Abstract
Necrostatin-1 inhibits receptor-interacting protein (RIP)-1 kinase and programmed necrosis and is neuroprotective in adult rodent models. Owing to the prominence of necrosis and continuum cell death in neonatal hypoxia–ischemia (HI), we tested whether necrostatin was neuroprotective in the developing brain. Postnatal day (P)7 mice were exposed to HI and injected intracerebroventricularly with 0.1 μL of 80 μmol necrostatin, Nec-1, 5-(1H-Indol-3-ylmethyl)-(2-thio-3-methyl) hydantoin, or vehicle. Necrostatin significantly decreased injury in the forebrain and thalamus at P11 and P28. There was specific neuroprotection in necrostatin-treated males. Necrostatin treatment decreased necrotic cell death and increased apoptotic cell death. Hypoxia–ischemia enforced RIP1–RIP3 complex formation and inhibited RIP3–FADD (Fas-associated protein with death domain) interaction, and these effects were blocked by necrostatin. Necrostatin also decreased HI-induced oxidative damage to proteins and attenuated markers of inflammation coincidental with decreased nuclear factor-κB and caspase 1 activation, and FLIP ((Fas-associated death-domain-like IL-1β-converting enzyme)-inhibitory protein) gene and protein expression. In this model of severe neonatal brain injury, we find that cellular necrosis can be managed therapeutically by a single dose of necrostatin, administered after HI, possibly by interrupting RIP1–RIP3-driven oxidative injury and inflammation. The effects of necrostatin treatment after HI reflect the importance of necrosis in the delayed phases of neonatal brain injury and represent a new direction for therapy of neonatal HI.
Keywords: apoptosis-necrosis cell death continuum, cytokines, delayed neurodegeneration, NFκB, programmed necrosis, receptor-interacting protein (RIP)
Introduction
Cells appear to be able to switch or coactivate alternative biochemical and structural processes of cell death if the initially activated mechanisms are occluded genetically, pharmacologically, or physiologically. The coactivation of apoptotic and necrotic cell deaths is prominent in the developing brain after hypoxia–ischemia (HI) and excitotoxicity (Northington et al, 2007; Portera-Cailliau et al, 1997). Cells undergoing this hybrid form of demise display morphologic features of both apoptosis and necrosis, which we have termed ‘continuum' cell death (Portera-Cailliau et al, 1997) and is similar to programmed necrosis, which has been described in vitro (Festjens et al, 2006). This hybrid form of cell death occurs upon cytokine-mediated death receptor activation, in the setting of caspase inhibition and seems to be under the control of the adaptor proteins receptor-interacting protein (RIP)1 and RIP3 kinase and prominently involves reactive oxygen species (ROS) (Kim et al, 2007; Zhang et al, 2009).
Necrostatin, 5-(1H-indol-3-ylmethyl)-3-methyl-2-sulfanylideneimidazolidin-4-one, a small molecule inhibitor of programmed cell necrosis, has shown promise as a neuroprotectant in adult rodent models of myocardial ischemia as well as traumatic and ischemic brain injury (Degterev et al, 2005; Lim et al, 2007; You et al, 2008). Necrostatin protects by inhibiting RIP1 kinase activation (Degterev et al, 2008) with possible downstream antiinflammatory effects (You et al, 2008) and perhaps other mechanisms. Although necrostatin has no intrinsic antioxidant activity, it blocks tumor necrosis factor (TNF)-induced activation of NADPH (nicotinamide adenine dinucleotide phosphate) oxidase (Kim et al, 2007) and nitric oxide-mediated necrosis (Davis et al, 2010) by the inhibition of both Nox1 and mitochondrial-derived oxygen-free radicals. In vitro, necrostatin has little effect on classic apoptotic cell death, but rather, strongly inhibits programmed cellular necrosis (Degterev et al, 2005). In neonatal brain HI, the prominence of necrosis and the hybrid ‘continuum' cell death (Northington et al, 2007), the known role of death receptor activation (Graham et al, 2004; Northington et al, 2001a), and the importance of oxidative injury and inflammation (Barks et al, 2008; Sheldon et al, 2004) led us to test the possibility that necrostatin could inhibit these mechanisms. We tested the efficacy of necrostatin to block neurodegeneration and cell death after neonatal HI and sought to identify the possible molecular mechanisms of action. This study shows that necrostatin could be an important translational neuroprotective drug for treating brain injury in newborns after HI by blocking a toxic cascade involving RIP1–RIP3 complex formation, oxidative injury, and inflammation.
Materials and methods
Animal Model
Animal studies were performed with the approval of the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine and carried out with standards of care and housing in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services 85–23, 1985.
Hypoxic-Ischemic Injury and Drug Administration
Postnatal day (P)7 mice were exposed to HI as described previously using the Vannucci model as adapted for neonatal mice (permanent ligation of the right common carotid and FiO2=0.08 minutes × 45 minutes) (Ditelberg et al, 1996; Graham et al, 2004). Fifteen minutes after the hypoxic exposure, the pups were briefly reanesthetized with isofluorane and 0.1 μL of 80 μmol necrostatin, Nec-1, 5-(1H-Indol-3-ylmethyl)-(2-thio-3-methyl) hydantoin (Alexis Biochemical, Plymouth Meeting, PA, USA) or vehicle, methyl-β-cyclodextrin (Sigma, St Louis, MO, USA) was injected intracerebroventricularly. The pups were returned to the dam until their killing (n=7 to 12 per group) at 3 hours, 24 hours (P8), P11, or P28 for histologic (n=7 to 12 per group) or biochemical analysis (n=6 per group). Additional groups included naive controls (n=6) and pups injected with either 0.1 or 0.2 μL of 80 μmol necrostatin or vehicle (n=6 each) for histologic analysis at P28. The necrostatin or vehicle-only groups were used to determine whether the drug alone causes deleterious effects on normal brain development.
Tissue Preparation and Histology
Histology
Animals were killed with an overdose of pentobarbital, 65 mg/kg intraperitoneally, and exsanguinated with cold 0.1 mol/L phosphate-buffered saline (pH 7.4) by intracardiac perfusion. Mice were perfusion fixed with 4% paraformaldehyde in phosphate-buffered saline for 20 minutes at 4 mL/min by intracardiac perfusion. The brains were postfixed in 4% paraformaldehyde for 12 to 16 hours, then cryoprotected in 20% glycerol, and frozen in isopentane (−30°C). Coronal sections of 60-μm thickness were cut on a sliding microtome for cresyl violet (CV) staining for injury analysis.
Injury Analysis
iVision software (iVision, Atlanta, GA, USA) was used to quantitate the percentage injury in each of the regions examined. Percentage injury was determined by measuring the total area of the contralateral cortex, hippocampus, or thalamus and comparing it with the uninjured area in the corresponding ipsilateral region. Injury is measured at four coronal levels for each region and combined for a single percentage injury score for each region of interest in each brain. As an error could be introduced into the measurements with uneven coronal sectioning of the brain, six naive sham brains were evaluated with the above-mentioned procedure and found to have side-to-side differences in area of 0.5% to 2.0%, suggesting that sectioning does not introduce a significant error into the measurements. As an additional assessment of injury at P8, optical densitometry (OD) of CV staining at multiple levels in the hippocampus was measured using the iVison software, corrected for background, and compared with OD of the contralateral hippocampus. The ipsilateral/contralateral OD ratio was compared between necrostatin- and vehicle-treated mice. Injection-only animals were analyzed to determine the consistency of CV staining and ipsilateral/contralateral OD ratios in these animals without visible injury were >0.95.
To determine whether there was a gender effect of necrostatin on neonatal HI injury, cerebellar and brain stem sections were stained for SRY (SRY C-17, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then reanalyzed to determine whether sex was a determinate of necrostatin neuroprotection.
To determine whether there was an effect of necrostatin treatment on different cell death phenotypes after neonatal HI, CV-stained sections from necrostatin- and vehicle-treated mice at P11 were used to photograph the cerebral cortex (sensorimotor cortex) in random nonoverlapping fields, under oil immersion. The morphology of individual dying cells in five high-resolution digital images were classified as apoptotic, necrotic, and continuum, using specific criteria as described previously (Martin et al, 1998) and then counted by an observer blinded to the treatment group.
OxyBlot Analysis, Immunoprecipitation, and Protein Immunoblotting
Ipsilateral forebrain samples were obtained immediately after pentobarbital overdose and then frozen (n=6 per group at 3 and 24 hours (P8) after HI). Cytosolic extracts obtained using NE-PER extraction reagents (Pierce Biotechnology, Rockford, IL, USA) were analyzed using the OxyBlot detection kit, (Chemicon International, Temecula, CA, USA) for detection of carbonyl modification of proteins as a measure of oxidative injury as described previously (Mueller-Burke et al, 2008). Films were scanned using Adobe Photoshop (San Jose, CA, USA) and quantitated with NIH Image J Software (NIH, Bethesda, MD, USA). The reliability of sample loading and protein transfer was evaluated by staining nitrocellulose membranes with Ponceau S before immunoblotting and by quantification of Coomassie-stained gels with optical densitometry.
Forebrain cytosolic extracts (250 μg of protein) from vehicle- and necrostatin-treated (3 and 24 hours) and control mice were combined with anti-RIP3 and incubated overnight, gently mixing at 4°C. Immobilized Protein A (Thermo Scientific, Rockford, IL, USA), 100 μL, was added to the antigen–antibody complex and gently mixed at room temperature for 2 hours. To remove any unbound protein, the samples were washed 4 times with 0.5 mL of immunoprecipitation buffer (Thermo Scientific) and centrifuged for 3 minutes at 2,500 × g. The supernatant was discarded after each wash. The pellet was washed with 0.5 mL distilled H2O, centrifuged for 3 minutes at 2,500 × g, and the supernatant discarded. The beads were resuspended in 50 μL of 2 × treatment buffer and boiled for 5 minutes, then centrifuged at 14,000 × g for 5 seconds. The supernatant (20 μL per lane) was loaded onto a gel for SDS-PAGE. Blots were incubated overnight at 4°C with anti-RIP1, exposed to a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (0.2 μg/mL) and developed with enhanced chemiluminescence.
To determine the specificity of anti-RIP3 immunoprecipitation, 2.5 μg of antibody was preincubated with 5 μg of recombinant RIP3 (Abnova, Taipei, Taiwan), and then immunodepleted by pull down with protein A-agarose beads. The cleared supernatant was then used to immunoprecipitate another aliquot of the sample from a 24-hour vehicle mouse determined to have abundant RIP3–RIP1 interaction in the immunoprecipitation studies.
Protein immunoblotting experiments for FLIP ((Fas-associated death-domain-like interleukin (IL)-1β-converting enzyme)-inhibitory protein), TNFα, RIP1, and RIP3 were performed as described previously (Graham et al, 2004).
Antibodies
Our use of anti-FLIP (G-11) and anti-Fas-associated protein with death domain (FADD) (H181) antibodies (Santa Cruz Biotechnology) has been described previously (Graham et al, 2004). Anti-RIP1, (0.2 μg/mL) (H-207, Santa Cruz Biotechnology) is a rabbit polyclonal antibody that does not cross-react with RIP2 or RIP3. Anti-RIP3 (2 μg/mL) (Abcam, Cambridge, MA, USA) is a rabbit polyclonal antibody used for both immunoprecipitation and immunoblotting experiments. Anti-TNFα (0.1 μg/mL) (Millipore, Billerica, MA, USA) is a rabbit polyclonal antibody raised against recombinant mouse TNFα.
Determination of Gene Expression by Real-Time Quantitative Reverse Transcriptase-PCR
Total RNA was extracted from the forebrain using the PureLink Micro-to-midi total RNA purification system (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's specifications. Approximately 1 μg of total RNA was used for the generation of complementary DNA (cDNA) using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Reverse-transcription protocol included 5 minutes at 25°C, 30 minutes at 42°C, and 5 minutes at 85°C. Complementary DNA was then used to amplify FLIP, TNFα, IL-1β, IL-6, and IL-12p35 genes by real-time quantitative reverse transcriptase-PCR using 300 nmol/L concentration of specific primers designed for mouse sequences (Table 1). The amplification protocol included 40 cycles of 1 minute at 95.0°C, 1 minute at 60°C, and 2 minutes at 72°C. Fold difference in gene expression was corrected to G6PDH (reference gene) using the method of Pfaffl (2001). Melting curves confirm the amplification of a single gene product of the expected molecular weight.
Table 1. Primers used for real time qRT-PCR.
| Gene | Direction | Primer set | UniSTS |
|---|---|---|---|
| FLIP | Sense | 5′-GGAGTCATTACATTAAAGATTATTTCT-3′ | 207162 |
| Antisense | 5′-GATTAAAAAGACACAGATAGATTGTTT-3′ | ||
| IL-1β | Sense | 5′-TTCAAGGGGACATTAGGCAG-3′ | 125786 |
| Antisense | 5′-TGTGCTGGTGCTTCATTCAT-3′ | ||
| IL-6 | Sense | 5′-TCCTTTTTCCTTATCTCTTTGCC-3′ | 130584 |
| Antisense | 5′-GCCTCTAACTCACAGAGATCTTCC-3′ | ||
| IL12p35 | Sense | 5′-TCACATCTCATCTCCCCAAA-3′ | 159124 |
| Antisense | 5′-TCTGCTAACACATTGAGGGG-3′ | ||
| TNFα | Sense | 5′-GGGACAGTGACCTGGACTGT-3′ | 144644 |
| Antisense | 5′-CTCCCTTTGCAGAACTCAGG-3′ | ||
| G6PDH | Sense | 5′-GAAGCCTGGCGTATCTTCAC-3′ | Ref. |
| Antisense | 5′-GTGAGGGTTCACCCACTTGT-3′ |
Ref, reference gene.
Caspase Activity Assay
Cytosolic extracts obtained from the forebrain were assayed for caspase 1 or caspase 3 activity using the respective Colorimetric Activity Assay Kits, (APT161 and APT165; Millipore). Cytosolic extracts (400 μg) were reconstituted in cell lysis buffer and diluted in the same volume of dithiothreitol (10 mmol/L) for caspase 1 and in an assay buffer for caspase 3 assay, as recommended by the manufacturer. YVAD-ρNA (caspase 1, 200 μmol/L) or Ac-DEVD-pNA (caspase 3, 30 μg/dL) substrate was added to the mix and incubated for 60 minutes at 37°C. The chromophore ρ-NA (p-nitroaniline) produced after cleavage from the labeled substrate was measured at 405 nm using a Model 680 Microplate Reader (Bio-Rad). For caspase 3, a standard curve was generated using recombinant human caspase 3 protein (CC119, Millipore, Temecula, CA, USA). A unit of caspase 3 is defined as the amount of enzyme that cleaves 1.0 nmol of the labeled substrate per hour at 37°C.
Nuclear Factor-κB p65 Transcription Factor Assay
Nuclear extracts, obtained by NE-PER extraction reagents (Pierce Biotechnology), were used to determine concentrations of the active nuclear factor (NF)κB attached to the consensus sequence (5′-GGGACTTTCC-3′) in the biotinylated capture probe bound to the streptavidin plate and detected by a primary rabbit anti-NFκB p65 antibody (1:1,000) followed by a horseradish peroxidase-conjugated goat anti-rabbit secondary detection antibody (1:500). Positive (TNFα-treated HeLa whole-cell extract), specific competitor (NFκB competitor oligonucleotide), and negative controls were used to establish accuracy of the results. Absorbance at 450 nm was determined using a microplate reader. Nuclear extracts (1 μL) were visualized at × 400 to confirm the presence of intact spherical nuclei.
Statistical Analysis
Mann–Whitney U-test was used to compare percentage injury, ipsilateral/contralateral OD ratios, cell death phenotype, and results from gene expression studies in necrostatin- and vehicle-treated mice, whereas Kruskal–Wallis ANOVA (analysis of variance) was used to determine progression of injury over time. Results were reported as medians with interquartile range (25th to 75th percentile) and, in most cases, represented as box-and-whisker plots in which 75th and 25th percentiles (interquartile range) define the upper and lower limits of the box, respectively, and the median is represented as the line inside the box. Optical densitometry measurements of carbonyl-modified proteins were analyzed using paired analysis, whereas NFκB p65, caspase activity, as well as TNFα and FLIP protein expression were analyzed with unpaired t-tests. Significance was assigned to P-values <0.05, and Bonferroni's correction was applied when multiple comparisons were performed. Statistical analyses were performed using Stata version 10.0 (Stata, College Station, TX, USA) and SPSS 14.0 (SPSS, Chicago, IL, USA).
Results
Necrostatin Provides Delayed and Persistent Neuroprotection by Blocking Injury Progression after P8 in the HI-Injured Ipsilateral Forebrain, Hippocampus, and Thalamus
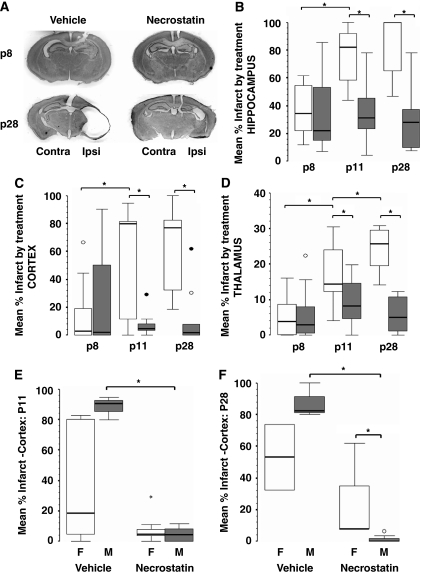
Unilateral carotid artery ligation followed by 45 minutes of hypoxia produces a moderate-to-severe brain injury in immature mice (Ditelberg et al, 1996; Graham et al, 2004) as shown in this study in vehicle-treated mice (Figure 1A). This injury is attenuated by necrostatin when administered immediately after HI (Figure 1A). Injury within the ipsilateral hippocampus is evident by 24 hours (P8), in both necrostatin- and vehicle-treated controls and is not different between groups (Figure 1B). After 24 hours, injury in vehicle-treated mice progresses to a large area of porencephalic damage in the ipsilateral cortex and hippocampus, and the ipsilateral thalamus atrophies (Figures 1B to 1D). Progression of injury after P8, in necrostatin-treated mice is markedly attenuated with a much smaller area of porencephaly and significant preservation of the ipsilateral cortex, hippocampus, and thalamus when compared with vehicle-treated mice at P28 (Figure 1A).
Figure 1.
Necrostatin provides delayed and persistent neuroprotection after neonatal HI with possible gender effects. (A) Representative coronal photomicrographs at the level of anterior hippocampus at postnatal day P8 and P28 comparing the degree of infarct after hypoxia–ischemia (HI) with subsequent injection of vehicle or necrostatin at P7. No difference is evident at P8; however, significant neuroprotection is seen at P28 after post-HI treatment with necrostatin. Percentage infarct at P8, P11, and P28 in the (B) hippocampus, (C) cerebral cortex, (D) and thalamus after HI and treatment with vehicle (white) or necrostatin (gray). Percentage cortical infarct by gender is also shown at (E) P11 and (F) P28 comparing females (white) and males (gray). Data are represented as box-and-whisker plots with 75th and 25th percentiles (interquartile range) defining the upper and lower limits of the box, respectively, and the median is represented by the line inside the box. *P≤0.02 (Mann–Whitney U-test corrected by Bonferroni). ○, outliers; •, extremes. n=7 to 12 per group, n=3 to 8 per group when stratified by gender).
There is no difference in percentage injury in necrostatin versus vehicle treatment when measured at 24 hours after HI (P8) in the hippocampus (Figure 1B), cerebral cortex (Figure 1C), or thalamus (Figure 1D). When P8 hippocampal injury was further analyzed with OD for loss of CV staining as the marker of injury instead of percentage injury based on area measurements, no difference was found between necrostatin- and vehicle-treated mice (median ipsilateral/contralateral OD; median: necrostatin=0.79, vehicle=0.86, P=0.34), thus confirming the measures of injured area. After P8, injury progresses in all regions in vehicle-treated mice but not in necrostatin-treated mice, and by P11, there is detectable neuroprotection in necrostatin-treated mice compared with those receiving vehicle, in the cortex (P=0.01), hippocampus (P<0.001), and thalamus (P=0.003). Owing to the severe degree of injury in the cerebral cortex and hippocampus in vehicle-treated mice, no further progression of injury is seen between P11 and P28. Although less severely injured, there is no progression of injury in the cerebral cortex or hippocampus in necrostatin-treated mice either; thus, the neuroprotection evident at P11 in necrostatin-treated mice persists at P28. In contrast, thalamic injury continues to increase from P11 to P28 in vehicle-treated mice (Figure 1D) (P<0.001). No additional injury in the thalamus is seen in necrostatin-treated mice after P8, thus increasing the difference between vehicle- and necrostatin-treated groups at P28 in the thalamus (Figure 1D).
When percentage injury in the cortex was stratified by gender, no effect was evident at P8, but significant neuroprotection was found in necrostatin-treated males at P11 (Figure 1E) (P=0.02) and at P28 (Figure 1F) (P=0.01). The median injury score in necrostatin-treated females and males was equivalent at P11, but necrostatin-treated females were not different from vehicle-treated females which showed wide variation in injury. In fact, females account for almost all of the variability in the combined data at both P11 and P28 (comparing Figure 1C with Figures 1E and 1F). At P28, in addition to having less injury than vehicle-treated males, necrostatin-treated males also had less cortical injury than necrostatin-treated females ((P=0.01) Figure 1F).
Necrostatin Blocks Necrotic Cell Death and Shifts the Cell Death Phenotype After Neonatal Hypoxia–Ischemia
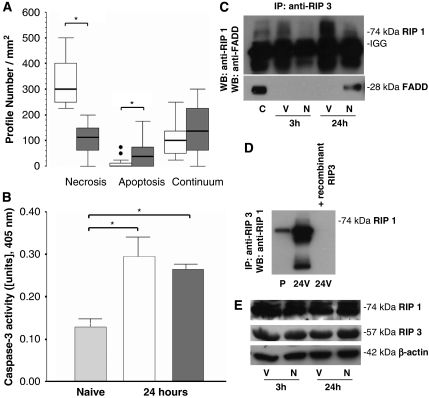
In the sensorimotor cortex of vehicle-treated HI mice at P11, cells were found undergoing classic necrosis, apoptosis, and continuum forms of cell death (Figure 2A). Cellular necrosis was the dominant phenotype in the HI cerebral cortex at P11 (Figure 2A). Necrostatin significantly decreased the number of cells undergoing necrosis in the cortex at P11 after neonatal HI (median; necrostatin=112.5 (dark gray) versus vehicle=300 (white), P<0.001 versus vehicle). In contrast, there was an increase in apoptotic cells in necrostatin-treated mice (Figure 2A) (median; necrostatin=37.5 versus vehicle=0, P=0.01 versus vehicle). Surprisingly, the number of cells classified as continuum did not differ between vehicle- and necrostatin-treated mice (Figure 2A). Caspase 3 activity in cytosolic extracts of the ipsilateral forebrain increased significantly in both vehicle- (white) or necrostatin (dark grey)-treated mice at 24 hours after HI compared with naive (light grey), but necrostatin and vehicle mice did not differ significantly (P=0.01 naive versus vehicle, P=0.001 naive versus necrostatin treated) (Figure 2B).
Figure 2.
Necrostatin shifts cell death phenotype and blocks RIP1–RIP3 interaction after neonatal HI. (A) Cell death phenotype analysis at postnatal day P11 after neonatal hypoxia–ischemia (HI) and treatment with either necrostatin or vehicle. Counts are no. of profiles per mm3 with necrotic, apoptotic, or continuum (hybrid) appearance viewed under oil immersion. Vehicle (white) versus necrostatin (dark gray): no. of necrotic profiles—*P<0.001; no. of apoptotic profiles—*P=0.01. Data are represented as box-and-whisker plots with 75th and 25th percentiles (interquartile range) defining the upper and lower limits of the box, respectively, and the median is represented by the line inside the box (Mann–Whitney U-test corrected by Bonferroni). •, extremes. (B) Caspase 3 activity in cytosolic extracts at 24 hours after HI and treatment with vehicle (white) or necrostatin (dark gray) versus naive (light gray) mice represented as bars (mean±s.e.m.). *P=0.01 naive versus vehicle, P=0.001 naive versus necrostatin treated, n=6 per group. (C) Necrostatin inhibits interaction of receptor-interacting protein (RIP)3 and RIP1, after neonatal HI. Immunoprecipitation with anti-RIP3 reveals a small increase in interaction with RIP1 at 3 hours in vehicle-treated mice and a larger increase in RIP3–RIP1 interaction at 24 hours. Minimal RIP3–RIP1 interaction is detected in necrostatin-treated mice. In naive mice, significant amounts of RIP3 interact with FADD; HI blocks this interaction in both vehicle- and necrostatin-treated mice. At 24 hours after HI, there is detectable RIP3–FADD interaction in necrostatin-treated mice only. (D) Anti-RIP3 is specific for detection of RIP3. Immunodepletion with recombinant RIP3 blocks the ability to detect RIP3–RIP1 immunocomplexed proteins (lane 3 versus lane 2). Aliquots from the same 24 hours vehicle-treated sample were used for lanes 2 and 3. (E) Immunoblot for RIP1 and RIP3 proteins in the neonatal mouse brain. Both RIP1 and RIP3 are abundantly expressed in necrostatin- and vehicle-treated mice at 3 and 24 hours after HI/treatment. β-Actin is shown as a loading control.
Necrostatin Inhibits Interaction of Receptor-Interacting Protein-3 and Receptor-Interacting Protein-1 After Neonatal Hypoxia–Ischemia
Coimmunoprecipitation showed that the neonatal mouse brain after HI has enforced interaction between RIP1 and RIP3 (Figure 2C). There was a small increase in RIP3–RIP1 interaction at 3 hours and a large increase at 24 hours in vehicle-treated mice (Figure 2C). Necrostatin largely occluded the RIP1–PIP3 interaction detected after HI (Figure 2C). Only a minimal amount of RIP3–RIP1 interaction is detected in necrostatin-treated mice at either 3 or 24 hours (Figure 2C). As a validation of this assay, anti-RIP3 antibody was preincubated with recombinant RIP3 protein, and then used for the immunoprecipitation assay; there was minimal pull down of RIP1 (Figure 2D). In naive mice, significant amounts of RIP3 interact with FADD, and HI blocks this interaction initially in both vehicle- and necrostatin-treated mice (Figure 2C). By 24 hours, there is again detectable RIP3–FADD interaction, but only in necrostatin-treated mice (Figure 2C). Differences in RIP3–RIP1 interaction are not due to significant differences in levels of the two proteins after HI. Receptor-interacting protein-1, the pharmacologic target of necrostatin-1 (Degterev et al, 2008) and RIP3 are highly expressed in the developing brain. Abundant expression of 74 kDa full-length RIP1 and 57 kDa RIP3 is found in the neonatal forebrain at both 3 and 24 hours after HI in vehicle- and necrostatin-treated mice, respectively (Figure 2E).
Protein Oxidation is Attenuated by Necrostatin After Neonatal Hypoxia–Ischemia
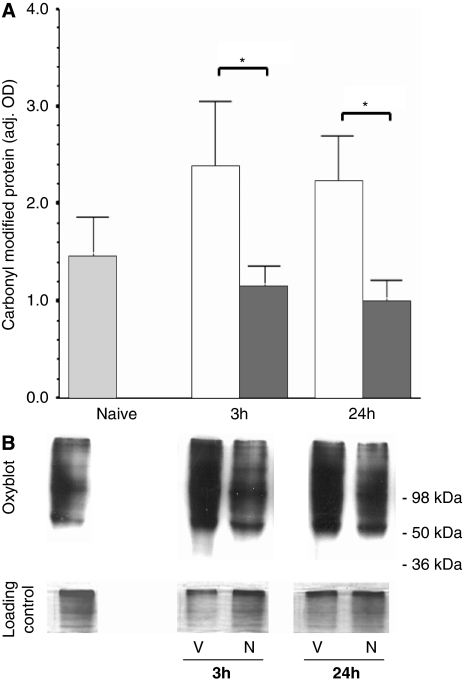
Proteins of multiple molecular weights are extensively oxidatively damaged after neonatal HI (Figure 3B). In vehicle-treated mice (dark gray bars; Figure 3A) at 3 and 24 hours after HI, there was a 63 and 51% increase in carbonyl-modified proteins, respectively (versus naive controls (light gray bars)); however, these differences did not reach significance. Treatment with necrostatin (white bars; Figure 3A) blocked this increase at both 3 and 24 hours after HI and necrostatin mice had 51% (P=0.03) and 55% (P=0.008) less oxidatively modified proteins than did vehicles at the same time point.
Figure 3.
Protein oxidation is suppressed by necrostatin treatment. (A) Carbonyl-modified protein in the forebrain from naive animals (light gray) and at 3 and 24 hours after hypoxia–ischemia (HI) and treatment with vehicle (white) or necrostatin (dark gray). Data represented as bars (mean±s.e.m.). *P<0.05. (B) Representative OxyBlots with corresponding loading controls are shown for vehicle (V) and necrostatin (N) at 3 and 24 hours after HI and treatment. Position of molecular weight standards is shown for reference.
Cytokine Gene and Protein Expression, Nuclear Factor-κB Activity, and Caspase 1 Activation are Suppressed in Necrostatin-Treated Neonatal Mice After Hypoxia–Ischemia
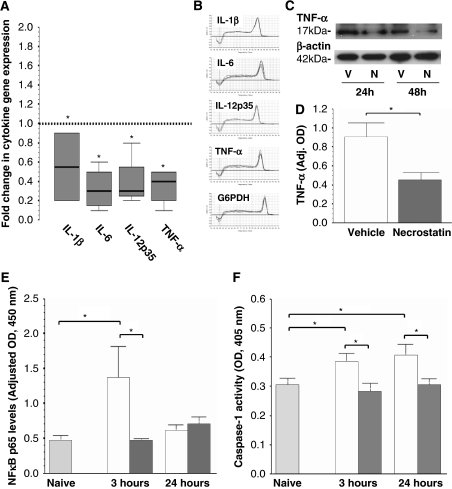
Cytokines increase in response to HI in the developing brain (Silverstein et al, 1997). When compared with vehicle-treated animals, necrostatin produced a 43, 73, 72, and 64% reduction in IL-1β, IL-6, IL-12p35, and TNFα gene expression in the forebrain, respectively (P<0.05; Figure 4A). Melting curves for each of the cytokines and the housekeeping gene showed that a single gene product was obtained in each case (Figure 4B). Immunoblots of crude homogenates used for the gene expression studies confirm that necrostatin suppresses TNFα protein expression after neonatal HI by ∼50% (Figures 4C and 4D, vehicle: white bars, necrostatin: dark gray bars, P=0.02 versus vehicle, n=3 per group).
Figure 4.
Cytokine gene and protein expression, NFκB and caspase 1 activity after HI and necrostatin treatment. (A) Fold change in interleukin (IL)-1β, IL-6, IL-12p35, and tumor necrosis factor (TNF)α gene expression in the forebrain 24 hours after HI and necrostatin treatment versus vehicle (reference line sitting at 1). Data represented as a box-and-whisker plot in which the 75th and 25th percentiles (interquartile range) define the upper and lower limits of the box, respectively, and the median is represented as the line inside the box. (B) Melting curves confirm single PCR product for all genes of interest. (C) Immunoblot for TNFα in protein fractions at 24 and 48 hours after hypoxia–ischemia (HI) (N: necrostatin treated, V: vehicle treated). β-Actin is shown as loading control. (D) TNFα protein expression is represented as bars (vehicle: white, necrostatin: dark gray, mean±s.e.m., *P=0.02 versus vehicle, n=3 per group). (E) Nuclear factor (NF)κB p65 levels in nuclear extracts and (F) caspase 1 activity in cytosolic extracts at 3 and 24 hours after HI and treatment with vehicle (white) or necrostatin (dark grey) versus naive (light gray) animals represented as bars (mean±s.e.m.). *P<0.05, n=5 to 6 per group.
An approximately three-fold increase in nuclear NFκB p65/50 levels occurs at 3 hours after HI in vehicle-treated mice (white bars, P=0.004) versus naive controls (light gray bars), and necrostatin (dark gray bars) blocks this increase, bringing levels of NFκB activity back to basal levels (P=0.02, versus 3 hours vehicle, Figure 4D). Levels of NFκB activity at 24 hours are not different between treatment groups or compared with controls. Caspase 1 activity increases by 23% at 3 hours (P=0.05) and by 32% at 24 hours after HI (P=0.03) in vehicle-treated mice (white bars, versus naive controls), and necrostatin treatment attenuates this response by 24% at 3 hours (P=0.04) and 25% at 24 hours after HI (P=0.04, dark gray bars versus vehicle, Figure 4E).
FLIP Gene and Protein Expression is Suppressed in Necrostatin-Treated Neonatal Mice After Hypoxia–Ischemia
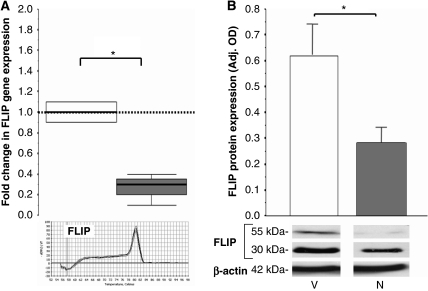
FLIP protein turnover is high and posttranscriptional modification is minimal; therefore, FLIP protein levels are highly dependent on levels of gene expression (Kataoka et al, 2002), which is under the transcriptional control of NFκB (Ichikawa et al, 2005). There is a 70% reduction in FLIP gene expression (range 90% to 60%, P=0.01, Figure 5A) and a >50% reduction in FLIP protein levels (P=0.04, Figure 5B) in necrostatin-treated mice (dark gray bars) versus vehicle-treated mice (white bars). Both the 55 and 30 kDa FLIP isoforms are identified by the antibody and are similarly affected.
Figure 5.
FLIP gene and protein expression after neonatal HI and necrostatin treatment. Change in (A) FLIP gene and (B) protein expression in the forebrain 24 hours after hypoxia–ischemia (HI) and treatment with vehicle (white) or necrostatin (gray). Gene expression is represented as box-and-whisker plots in which 75th and 25th percentiles (interquartile range) define the upper and lower limits of the box, respectively, and the median is represented as the line inside the box. Melting curves for FLIP confirm a single PCR product. Protein expression is represented as bars (mean±s.e.m.). Representative SDS-PAGE for FLIP protein expression is shown with β-actin as loading control in panel B. *P<0.05 in all cases. n=3 to 6 per group.
Discussion
Necrostatin, when administered after neonatal HI, provides robust and long-lasting neuroprotection primarily by blocking necrotic cell death in the mouse brain. Necrostatin blocks the interaction of RIP1 and RIP3 and facilitates the restoration of FADD and RIP3 binding. These necrostatin-mediated alterations in brain cell death and molecular signaling are associated with decreases in protein oxidation, decreases in NFκB activation, caspase 1 activity, FLIP gene and protein expression, and decreases in gene and protein expression of proinflammatory cytokines.
This is the first study to show the neuroprotective efficacy of necrostatin in any model of neonatal brain injury. Previous studies have shown that necrostatin is neuroprotective in adult mouse stroke and traumatic brain injury models as shown by histologic and behavioral outcomes (Degterev et al, 2005; You et al, 2008). Protection in necrostatin-treated neonatal mice is demonstrable in the ipsilateral forebrain and thalamus at 4 days (P11) and persists at 21 days (P28) after HI. Our techniques may lack the sensitivity for detecting small histologic/structural differences in necrostatin-treated mice at P8 due to the variability inherent in this model. However, necrostatin-treated mice did show prominent neuroprotection against biochemical damage at 3 and 24 hours after HI as revealed by OxyBlot and coimmunoprecipitation analyses. The protection against the delayed progressive injury in the thalamus may result from necrostatin-mediated preservation of the forebrain and interruption of system-directed neurodegeneration (Northington et al, 2001b; Stone et al, 2008).
Gender differences have been found in neonatal rodent models of HI brain injury (Zhu et al, 2006). We analyzed cohorts of male mice and found that necrostatin provided significant neuroprotection in males when necrostatin-treated mice were compared with vehicle-treated mice. This study was not designed to determine the effect of gender, and the numbers in each group were small when separated for gender (the female group sizes were too small to analyze); nevertheless, a strong neuroprotective effect of necrostatin resolved in males. Necrostatin neuroprotection in vivo is associated with a block in translocation of apoptosis-inducing factor (AIF) to the nucleus (Asare et al, 2009). After HI, AIF nuclear translocation is enhanced in neonatal males in comparison with females (Zhu et al, 2006). Our results showing that necrostatin blocks RIP1–RIP3 interaction, oxidative damage, and inflammation could reflect mechanisms of action upstream of AIF translocation in males.
Necrostatin is a small molecule chosen from an extensive chemical library for its ability to inhibit cell death caused by TNFα stimulation in the setting of caspase inhibition (Degterev et al, 2005). It is an allosteric inhibitor of RIP1 (Degterev et al, 2005), which is a key signaling intermediate in the form of cell death described as programmed necrosis (Holler et al, 2000). In vitro, inhibition of RIP1 kinase blocks cell death caused by death receptor signaling in the presence of caspase inhibition (Degterev et al, 2008). We have described previously that a morphologic hallmark of delayed-ongoing neurodegeneration in the neonatal rodent brain after HI is apoptotic-necrotic continuum or hybrid cell death (Northington et al, 2007), which has similarities to programmed necrosis. Classic cellular necrosis also contributes significantly to the neurodegeneration caused by HI (Northington et al, 2001b). ‘Continuum' cell neurodegeneration may result from failure of completion of caspase-dependent apoptotic cell death after neonatal HI (Northington et al, 2007). There is an abundance of biochemical evidence for activation of caspase-dependent pathways after neonatal HI (Blomgren et al, 2001; Nakajima et al, 2000; Northington et al, 2001a; Zhu et al, 2006), but there is a paucity of evidence that apoptosis is fully executed during the acute phase of neonatal brain injury induced by HI (Blomgren et al, 2007; Ishimaru et al, 1999; Martin et al, 2000; Northington et al, 2001b), thus resulting in a preponderance of continuum cell death driven by failure of completion of caspase-dependent apoptotic cell death after neonatal HI (Northington et al, 2007). When cell death phenotype analysis was conducted, we once again found a prominent role for necrotic cell death in neurodegeneration after HI (Northington et al, 2001b), and found that necrostatin significantly blocked this necrotic cell death with a three-fold reduction in the number of necrotic profiles. As a result, there was a major overall increase from <33% to ∼60% in the contribution of apoptotic and continuum forms of cell death in necrostatin-treated animals. This finding was surprising, given that we engaged the study with the hypothesis that necrostatin would act on programmed necrosis or continuum cell death in the neonatal HI brain. Nevertheless, this result validates the concept of the apoptosis-necrosis cell death continuum and further supports the idea that morphologically defined classic necrosis and continuum cell death are distinct entities, and that mechanistically classic necrosis and programmed necrosis might be more similar than previously realized. In this model, consistent with previous reports, there is no effect of necrostatin on caspase activity (Degterev et al, 2005), and in necrostatin-treated mice, there is an increase in the number of cells with an apoptotic profile. The decrease in FLIP gene and protein expression in necrostatin-treated mice may also support increased apoptosis (Kreuz et al, 2001) because FLIP normally acts as a dominant negative for caspase 8 (Micheau et al, 2001). The shift to a greater incidence of apoptosis in necrostatin-treated mice after HI is consistent with these findings. Overall, these findings support the interpretation that necrostatin acts directly on and reduces the predominant cellular necrosis after neonatal HI and favors an overall cell death profile that is ‘cleaner' and less likely to result in proinflammatory cell disruption. A similar mechanism of neuroprotection in the injured neonatal brain may be operative with mild hypothermia (Mueller-Burke et al, 2008).
The receptor-interacting protein kinases, RIP1 and especially RIP3, have major roles as a molecular switch between programmed necrosis and apoptosis (Zhang et al, 2009), and possibly, on the basis of data presented in this study, between classic necrosis and apoptosis. Similar to RIP1, RIP3 contains an N-terminal kinase domain with ∼40% homology to RIP1 and an RIP homotypic interaction motif, but does not contain a death domain (Cho et al, 2009), and both proteins are abundantly expressed in the neonatal brain. Both RIP3 and the kinase activity of RIP1 are essential for stable formation of the RIP1–RIP3 pronecrotic complex (complex II), and induction of complex II kinase activity is specifically required for programmed necrosis. Necrostatin can abolish RIP1–RIP3 interaction by the inhibition of RIP3 phosphorylation and can potently inhibit complex II kinase activity and thus programmed necrosis (Cho et al, 2009) or classic necrosis as seen in this study. Receptor-interacting protein-3 also regulates ROS production (Cho et al, 2009) by stimulation of metabolic pathways that provide substrates for the respiratory chain and mitochondrial ROS production (Zhang et al, 2009). Our finding that necrostatin attenuates cellular necrosis and oxidative damage to proteins after neonatal HI is consistent with an anti-ROS/antinecrosis mechanism of action in vivo.
Both RIP1 and RIP3 interact with FADD (Cho et al, 2009). When the RIP kinases are complexed with FADD and exposed to TNFα, apoptosis seems to be the predominant form of cell death. Receptor-interacting protein-1 is recruited to FADD in a TNF-dependent manner, whereas RIP3 is more constitutively associated with FADD (Cho et al, 2009). Our results are consistent with these in vitro studies. We find that RIP3 and FADD coimmunoprecipitate in the normal developing brain; RIP1 is recruited to complex with RIP3 after HI, and HI disrupts the association of FADD and RIP3. Importantly, necrostatin markedly reduces RIP1–RIP3 complex formation after HI, and by 24 hours after HI, partially restores the association of RIP3 and FADD. These findings are consistent a central role for pronecrotic RIP1–RIP3 complex formation in response to neonatal HI and with inhibition of RIP1–RIP3 activity as a major mechanism of necrostatin-mediated neuroprotection. The results of RIP3–FADD coimmunoprecipitation studies may indicate a generalized permissivity of the developing brain to apoptotic cell death and a shift toward a molecular environment more permissive for apoptosis and inhibitory to classic necrosis in necrostatin-treated mice after neonatal HI.
Our study shows that a single dose of necrostatin significantly decreases the oxidative damage to proteins during the first 24 hours after neonatal HI. We do not know how necrostatin acts to block oxidative protein modification, nor do we know the source of the ROS responsible for the oxidative damage to proteins. However, this oxidative injury to proteins can be attenuated dramatically by immediate post-HI treatment with necrostatin, as is also the finding with hypothermia (Mueller-Burke et al, 2008). Receptor-interacting protein-1 has been implicated in signaling cascades important to both mitochondrial and nonmitochondrial ROS production (Lin et al, 2004; Shen et al, 2004). There is no known direct antioxidant effect of necrostatin, but it does modulate redox mechanisms in experimental systems (Xu et al, 2007). Necrostatin increases glutathione levels and decreases ROS production (Xu et al, 2007), blocks nitric oxide-mediated cell necrosis and mitochondrial ROS production (Davis et al, 2010) and may also delay the opening of mitochondrial permeability transition pore (Lim et al, 2007). These effects may be reflected in the decrease in protein oxidation in the first 24 hours and the subsequent delayed appearance of neuroprotection after HI and necrostatin administration in our model.
We also determined that necrostatin treatment suppresses important brain proinflammatory cytokines and proximal regulators of cytokine expression after neonatal HI. In normal physiology, a primary function of RIP1 kinase is to transduce the NFκB signal, and although apparently normal at birth, RIP-deficient mice fail to thrive and die during the first 3 days after birth with extensive lymphoid apoptosis associated with failure to activate NFκB (Kelliher et al, 1998). A single intracerebroventricular dose of necrostatin does not have this effect because our mice survived for 3 weeks after injection of necrostatin, and in drug-only controls, there was no abnormality of the brain seen on CV sections (Supplementary figure available online). In vitro studies establishing necrostatin as an inhibitor of RIP1 kinase failed to show an effect on NFκB activation (Degterev et al, 2008), but the molecular actions of necrostatin in vivo are unknown. As FLIP is under the transcriptional control of NFκB, the effects on FLIP gene and protein expression in necrostatin-treated mice serve as a reporter of decreased NFκB activity in these mice, whether decreased NFκB is a direct or indirect result of necrostatin treatment. Early inhibition of NFκB is neuroprotective in this model (Nijboer et al, 2008). We find that HI induced a three-fold increase in NFκB activity in vehicle-treated mice, which is not seen in necrostatin-treated mice. This suppression in NFκB activity could contribute to the decrease in cytokine gene expression found in necrostatin-treated mice or result from the decreased cytokine expression. The 32% increase in caspase 1 activity after HI in vehicle-treated mice suggests a significant activation of the inflammasome (Bryant and Fitzgerald, 2009), which also signals for cytokine production. The effect of necrostatin to suppress this HI-induced increase in caspase 1 activity may act in conjunction with early inhibition of NFκB activity to further suppress cytokine gene and protein expression (Bryant and Fitzgerald, 2009). We do not show that these protein, enzyme, and gene expression effects are a direct result of necrostatin treatment, and that the decrease in inflammatory markers may be related indirectly to necrostatin inhibition of necrosis. These results, overall, are consistent with the decreased neutrophil influx and microglial activation found in necrostatin-treated adult mice after traumatic brain injury (You et al, 2008).
Conclusions
Necrostatin is a potent neuroprotective drug for treating neonatal HI brain injury in mice by blocking necrotic cell death, previously believed to be unregulated and untreatable. The mechanisms of operation of necrostatin are related to the inhibition of RIP1–RIP3 interaction, decreased oxidative injury, and suppression of upstream proinflammatory signaling and multiple cytokines. Our results emphasize the importance of necrosis in neonatal HI and suggest a plausible biochemical mechanism that may underlie expression of the apoptosis-necrosis continuum.
Acknowledgments
The authors acknowledge the expert technical assistance of Debra L Flock, Devin W Mack, Rajni Ahlawat, MD, and Lindsey Zemeir.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
These studies are funded by the March of Dimes Foundation (6-08-275) NS 059529 (FJN), AG016282 (LJM), and by a grant from Lehmann Brothers Foundation (FJN).
Supplementary Material
References
- Asare N, Tekpli X, Rissel M, Solhaug A, Landvik N, Lecureur V, Podechard N, Brunborg G, Lag M, Lagadic-Gossmann D, Holme JA. Signalling pathways involved in 1-nitropyrene (1-NP)-induced and 3-nitrofluoranthene (3-NF)-induced cell death in Hepa1c1c7 cells. Mutagenesis. 2009;24:481–493. doi: 10.1093/mutage/gep032. [DOI] [PubMed] [Google Scholar]
- Barks JD, Liu YQ, Shangguan Y, Li J, Pfau J, Silverstein FS. Impact of indolent inflammation on neonatal hypoxic-ischemic brain injury in mice. Int J Dev Neurosci. 2008;26:57–65. doi: 10.1016/j.ijdevneu.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12:993–1010. doi: 10.1007/s10495-007-0754-4. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia- ischemia: a mechanism of ‘pathological apoptosis'. J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CW, Hawkins BJ, Ramasamy S, Irrinki KM, Cameron BA, Islam K, Daswani VP, Doonan PJ, Manevich Y, Madesh M. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Rad Biol Med. 2010;48:306–317. doi: 10.1016/j.freeradbiomed.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Ditelberg JS, Sheldon RA, Epstein CJ, Ferriero DM. Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatr Res. 1996;39:204–208. doi: 10.1203/00006450-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Graham EM, Sheldon RA, Flock DL, Ferriero DM, Martin LJ, O'Riordan DP, Northington FJ. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17:89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Takada Y, Murakami A, Aggarwal BB. Identification of a novel blocker of I kappa B alpha kinase that enhances cellular apoptosis and inhibits cellular invasion through suppression of NF-kappaB-regulated gene products. J Immunol. 2005;174:7383–7392. doi: 10.4049/jimmunol.174.11.7383. [DOI] [PubMed] [Google Scholar]
- Ishimaru MJ, Ikonomidou C, Tenkova TI, Der TC, Dikranian K, Sesma MA, Olney JW. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J Comp Neurol. 1999;408:461–476. [PubMed] [Google Scholar]
- Kataoka T, Ito M, Budd RC, Tschopp J, Nagai K. Expression level of c-FLIP versus Fas determines susceptibility to Fas ligand-induced cell death in murine thymoma EL-4 cells. Exp Cell Res. 2002;273:256–264. doi: 10.1006/excr.2001.5438. [DOI] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC.2007The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore Cardiovasc Drugs TherSponsored by the International Society of Cardiovascular Pharmacotherapy21467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Price AC, Kaiser A, Agnew DM, Ichord RN, Traystman RJ. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol Dis. 2000;7:169–191. doi: 10.1006/nbdi.2000.0282. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Burke D, Koehler RC, Martin LJ. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurodegeneration after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int J Dev Neurosci. 2008;26:67–76. doi: 10.1016/j.ijdevneu.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000;20:7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. A dual role of the NF-kappaB pathway in neonatal hypoxic-ischemic brain damage. Stroke. 2008;39:2578–2586. doi: 10.1161/STROKEAHA.108.516401. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia–ischemia is apoptosis. J Neurosci. 2001a;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001b;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Zelaya ME, O'Riordan DP, Blomgren K, Flock DL, Hagberg H, Ferriero DM, Martin LJ. Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as ‘continuum' phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience. 2007;149:822–833. doi: 10.1016/j.neuroscience.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. Excitoxic neuronal death in the immature brain is an apoptosis-necrosis morphological continuum. J Comp Neurol. 1997;378:70–87. [PubMed] [Google Scholar]
- Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Tauber MG, Ferriero DM. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res. 2004;56:656–662. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- Shen HM, Lin Y, Choksi S, Tran J, Jin T, Chang L, Karin M, Zhang J, Liu ZG. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol. 2004;24:5914–5922. doi: 10.1128/MCB.24.13.5914-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein FS, Barks JD, Hagan P, Liu XH, Ivacko J, Szaflarski J. Cytokines and perinatal brain injury. Neurochem Int. 1997;30:375–383. doi: 10.1016/s0197-0186(96)00072-1. [DOI] [PubMed] [Google Scholar]
- Stone BS, Zhang J, Mack DW, Mori S, Martin LJ, Northington FJ. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol. 2008;64:535–546. doi: 10.1002/ana.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chua CC, Kong J, Kostrzewa RM, Kumaraguru U, Hamdy RC, Chua BH. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, Moskowitz MA, Whalen MJ. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science (New York, NY) 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.