixture of 70% N2O, 30% O2. Rats were secured in a stereotactic holder (Rapid Biomedical) using ear bars and a bite bar, while anesthesia was delivered through a nose cone. Breath rate was continually measured throughout imaging by a pressure probe between the rat and the holder. The breathing rate was kept between 62 and 68 breaths per minute by adjusting the anesthesia concentration to 0.7% to 1.7%. The holder was electronically heated to sustain a temperature of 37°C throughout imaging.
Anatomic Imaging
T2-weighted images were acquired using a spin-echo sequence, whereby the time to echo=70 mseconds, repetition time=2,500 milliseconds, and field of view=4 × 4 cm2 covered with 128 × 256 points. Data sets comprised of 15 stacked slices (thickness=1 mm each). The center of the stack was positioned at −3.6 mm from the bregma with the help of axial pilot images and a rat brain atlas (Paxinos and Watson, 1998). The estimated slice positioning error between repeated scans is <50% of the slice thickness. The total anatomic imaging time was 10.5 minutes.
Cerebral Blood Flow
Absolute CBF was quantified using continuous ASL as described previously (Williams et al, 1992), with a fast spin-echo read out with a field of view=4 × 4 cm2, 128 × 128 points, slice thickness=2 mm, repetition time=6 seconds, echo spacing=7 milliseconds, and number of echoes=16. The duration and amplitude of the square labeling pulse causing flow-driven adiabatic inversion were 3 seconds and 0.1 G, respectively, with a postlabeling delay of 800 milliseconds. The labeling pulse was positioned on the neck 2 cm from the imaging slice, and the control image was acquired with an identical radiofrequency pulse positioned symmetrically opposite the imaging slice. Subtraction images from six pairs of label and control images were averaged, and used to provide a CBF map from one hippocampal slice, which was equivalent to the center of the T2-weighted image stack.
As CBF measured by ASL is influenced by T1 variations in tissue, T1 was mapped in the same coronal slice as CBF using an inversion recovery fast-spin-echo sequence (repetition time=4 seconds, echo spacing=13 milliseconds, 4 to 8 echoes per excitation, field of view=4 × 4 cm2 with 64 × 64 points, slice thickness=2 mm, incremented inversion times=5,300, 600, 1,000, and 1,500 milliseconds). The total ASL imaging time was 25 minutes.
Cerebral Blood Volume
Cerebral blood volume was studied at 2 days and 2 weeks after TBI. Before imaging, one catheter was inserted into the femoral vein for contrast agent delivery, and another into the femoral artery for blood sampling and subsequent blood testing. The first blood sample was collected ∼1 hour after imaging began and the second ∼45 minutes later, once postcontrast imaging was completed.
After CBF and anatomic T2-weighted imaging, a bolus of Sinerem MION contrast agent (6 mg/kg, Geurbet, Villepinte, France) was administered intravenously. Three minutes later, T2-weighted imaging was repeated, allowing ΔR2 to be calculated for each data set (R2=1/T2) and ΔR2 to be mapped or all slices. ΔR2 was assumed to be directly proportional to CBV (Boxerman et al, 1995; Dunn et al, 2004). Blood samples were collected and analyzed immediately before contrast agent delivery and after imaging was completed.
Magnetic Resonance Imaging Data Analyses
All analytical tools were provided by our in-house software (Aedes, Kuopio, Finland; http://aedes.uku.fi/) written in Matlab 7.1 (MathWorks, Natick, MA, USA). The outlines of ROIs were defined according to the rat brain atlas of Paxinos and Watson (1998). They were then drawn manually on the anatomic T2-weighted images from each imaging session and copied to the CBF maps and CBV maps in Aedes (Figure 2).
T1 maps were calculated from the inversion recovery MRI data using a standard two-parameter fitting, and then used for CBF calculation (Barbier et al, 2001). Region of interest-based analyses of CBF and CBV maps provided quantitative CBF and CBV data, respectively. Coupling between CBF and CBV was investigated by testing the relationship CBV=0.5 × CBF0.5 in a regional manner (van Zijl et al, 1998).
Histology
Samples for histology were collected from rats imaged at 2 weeks (group A; nine TBI rats, four sham controls) or 6 hours after TBI (group B; six TBI rats, two sham controls), in regions where the cerebral hemodynamic parameters were studied by MRI.
Tissue Fixation and Processing
Rats were transcardially perfused according to a paraformaldehyde fixation protocol described previously (Kharatishvili et al, 2007). The brains were postfixed in 4% paraformaldehyde for 4 hours and then cryoprotected for 24 hours in a solution containing 20% glycerol in 0.02 mol/L potassium phosphate-buffered saline (KPBS, pH 7.4), frozen in dry ice, and stored at −70°C. The frozen sections were cut at the coronal plane using a sliding microtome (30 μm thick, 1-in-5 series) and stored in tissue-collecting solution (30% ethylene glycol, 25% glycerol in 0.05 mol/L sodium phosphate buffer) at −20°C.
Rat Endothelial Cell Antibody-1 (RECA-1) Immunohistochemistry
To visualize and quantify the morphology and density of blood vessels, we stained one series of sections from each rat with an antibody raised against RECA-1 as described previously (Ndode-Ekane et al, 2010). Briefly, sections were washed three times in KPBS for 10 minutes per wash and incubated in 1% hydrogen peroxide for 15 minutes at room temperature. After six 5-minute washes in KPBS, the sections were blocked and incubated in 5% horse serum (NHS) and 0.4% Triton-X 100 in KPBS for 2 hours at room temperature. Next, sections were incubated with the mouse anti-RECA-1 (1:5,000) prepared in blocking solution (5% NHS and 0.4% Triton-X in KPBS) for 48 hours at 4°C. Sections were then given three 10-minute washes in 2% NHS prepared in KPBS before 2 hours incubation with the goat anti-mouse IgG (1:200, room temperature). After a further three 10-minute KPBS washes, sections were incubated with an avidin–biotin complex (1:100) solution for 1 hour, washed again, and recycled in the secondary antibody solution for 45 minutes (room temperature). Sections were thoroughly washed in KPBS and finally incubated in diaminobenzidine solution for 2 to 4 minutes. Sections were coverslipped with DePeX (BDH, Laboratory Supplies, England, UK) and left to dry overnight.
Assessment of Vessel Density
To assess the density of blood vessels, we analyzed three consecutive RECA-1-immunostained sections (150 μm apart from each other, slice thickness 450 μm) corresponding to the slices used to measure CBF and CBV with MRI from the same animals. Regions of interest were drawn and vessel counting was performed using Stereo Investigator software (MBF Bioscience, Williston, VT, USA) connected to an Olympus BX50 microscope (Olympus, Tokyo, Japan) using systematic random sampling. First, an ROI was outlined and a sampling grid was laid on the section (cortex 150 × 150 μm2, hippocampus 250 × 250 μm2, stratum oriens 100 × 100 μm2, and thalamus 320 × 320 μm2). The number of hit points (Q) was then recorded using a counting frame (cortex 50 × 50 μm2, hippocampus 70 × 70 μm2, stratum oriens 60 × 60 μm2, and thalamus 70 × 70 μm2). For each region, vessel density (D) was calculated in each section using a formula D=∑Q·1/asf, where asf is the area sampling fraction (the area of counting frame divided by area of sampling grid). For statistical analyses, a mean density from the three sections for each region for each rat was calculated. Mean density values from all control rats (6 hours and 2 weeks) did not differ and were pooled (n=6) for analyses. Sections from two rats were unavailable due to inadequate staining.
Statistics
Statistical analyses were performed using SPSS for Windows (v. 14.0, Chicago, IL, USA). Differences between groups were tested using the Kruskal–Wallis test, followed by Mann–Whitney U-testing. Differences within groups were tested using Freidman's test with post hoc Wilcoxon's tests corrected for any multiple comparisons (Bonferroni). All values are given as mean±s.e.m. The level of statistical significance was P<0.05.
Results
Mortality
A total of 29 rats were used in this study (23 LFPI, 6 sham-operated controls). Mortality within 72 hours after injury was 30% (7/23), corresponding to severe injury (Pitkanen and McIntosh, 2006). Five injured rats died before imaging was completed within 6 hours after LFPI. Two injured rats died during the 24 hours imaging session. Overall, data obtained from 15 TBI rats (group A, 9; group B, 6) and 6 sham-operated control rats (group A, 4; group B, 2) are reported.
Blood Gases and pH During Magnetic Resonance Imaging
Blood gases and pH analyzed from samples collected before MION administration and immediately after imaging were within normal range (Table 1). There was no difference between the LFPI and control groups.
Table 1. Arterial pH, pCO2, and pO2 during extended imaging sessions.
|
Day 2 |
2 Weeks |
|||||
|---|---|---|---|---|---|---|
| pH | pCO2 (mm Hg) | pO2 (mm Hg) | pH | pCO2 (mm Hg) | pO2 (mm Hg) | |
| Sample 1 | ||||||
| Control | 7.39±0.01 | 51.5±2.5 | 142.3±6.0 | 7.41±0.01 | 44.5±1.7 | 131.7±12.9 |
| Trauma | 7.38±0.02 | 50.8±1.3 | 170.3±11.7 | 7.40±0.01 | 46.8±1.7 | 139.3±10.4 |
| Sample 2 | ||||||
| Control | 7.38±0.02 | 51.9±3.1 | 135.5±5.8 | 7.40±0.01 | 46.0±1.3 | 131.7±5.1 |
| Trauma | 7.41±0.03 | 49.1±4.0 | 118.0±9.9 | 7.37±0.02 | 53.0±2.9 | 125.5±4.4 |
Values are presented as mean±s.e.m. Values in injured rats did not differ from those in controls.
Traumatic Brain Injury Induced Prolonged Regional Cerebral Blood Flow Changes in the Rat Brain
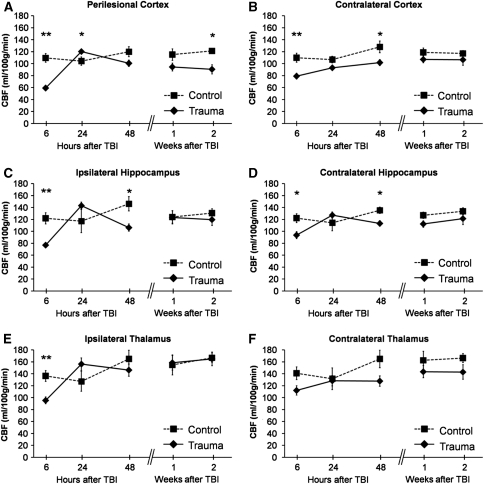
Alterations in CBF after TBI are summarized in Figures 2 and 3.
Figure 3.
Regional cerebral blood flow (CBF) quantified by arterial spin labeling (ASL) magnetic resonance imaging (MRI) over 14 days after traumatic brain injury (TBI). (A) Perilesional cortex, (B) contralateral cortex, (C) ipsilateral hippocampus, (D) contralateral hippocampus, (E) ipsilateral thalamus, and (F) contralateral thalamus. Data are shown as mean±s.e.m. The data at 6 hours come from 6 controls and 15 rats with TBI. For the analysis at later time points, there were four controls and nine TBI rats. Statistical significances: *P<0.05, **P<0.01 (Mann–Whitney U-test, control versus TBI at the same time point). Comparison of ipsilateral changes with those contralaterally (Wilcoxon's test) indicated that at 6 hours after injury, ipsilateral CBF was lower than contralateral CBF in the perilesional cortex, hippocampus, and thalamus (P<0.01). Twenty-four hours after injury, ipsilateral CBF was elevated compared with contralateral CBF in the perilesional cortex (P<0.01), hippocampus (P<0.05), and thalamus (P<0.01). Two days after injury, ipsilateral CBF was lower than contralateral CBF in the hippocampus (P<0.05) and thalamus (P<0.01). Compared with the contralateral cortex, CBF in the perilesional cortex became lower 1 week after injury (P<0.05) and remained so thereafter. At 1 and 2 weeks after injury, thalamic CBF was slightly greater ipsilaterally than contralaterally (P<0.05). The CBF in controls did not change between time points (Wilcoxon's test).
In all ipsilateral regions, CBF after TBI varied over time (P<0.001, Friedman's test). In the perilesional cortex, CBF was only 54% of that in controls (P<0.01) at 6 hours after injury (for absolute values and s.e.m., please see Figure 3A). It recovered by 24 hours after injury, being 15% above the control mean (P<0.05). However, during the next 2 weeks, the perilesional cortex again became hypoperfused as the CBF reduced to 75% of the control mean by 2 weeks (P<0.05). Within the TBI group, the recovery of perilesional hypoperfusion between 6 and 24 hours, and subsequent CBF decrease between 24 hours and 2 weeks, were significant (overall P<0.01).
The pattern of acute CBF changes in the ipsilateral hippocampus resembled those of the perilesional cortex, being 63% of that in controls at 6 hours (P<0.01) and 122% at 24 hours (P>0.05, Figure 3C). Unlike the perilesional cortex, the ipsilateral hippocampus became hypoperfused already by 48 hours after injury (73% of that in controls, P<0.05). At 1 and 2 weeks after injury, CBF had recovered to control levels. Within the TBI group, the recovery of ipsilateral hippocampal hypoperfusion between 6 and 24 hours was significant (P<0.05). At 48 hours, CBF was comparable with that at 6 hours (P>0.95). In the thalamus, a decrease in CBF was observed at 6 hours to 70% of that in controls (P<0.01, Figure 3E). Within the group, CBF had recovered by 24 hours (P<0.01). At later follow-up points, thalamic CBF in injured rats did not differ from that in controls or over that time within the TBI group.
The three phases of temporal changes (namely acute hypoperfusion, normalization, and secondary hypoperfusion) in CBF were evident ipsilaterally. Contralaterally, the pattern in CBF changes was similar but the fluctuations were milder. Cerebral blood flow after TBI varied over time in the contralateral cortex and hippocampus (P<0.01, Friedman's test) but not in the contralateral thalamus. In the contralateral cortex of injured rats, CBF was decreased to 72% of that in controls at 6 hours (P<0.01, Figure 3B). Hypoperfusion continued until 48 hours after injury (CBF was 79% of controls, P<0.05). Within the TBI group, CBF increased between 6 hours and 1 week after injury (P<0.05). Thereafter, no differences compared with controls or over time were found. In the contralateral hippocampus, CBF was also decreased at 6 and 48 hours (63 and 73% of that in controls, respectively, both P<0.05, Figure 3D), and was normal thereafter. Within the TBI group, contralateral hippocampal hypoperfusion recovered between 6 and 24 hours (P<0.01), yet CBF was not significantly decreased again at 48 hours when compared with CBF at 24 hours. In the contralateral thalamus, CBF in injured rats did not differ from controls at any follow-up point.
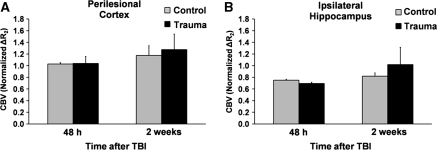
Cerebral Blood Volume After Traumatic Brain Injury Remained Unchanged
To investigate hemodynamic coupling between CBF and CBV after TBI, we investigated CBV at 48 hours and 2 weeks after TBI in all brain regions studied. Cerebral blood volume results from the perilesional cortex and ipsilateral hippocampus are shown in Figure 4. Unlike what was expected, in all regions studied, CBV did not differ from that in controls. For example, in the ipsilateral hippocampus, where a significant decrease in CBF was found at 48 hours after TBI, the CBV remained unchanged. Both CBV and CBF did not correlate according to the relationship CBV=0.5 × CBF0.5 (van Zijl et al, 1998) in any region studied in either TBI rats or sham-operated rats.
Figure 4.
Regional cerebral blood volume (CBV) in the (A) perilesional cortex and (B) ipsilateral hippocampus. CBV was measured at 48 hours and at 2 weeks after traumatic brain injury (TBI, n=9) or sham operation (n=4). Data are presented as mean±s.e.m. No statistically significant differences were found between the groups (Mann–Whitney U-test).
Blood Vessel Densities at 6 hours and 2 Weeks After Traumatic Brain Injury
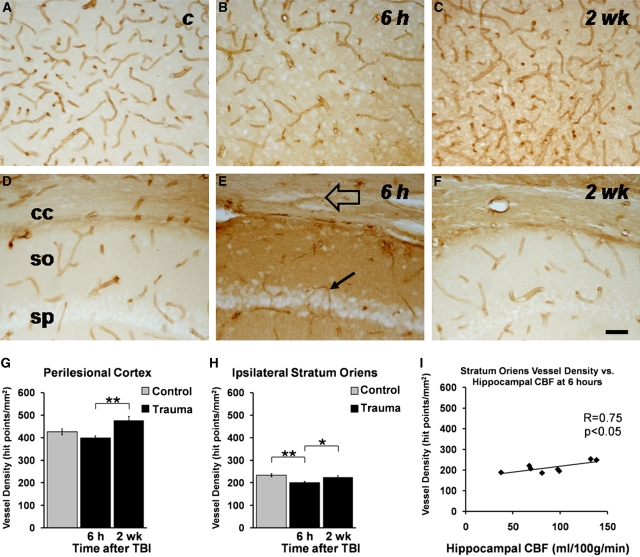
Vessel densities were quantified from histologic sections sampled from the levels corresponding to the MRI slice from which CBF and CBV were analyzed. Contralaterally, vessel density in injured rats did not differ from that in controls in any of the regions studied (Table 2). At 6 hours, vessel density in the perilesional cortex tended to become reduced to ∼90% of that in controls (P>0.05, Figures 5B and 5G). By 2 weeks after injury, vessel density tended to increase and was on average 112% of the control mean (P>0.05), yet most importantly it was 120% of that measured at 6 hours (P<0.01).
Table 2. Density of blood vessels in different brain areas after traumatic brain injury (TBI).
|
Blood vessel density (hit points per mm2) |
|||
|---|---|---|---|
| Control (n=6) | 6 hours after TBI (n=6) | 2 weeks after TBI (n=7) | |
| Cortex | |||
| Perilesional cortex | 426±13 | 399±10 | 477±18## |
| Contralateral cortex | 402±14 | 387±9 | 446±16 |
| Hippocampus (total) | |||
| Ipsilateral | 221±9 | 222±8 | 222±3 |
| Contralateral | 213±8 | 221±2 | 209±5 |
| CA1 stratum oriens | |||
| Ipsilateral | 233±7 | 201±5** | 225±7# |
| Contralateral | 244±8 | 238±9 | 231±5 |
| Thalamus | |||
| Ipsilateral | 368±22 | 412±16 | 431±31 |
| Contralateral | 357±25 | 384±9 | 381±15 |
The number of rats is in parentheses. Data are shown as mean±s.e.m. Vessel densities in the perilesional cortex and ipsilateral CA1 stratum oriens were significantly between the control group, 6-hours group, and 2-week group (P<0.05, Kruskal–Wallis test). Post hoc statistical significances are shown: **P<0.01 as compared with controls (Mann–Whitney U-test); #P<0.05, ##P<0.01 as compared with the 6-hour time point (Mann–Whitney U-Test). Control measures from each time point did not vary; thus, control data were pooled.
Figure 5.
Blood vessel density at 6 hours and 2 weeks after traumatic brain injury (TBI). Photomicrographs show blood vessels from representative sections from (A) cortex (control), (B) perilesional cortex 6 hours after injury, (C) perilesional cortex 2 weeks after injury, (D) stratum oriens of CA1 (control), (E) stratum oriens of CA1 6 hours after injury, and (F) stratum oriens of CA1 2 weeks after injury. The open arrow highlights postinjury tearing of the corpus callosum, and the solid arrow highlights a thin blood vessel at 6 hours after injury. Scale bar=50 μm and applies to all panels. Bar graphs show the quantitative analysis in (G) the perilesional cortex and (H) ipsilateral stratum oriens of CA1. **P<0.01, *P<0.05 (Mann–Whitney U-Test). (I) Correlation between the hippocampal blood flow (CBF) and vessel density in the ipsilateral stratum oriens of the CA1 at 6 hours after injury. The decrease in vessel density was associated with reduced CBF (P<0.05, Pearson's correlation test). c, control; cc, corpus callosum; so, stratum oriens of CA1; sp, stratum pyramidale of CA1. Density of vessels is presented as mean±s.e.m. in controls (n=6) and at 6 hours (n=6) or 2 weeks (n=7) after TBI.
When the density of vessels was quantified from the whole hippocampus, no difference to controls was observed at either time point. However, our visual analysis of sections showed an acute vessel loss in the CA1 subfield after injury. Quantification of vessel density focused on the stratum oriens of CA1 indicated that only 80% of vessels were remaining at 6 hours as compared with controls (P<0.01, Figures 5E and 5H). Interestingly, at 6 hours, the vessel density in the ipsilateral stratum oriens correlated with hippocampal CBF (r=0.753, P<0.05, Figure 5I). Vessel density returned to the control level by 2 weeks, being 112% of that measured at 6 hours (P<0.05, Figures 5F and 5H). Vessel density and CBF measures did not correlate in the other regions studied.
Discussion
This study was designed to test a hypothesis that postacute hemodynamic responses to TBI in rodents match those of patients. For this, we quantified absolute CBF and relative CBV changes induced by LFPI in rats during a 2-week follow-up period that is the time window for the initiation of brain tissue recovery. The data presented show several novel findings. First, at the acute phase (i.e., at 6 hours after injury), LFPI causes widespread ipsilateral and contralateral hypoperfusion, including both the cortex and hippocampus bilaterally, as well as the ipsilateral thalamus. Second, three phases of perilesional alterations in CBF, progressing from hypoperfusion to normal and back to hypoperfusion within 2 weeks, are shown for the first time in a rat TBI model. Finally, we show a loss of blood vessels at 6 hours in the ipsilateral stratum oriens and an increase in vessel density by 2 weeks both there and in the perilesional cortex as well. To our knowledge, this is the first time that hemodynamic profiles for several subregions of the brain have been described both during and beyond the acute phase of TBI in rats.
The Three Phases of the Hemodynamic Profile Around the Lesion
The perilesional cortex and ipsilateral hippocampus showed acute hypoperfusion, followed by normalization or overshoot at 24 hours, and then secondary hypoperfusion over the next 2 weeks when assessed by ASL MRI. Acute ipsilateral hypoperfusion occurring within a few hours after TBI is well described in rats by different methodologies, including autoradiography, radiolabeled microspheres, and laser Doppler flowmetry (Pasco et al, 2007; Yuan et al, 1988; Yamakami and McIntosh, 1989; Bryan et al, 1995; Nilsson et al, 1996; DeWitt et al, 1997; Thomale et al, 2002; Dietrich et al, 1998; Robertson et al, 2000). Our study extends these observations to show that it is particularly the perilesional cortex, ipsilateral hippocampus, and ipsilateral thalamus in which CBF is compromised acutely. Moreover, CBF reduction was associated with decreased vessel density in the stratum oriens at the acute time point.
In our study, by 24 hours after TBI, CBF recovered in all areas studied, including the perilesional cortex, ipsilateral hippocampus, and thalamus. This is in contrast with cortical hypoperfusion observed after severe temporal fluid percussion (Ishige et al, 1987). However, in this study, rats were subjected to prolonged hypoxia and TBI, which could explain the prolonged hypoperfusion observed. In line with our findings, Forbes et al (1997) used ASL MRI to measure CBF at 24 hours after CCI and pericontusional values were comparable with controls. In contrast, Thomale et al (2002) reported pericontusional hyperperfusion at 24 hours after CCI injury by using laser Doppler flowmetry, which we found only in the perilesional cortex at this time point. The discrepancy between CCI studies could be due to injury severity, as the former study used a 2.5-mm deformation depth, whereas the more recent results came after 0.5-mm deformation. The different measurement techniques may also explain the variation between studies.
From day 2, hypoperfusion returned to the perilesional cortex and ipsilateral hippocampus. To our knowledge, this has not been previously documented in animal models. In the perilesional cortex, it became most apparent at 2 weeks after TBI. In the ipsilateral hippocampus, however, CBF had recovered by 1 week after injury.
Taken together, the three-phase profile of hemodynamic changes can be seen in the regions known to undergo neurodegeneration and repair processes after LFPI, including the cortex, hippocampus, and thalamus (Thompson et al, 2005). Although each of these regions has a slightly different time course of CBF alterations, data obtained in the LFPI model match the general pattern observed in TBI patients (Marion et al, 1991; Bouma et al, 1991; Kelly et al, 1997; Martin et al, 1997).
Traumatic Blood Injury Also Invokes Alterations in Cerebral Blood Flow Contralaterally
Contralaterally, the three-phase profile of hemodynamic changes was detected in the cortex and hippocampus. However, contralateral hypoperfusion was less pronounced than in ipsilateral regions around the lesion, which is in line with previous reports in rat TBI models (Pasco et al, 2007; Yamakami and McIntosh, 1989; Bryan et al, 1995; Nilsson et al, 1996; DeWitt et al, 1997; Dietrich et al, 1998), and the hemodynamic profile was normal after 2 days after injury. Yet, unlike in this study, some previous studies noted a faster recovery of contralateral CBF occurring within only a few hours. For example, Yamakami and McIntosh (1989) reported contralateral hypoperfusion at 1 hour after moderate LFPI and it recovered by 2 hours. This difference may be due to the less severe injury than that of our study. After CCI TBI, contralateral hypoperfusion was reported to normalize by 4 hours after trauma (Bryan et al, 1995). Conversely, Ishige et al (1987) observed extensive contralateral hypoperfusion that extended to 24 hours after TBI. As noted before, in this study, rats were exposed to hypoxic conditions and severe temporal fluid-percussion injury. This study supports the idea of posttraumatic contralateral hypoperfusion after TBI, although its extent may be severity and model dependent.
Hemodynamic Disruption may Relate to Vascular Changes
This study found no significant CBV changes after TBI. The only similar previous study we know of was by Pasco et al (2007), who found a CBV decrease 1 to 5 hours after moderate LFPI in rats that coincided with hypoperfusion and was resolved by day 3. This contrasts with our study, which suggests that normal physiological coupling between CBV and CBF is lost after severe LFPI because hypoperfusion never coincided with decreased CBV. Moreover, CBV and CBF did not correlate in any region studied at either 48 hours or 2 weeks after TBI in our study. However, it is possible that the sensitivity of the relative CBV measurement was insufficient for such relationships to be tested conclusively between different data sets acquired during extended imaging sessions, because CBV and CBF measures did not correlate significantly in the same brain regions of control animals.
We report an increase in vessel density between 6 hours and 2 weeks after TBI both in the stratum oriens and the perilesional cortex. In the perilesional cortex, CBF measures did not correlate with vessel density, and it is possible that newly formed vessels may not be fully functional 2 weeks after TBI. A functional endothelium would initiate hyperemia in response to hypoxia and hypercapnia that occur because of low CBF. As shown in this study, there was a trend toward vascular loss in the perilesional cortex and ∼20% vessel density decrease in the stratum oriens of CA1 at 6 hours after injury. A recent immunohistochemistry study by Park et al (2009) showed cortical blood vessel loss 24 hours after LFPI, which recovered by 2 weeks in moderately injured rats but not in severely injured rats. Vessel loss and recovery may provide some explanation to the hemodynamic uncoupling we observed. However, we observed hypoperfusion in regions without significant vessel loss, such as the thalamus. Moreover, previous studies have shown that LFPI causes blood–brain barrier damage (Cortez et al, 1989; Tanno et al, 1992) and an endothelium that is incapable of detecting conditions requiring hyperemia (Muir et al, 1992; Forbes et al, 1997). In fact, vascular damage has been suggested to be a mechanism for the loss of cerebral autoregulation after TBI, resulting in CBV and CBF uncoupling (Greve and Zink, 2009), and thus vascular damage could partly explain the uncoupling at the acute phase found in this study.
Metabolic Changes and Vasoconstriction may Induce Hemodynamic Disruption
As hemodynamic changes can only be partly explained by cerebrovascular damage or vessel remodeling, we speculate that hypoperfusion and recovery after TBI are likely due to other cellular processes. Traumatic brain injury induces immediate metabolic uncoupling in rats, whereby cytotoxicity leads to increased cerebral glucose utilization while CBF remains low (Ginsberg et al, 1997; Richards et al, 2001). As early as 6 hours after TBI in rats, a period of depressed oxidative metabolism and reduced CBF may occur (Hovda et al, 1995). Decreased CBF results from vasoconstriction induced by endothelium-derived contracting factor signaling after TBI (reviewed in Golding, 2002). This may explain the initial cerebral hypoperfusion we observed in this study. Later, hyperemia may occur in response to hyperglycolysis (Martin et al, 1997) that occurs due to cellular efforts to reestablish ionic gradients (Hovda et al, 1995). Gliosis begins ∼24 hours after TBI (Gehrmann et al, 1995; Graham et al, 2000), which is an energetically demanding process and may in part be responsible for the recovery of CBF observed at this time point in many regions. Finally, vasospasm (Martin et al, 1997) or vasoconstriction caused by delayed vasogenic edema (Rangel-Castilla et al, 2008) may lead to the second hypoperfusion phase we observed. However, the mechanisms of hemodynamic responses to TBI are multifactorial and require further characterization (Park et al, 2009).
Methodological Concerns
In this study, rat mortality after LFPI was 30%. Our study did not investigate the causes of mortality but as many rats died very soon after LFPI, it is likely that severe hypotension or elevated intracranial pressure were responsible (Prins et al, 1996). Other research suggests that hypoxemia due to prolonged apnea may lead to mortality soon after injury (McIntosh et al, 1989).
One limitation of animal studies is that anesthesia is required for MRI. A combination of isoflurane and nitrous oxide, which is the most commonly used recovery anesthesia in injured rats, has been shown to increase baseline blood flow (Hansen et al, 1989; Iltis et al, 2005). Throughout all imaging sessions, anesthesia dosage was consistently kept as small as possible by measuring and maintaining the breathing rate throughout. Consequently, the physiological variables of the rats in this study remained within normal limits during the extended MRI sessions and there were no significant differences in the blood-derived parameters between injured and control rats.
Arterial spin labeling quantification is dependent on tissue T1, partition coefficient (λ) and the quality of the labeling B1 field. Cerebral blood flow values were corrected using T1 maps from individual animals at each time point, and the quality of B1 was controlled by B1 mapping. We cannot rule out the possibility that λ increases from 0.9 to 0.99 in the edematous tissue, which corresponds to a possible 9% uncertainty for CBF (Herscovitch and Raichle, 1985). Although CBF and CBV maps carry data regarding the lesion core, these data were not analyzed due to dramatic blood–brain barrier breakdown and tissue loss taking place in the core area, which likely leads to significant, uncontrollable changes in λ and leakage of the intravascular contrast agent.
Arterial spin labeling MRI provides completely noninvasive, absolute quantification of CBF. This allows consecutive imaging of individual subjects; thus, long-term animal studies of hemodynamic changes are feasible over many time points. This is not the case with autoradiography methods, which are limited to single time points per animal. As shown in this study, ASL also allows detailed delineation of regional CBF in many brain regions after each scan; thus, the spatial resolution is an improvement over minimally invasive Doppler methods.
Conclusions
Our results show for the first time that acute and subacute regional hemodynamic changes in a rat LFPI model follow a general pattern resembling that of TBI patients. This makes it feasible to use this model to study mechanisms behind hemodynamic changes and explore novel therapeutic targets and future treatments for secondary brain damage due to secondary hypoperfusion after TBI.
Acknowledgments
We thank Maarit Pulkkinen and Jarmo Hartikainen for providing technical assistance. We also thank Guerbert (Paris, France) for generously providing Sinerem contrast agent for the MRI studies.
The authors declare no conflict of interest.
Footnotes
This study was funded by a Marie Curie Early Stage Trainee Research Fellowship MEST-CT-2005-019217, by The Academy of Finland (AP, OG) and by The Sigrid Juselius Foundation (AP).
References
- Barbier EL, Lamalle L, Decorps M. Methodology of brain perfusion imaging. J Magn Reson Imaging. 2001;13:496–520. doi: 10.1002/jmri.1073. [DOI] [PubMed] [Google Scholar]
- Bonne O, Gilboa A, Louzoun Y, Kempf-Sherf O, Katz M, Fishman Y, Ben-Nahum Z, Krausz Y, Bocher M, Lester H, Chisin R, Lerer B. Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res. 2003;124:141–152. doi: 10.1016/s0925-4927(03)00109-4. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Bryan RM, Jr, Cherian L, Robertson C. Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth Analg. 1995;80:687–695. doi: 10.1097/00000539-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Cortez SC, McIntosh TK, Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989;482:271–282. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Prough DS. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Smith TG, Deyo DJ, Miller KR, Uchida T, Prough DS. L-arginine and superoxide dismutase prevent or reverse cerebral hypoperfusion after fluid-percussion traumatic brain injury. J Neurotrauma. 1997;14:223–233. doi: 10.1089/neu.1997.14.223. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Prado R, Zhao W, Dewanjee MK, Ginsberg MD.1998Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats Neurosurgery 43585–593.discussion 593–4 [DOI] [PubMed] [Google Scholar]
- Dunn JF, Roche MA, Springett R, Abajian M, Merlis J, Daghlian CP, Lu SY, Makki M. Monitoring angiogenesis in brain using steady-state quantification of DeltaR2 with MION infusion. Magn Reson Med. 2004;51:55–61. doi: 10.1002/mrm.10660. [DOI] [PubMed] [Google Scholar]
- Forbes ML, Hendrich KS, Kochanek PM, Williams DS, Schiding JK, Wisniewski SR, Kelsey SF, DeKosky ST, Graham SH, Marion DW, Ho C. Assessment of cerebral blood flow and CO2 reactivity after controlled cortical impact by perfusion magnetic resonance imaging using arterial spin-labeling in rats. J Cereb Blood Flow Metab. 1997;17:865–874. doi: 10.1097/00004647-199708000-00005. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Banati RB, Wiessner C, Hossmann KA, Kreutzberg GW. Reactive microglia in cerebral ischaemia: an early mediator of tissue damage. Neuropathol Appl Neurobiol. 1995;21:277–289. doi: 10.1111/j.1365-2990.1995.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Zhao W, Alonso OF, Loor-Estades JY, Dietrich WD, Busto R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol. 1997;272:H2859–H2868. doi: 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- Golding EM. Sequelae following traumatic brain injury. The cerebrovascular perspective. Brain Res Brain Res Rev. 2002;38:377–388. doi: 10.1016/s0165-0173(02)00141-8. [DOI] [PubMed] [Google Scholar]
- Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–651. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- Hansen TD, Warner DS, Todd MM, Vust LJ. Effects of nitrous oxide and volatile anaesthetics on cerebral blood flow. Br J Anaesth. 1989;63:290–295. doi: 10.1093/bja/63.3.290. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME. What is the correct value for the brain—blood partition coefficient for water. J Cereb Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Lee SM, Smith ML, Von Stuck S, Bergsneider M, Kelly D, Shalmon E, Martin N, Caron M, Mazziotta J. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotrauma. 1995;12:903–906. doi: 10.1089/neu.1995.12.903. [DOI] [PubMed] [Google Scholar]
- Iltis I, Kober F, Dalmasso C, Lan C, Cozzone PJ, Bernard M. In vivo assessment of myocardial blood flow in rat heart using magnetic resonance imaging: effect of anesthesia. J Magn Reson Imaging. 2005;22:242–247. doi: 10.1002/jmri.20352. [DOI] [PubMed] [Google Scholar]
- Ishige N, Pitts LH, Berry I, Carlson SG, Nishimura MC, Moseley ME, Weinstein PR. The effect of hypoxia on traumatic head injury in rats: alterations in neurologic function, brain edema, and cerebral blood flow. J Cereb Blood Flow Metab. 1987;7:759–767. doi: 10.1038/jcbfm.1987.131. [DOI] [PubMed] [Google Scholar]
- Kelly DF, Martin NA, Kordestani R, Counelis G, Hovda DA, Bergsneider M, McBride DQ, Shalmon E, Herman D, Becker DP. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg. 1997;86:633–641. doi: 10.3171/jns.1997.86.4.0633. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Immonen R, Grohn O, Pitkanen A. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain. 2007;130:3155–3168. doi: 10.1093/brain/awm268. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg. 1991;74:407–414. doi: 10.3171/jns.1991.74.3.0407. [DOI] [PubMed] [Google Scholar]
- Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, Shalmon E, Hovda DA, Becker DP. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Muir JK, Boerschel M, Ellis EF. Continuous monitoring of posttraumatic cerebral blood flow using laser-Doppler flowmetry. J Neurotrauma. 1992;9:355–362. doi: 10.1089/neu.1992.9.355. [DOI] [PubMed] [Google Scholar]
- Ndode-Ekane XE, Hayward N, Grohn O, Pitkanen A. Vascular changes in epilepsy: functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience. 2010;166:312–332. doi: 10.1016/j.neuroscience.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Gazelius B, Carlson H, Hillered L. Continuous measurement of changes in regional cerebral blood flow following cortical compression contusion trauma in the rat. J Neurotrauma. 1996;13:201–207. doi: 10.1089/neu.1996.13.201. [DOI] [PubMed] [Google Scholar]
- Park E, Bell JD, Siddiq IP, Baker AJ. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:575–584. doi: 10.1038/jcbfm.2008.151. [DOI] [PubMed] [Google Scholar]
- Pasco A, Lemaire L, Franconi F, Lefur Y, Noury F, Saint-Andre JP, Benoit JP, Cozzone PJ, Le Jeune JJ. Perfusional deficit and the dynamics of cerebral edemas in experimental traumatic brain injury using perfusion and diffusion-weighted magnetic resonance imaging. J Neurotrauma. 2007;24:1321–1330. doi: 10.1089/neu.2006.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GT, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press; 1998. [Google Scholar]
- Pitkanen A, McIntosh TK. Animal models of post-traumatic epilepsy. J Neurotrauma. 2006;23:241–261. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res Dev Brain Res. 1996;95:272–282. doi: 10.1016/0165-3806(96)00098-3. [DOI] [PubMed] [Google Scholar]
- Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus. 2008;25:E7. doi: 10.3171/FOC.2008.25.10.E7. [DOI] [PubMed] [Google Scholar]
- Richards HK, Simac S, Piechnik S, Pickard JD. Uncoupling of cerebral blood flow and metabolism after cerebral contusion in the rat. J Cereb Blood Flow Metab. 2001;21:779–781. doi: 10.1097/00004647-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Hendrich KS, Kochanek PM, Jackson EK, Melick JA, Graham SH, Marion DW, Williams DS, Ho C. Assessment of 2-chloroadenosine treatment after experimental traumatic brain injury in the rat using arterial spin-labeled MRI: a preliminary report. Acta Neurochir Suppl. 2000;76:187–189. doi: 10.1007/978-3-7091-6346-7_37. [DOI] [PubMed] [Google Scholar]
- Shen Y, Kou Z, Kreipke CW, Petrov T, Hu J, Haacke EM. In vivo measurement of tissue damage, oxygen saturation changes and blood flow changes after experimental traumatic brain injury in rats using susceptibility weighted imaging. Magn Reson Imaging. 2007;25:219–227. doi: 10.1016/j.mri.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Tanno H, Nockels RP, Pitts LH, Noble LJ. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: distribution and time course of protein extravasation. J Neurotrauma. 1992;9:21–32. doi: 10.1089/neu.1992.9.21. [DOI] [PubMed] [Google Scholar]
- Thomale UW, Kroppenstedt SN, Beyer TF, Schaser KD, Unterberg AW, Stover JF. Temporal profile of cortical perfusion and microcirculation after controlled cortical impact injury in rats. J Neurotrauma. 2002;19:403–413. doi: 10.1089/08977150252932361. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4:159–167. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakami I, McIntosh TK. Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J Cereb Blood Flow Metab. 1989;9:117–124. doi: 10.1038/jcbfm.1989.16. [DOI] [PubMed] [Google Scholar]
- Yuan XQ, Prough DS, Smith TL, Dewitt DS. The effects of traumatic brain injury on regional cerebral blood flow in rats. J Neurotrauma. 1988;5:289–301. doi: 10.1089/neu.1988.5.289. [DOI] [PubMed] [Google Scholar]