Abstract
Am80 (tamibarotene) is a retinoic acid receptor (RAR) agonist clinically available for treatment of acute promyelocytic leukemia. As intracerebral hemorrhage (ICH) accompanies inflammatory reactions in the brain and also because retinoids may suppress activation of microglia, we investigated the effect of Am80 on collagenase-induced experimental model of ICH in adult mice. Daily oral administration of Am80 (5 mg/kg) starting from 1 day before or from up to 6 hours after intrastriatal injection of collagenase significantly inhibited the decrease in the number of striatal neurons at 3 days after the insult. Am80 showed no significant effect on the hematoma size and the extent of edema associated with hemorrhage. Prominent expression of RARα was observed in activated microglia/macrophages, and the number of activated microglia/macrophages in the perihematoma region was lower in Am80-treated mice than in vehicle-treated mice. Am80 treatment also reduced areas affected by hemorrhage-associated oxidative stress as indicated by nitrotyrosine immunoreactivity, and attenuated heme oxygenase-1 expression in activated microglia/macrophages. Moreover, Am80-treated mice exhibited better recovery from hemorrhage-induced neurologic deficits than vehicle-treated mice. These results suggest that RAR is a promising target of neuroprotective therapy for ICH.
Keywords: heme oxygenase, inflammation, microglia, nitrotyrosine, retinoid, tamibarotene
Introduction
Intracerebral hemorrhage (ICH), representing 10% to 30% of all strokes, is a devastating disease frequently resulting in poor prognosis. Although several therapeutic strategies including regulation of osmotic pressure and invasive neurosurgical evacuation of hematoma are in clinical practice (Qureshi, 2009), virtually no therapeutic strategies intended for neuroprotection are available.
Regulation of inflammatory processes may provide an opportunity for restricting expansion of ICH-induced brain tissue damage (Wang and Dore, 2007b). In the brain, inflammatory responses involve robust activation of microglia that promotes neurodegeneration through production of deleterious factors including inflammatory cytokines, reactive oxygen species, and nitric oxide. Indeed, accumulation of activated microglia/macrophages is observed in the perihematoma region, and treatments aimed to inhibit microglial activation improve neuropathological outcome after ICH (Ohnishi et al, 2007).
In this study, we focused on the effect of retinoids (Maden, 2007). Retinoids refer to natural or synthetic compounds related to retinoic acid that act on retinoic acid receptors (RARs) and retinoid X receptors. Both RARs and retinoid X receptors include three subtypes designated as α, β, and γ, and expression of these receptor subtypes is observed in the adult central nervous system (Krezel et al, 1999; Zetterstrom et al, 1999). Details of physiological and pathophysiological roles of retinoids remain to be investigated, but several lines of evidence indicate that retinoids may protect neurons from inflammation-associated injury (Mey, 2006; Malaspina and Michael-Titus, 2008). For example, all-trans-retinoic acid inhibits expression of inducible nitric oxide synthase (iNOS) and several proinflammatory cytokines in activated microglia in culture (Dheen et al, 2005; Xu and Drew, 2006). A synthetic RAR agonist Am80 (tamibarotene) ameliorates the symptoms of experimental autoimmune encephalomyelitis (Wang et al, 2000; Klemann et al, 2009). In addition, we have recently reported that Am80 prevents inflammatory degeneration of midbrain dopaminergic neurons induced by lipopolysaccharide both in vitro and in vivo (Katsuki et al, 2009). Here, we examined the effect of Am80 on various pathological parameters in collagenase-induced ICH in mice.
Materials and methods
Induction of Intracerebral Hemorrhage and Am80 Treatment
All procedures were approved by our institutional ethical committee concerning animal experiments, and animals were treated in accordance with the Guidelines of the United States National Institutes of Health regarding the care and use of animals for experimental procedures. Male C57BL/6J mice at 8 to 10 weeks of age weighing 21 to 28 g were used to produce collagenase-induced model of ICH (Lee et al, 2007; Wang et al, 2003). Animals were maintained at constant ambient temperature (22°C±1°C) under a 12-hour light/dark cycle (lights on between 0800 and 2000 hours). After intraperitoneal injection of 50 mg/kg pentobarbital, mice were placed in a stereotaxic frame. A 30-gauge needle was inserted through a burr hole on the skull into the striatum (stereotaxic coordinates; 2.3 mm lateral to the midline, 0.2 mm anterior to the bregma and 3.5 mm depth below the skull). The ICH was induced by injection of 0.025 U collagenase type VII (Sigma, St Louis, MO, USA) in 0.5 μL saline, at a constant rate of 0.20 μL/min with a microinfusion pump. Sham-operated mice received injection of the same volume of saline. Body temperature was maintained at 37°C during surgery.
Am80 (0.5 or 1.5 mg/mL) was suspended in 0.5% carboxymethyl cellulose solution and orally administered to mice at 5 or 15 mg/kg once per day. In pretreatment studies, animals received collagenase injection on the next day (0900 to 1100 hours) of the first oral administration of Am80 (1900 to 2300 hours), and then received Am80 orally for consecutive 3 days (1900 to 2000 hours). In posttreatment studies, oral administration of Am80 was first performed 2, 6, or 24 hours after collagenase injection, and then daily at 24-hour intervals (total three times in the case of 2 and 6 hours posttreatment, and total twice in the case of 24 hours posttreatment). Control animals received oral administration of the same volume of 0.5% carboxymethyl cellulose solution.
Immunohistochemistry
Three days after ICH, mice were anesthetized again with pentobarbital and perfused transcardially with 30 mL of ice-cold phosphate-buffered saline (PBS) followed by 30 mL of 4% paraformaldehyde. Brains were isolated and fixed in 4% paraformaldehyde overnight and then soaked in 15% sucrose overnight at 4°C. After freezing, they were cut into sections of 30 μm thickness, and four sections around the injection site were collected every 120 μm and mounted onto slides. Antigen retrieval was achieved by soaking specimens in 10 mmol/L citric acid buffer (pH 8.5) for 30 minutes at 80°C and incubation for 1 hour at room temperature. After rinsing with PBS containing 0.3% Triton X-100 (tPBS), specimens were treated with tPBS containing blocking serum for 1 hour at room temperature, then incubated with primary antibodies overnight at 4°C. Primary antibodies were mouse anti-NeuN (1:500, Millipore, Bedford, MA, USA), rabbit anti-nitrotyrosine (1:500, Millipore), rabbit anti-heme oxygenase-1 (1:500, Assay Designs, Ann Arbor, MI, USA), rabbit anti-RARα (1:200, Santa Cruz Biotech., Santa Cruz, CA, USA), and rabbit anti-RARβ (1:200, Santa Cruz Biotech.). After rinsing with tPBS, specimens were incubated with corresponding secondary antibodies for 2 hours at room temperature. Biotinylated goat anti-mouse IgG (1:200, Vector Laboratories, Burlingame, CA, USA) and biotinylated goat anti-rabbit IgG (1:200, Vector Laboratories) were used as secondary antibodies. Microglia/macrophages were labeled by overnight incubation with biotinylated Griffonia simplicifolia isolectin B4 (1:100, Vector Laboratories; Lee et al, 2006a).
After incubation with biotinylated conjugates, specimens were treated with avidin–biotinylated horseradish peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories) and then peroxidase was visualized with diaminobenzidine and H2O2. The number of NeuN-positive cells per 230 × 340 μm2 was counted at the central and the peripheral regions of hematoma in the striatum as described (Ohnishi et al, 2007). Here, the central region of hematoma refers to the region adjacent to the collagenase injection site defined by a track of cannula. The peripheral region of hematoma means the region adjacent to the edge of hematoma defined by low NeuN immunoreactivity. Four coronal sections collected every 120 μm from around the injection site in each mouse were examined for cell counting, and the averaged number of cells from these sections was taken as a value of each mouse. The number of isolectin B4 binding- or HO-1-positive cells per 230 × 340 μm2 was counted at the peripheral region of hematoma. Concerning isolectin B4 binding-positive cells, only cells exhibiting morphology of activated microglia/macrophages, such as amoeboid appearance with short and thick processes, were incorporated in cell counting. For nitrotyrosine immunoreactivity, threshold-based quantification of immunopositive area was conducted with ImageJ in a section containing a track of cannula. Nitrotyrosine-positive area was presented as a total value obtained from four fields of 0.25 mm2. As this method of quantification discards information on variable staining intensities, integrated values of intensity of nitrotyrosine immunoreactivity were also obtained from the same fields, with the use of ImageJ software.
Double fluorescence histochemistry was performed for combinations of isolectin B4 binding with immunoreactivity against RARα, Iba1, iNOS, or HO-1. Rabbit anti-RARα (1:200), rabbit anti-Iba1 (1:200, Wako Chemicals, Osaka, Japan), mouse anti-iNOS (1:500, BD Transduction Laboratories, Franklin Lakes, NJ, USA), and rabbit anti-HO-1 (1:200) were used as primary antibodies, along with biotinylated G. simplicifolia isolectin B4 (1:100). Alexa Fluor 594-conjugated donkey anti-rabbit IgG(H+L) (1:500, Molecular Probes, Eugene, OR, USA) or Alexa Fluor 594-conjugated goat anti-mouse IgG(H+L) (1:500, Molecular Probes), and Alexa Fluor 488-conjugated streptavidin (1:2000, Molecular Probes) were used to detect localization. Double immunofluorescence staining was also performed for iNOS and myeloperoxidase, a neutrophil marker. For myeloperoxidase immunostaining, rabbit antimyeloperoxidase (1:500, Dako, Glostrup, Denmark) and Alexa Fluor 488-conjugated goat anti-rabbit IgG(H+L) (1:500, Molecular Probes) were used. Confocal images were obtained with the usage of Fluoview FV300 system (Olympus, Tokyo, Japan).
Estimation of Lesion Size
According to the procedures by Ohnishi et al (2007), the area with low NeuN immunoreactivity in a section containing a track of cannula for collagenase injection was taken as an area invaded by hematoma, which was quantified with ImageJ software.
Lesion volume was also estimated by Nissl staining with Cresyl Violet (Wang and Dore, 2007a; Rynkowski et al, 2009) of 30-μm coronal frozen brain sections obtained every 210 μm. Injured hemorrhage areas in sections spanning the entire hematoma were measured by ImageJ software. Lesion volume (in mm3) was determined by integration of the injured area in each section over the section depth.
Estimation of Hemorrhage Volume
Hemorrhage volume was estimated by spectrophotometric assay of hemoglobin content using Drabkin's reagent (Sigma), essentially according to the procedures described earlier (Lee et al, 2006b). Hemispheres ipsilateral and contralateral to the hemorrhage were harvested as separate samples. Calibration of the blood volume was made by addition of a defined volume of homologous blood to assay samples. The value of hemorrhage volume was obtained by subtracting the value of the contralateral side from that of the ipsilateral side.
Measurement of Brain Water Content
Three days after ICH, mice were decapitated under deep anesthesia with pentobarbital. Brain was removed from the skull and a coronal brain slice of 4 mm thickness was obtained 2 mm posterior from the frontal pole. The brain slice was divided into the ipsilateral and the contralateral sides along the midline. The cerebellum was also collected as a control. After wet weight of the tissues was obtained, tissues were dried at 75°C for 12 hours to give the dry weight. The water content was calculated by the following formula: (wet weight−dry weight)/wet weight) × 100 (%).
Behavioral Tests
Sensorimoter functions were evaluated by means of beam-walking test, rotarod test, and modified limb-placing test at 1, 3, 7, and 14 days after surgery. These tests were conducted by an experimenter blinded to the treatments.
In the beam-walking test, mouse was placed on a beam (1.2 m long, 1.5 cm wide, and 50 cm high), and usage of hindlimb during crossing the beam was analyzed on the basis of an eight-point scale as well as a fault rate. A score of 0 was given when mouse could not balance on the beam (<5 seconds); 1 was given when mouse remained on the beam for >5 seconds but could not cross the beam; 2 was given when mouse could balance on the beam but not traverse it; 3 was given when mouse traversed the beam with the affected limb extended and not reaching the surface of the beam, or when mouse made a turn on the beam; 4 was given when mouse traversed the beam with 100% footslips; 5 was given when mouse traversed the beam with >50% but <100% footslips; 6 was given when mouse traversed the beam with <50% footslips; 7 was given when mouse traversed the beam with two or less footslips. Performance on each day was expressed as a summated score of three trials. Fault rate was presented as an average of three trials.
In the rotarod test, mouse was placed on a rotarod cylinder, and the duration for which the mouse remained on the rotarod was recorded. The rotation speed was slowly increased from 4 to 40 revolutions/min within a period of 5 minutes. The trial was ended if the animal fell off the rung or gripped the device and spun around for two consecutive revolutions. Animals were trained for 3 days before surgery of collagenase injection. The maximum duration was obtained with three measurements before ICH induction. Data are presented as percentages of the maximal duration compared with the internal baseline control (before ICH).
Modified limb-placing test consists of two limb-placing tasks that assess the sensorimotor integration of the forelimb and the hindlimb, by testing responses to tactile and proprioceptive stimuli (Chu et al, 2004). First, mouse was suspended 10 cm over a table, and the stretch of the forelimbs toward the table was observed and evaluated: normal stretch, 0 point; abnormal flexion, 1 point. Next, the mouse was positioned along the edge of the table, and forelimbs were placed out of the table, suspended over the edge and allowed to move freely. Each forelimb was gently pulled down and retrieval and placement was observed. Finally, the mouse was placed toward the table edge to check lateral placement of the forelimb. The three tasks were scored in the following manner: normal performance, 0 point; performance with a delay (2 seconds) and/or incomplete performance, 1 point; no performance, 2 points; 7 points means maximal neurologic deficit and 0 point means normal performance.
Statistical Analysis
All data are presented as means±s.e.m. Data were statistically analyzed by Student's t-test for two group comparison (data in Figures 5 and 6). If the distribution of data points was not suitable for t-test, nonparametric Mann–Whitney U-test was used. When data sets included more than two groups (data in Figures 1,2,3), one-way analysis of variance followed by post hoc comparisons by Student–Newman–Keuls test was used. Behavioral data (Figure 7) were analyzed by two-way analysis of variance with repeated measures, followed by post hoc comparisons with Bonferroni method. Two-tailed probability values <0.05 were considered significant.
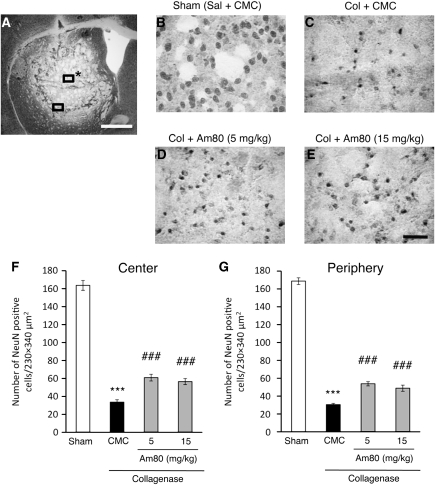
Figure 1.
Effect of Am80 on intracerebral hemorrhage (ICH)-induced neuronal damage. (A) A representative image of a NeuN-immunostained coronal section obtained 3 days after collagenase injection. The central region and the peripheral region for cell counts were denoted by rectangles with and without an asterisk, respectively. Scale bar=1 mm. (B–E) Representative images of the area corresponding to the center of hematoma in NeuN-immunostained coronal sections obtained 3 days after induction of ICH. Mice received intrastriatal injection of saline (Sal; B) or collagenase (Col; C–E). Oral administration of vehicle (0.5% carboxymethyl cellulose (CMC); B and C) or Am80 at indicated doses (D and E) was performed once daily for 4 days starting from the day before induction of ICH. Scale bar=50 μm. (F and G) The number of NeuN-positive cells in the central (F) and the peripheral (G) regions of hematoma was quantified. n=6 to 15 for each condition. ***P<0.001 versus sham group; ###P<0.001 versus CMC group (analysis of variance results: for E, F3,36=191.95, P<0.0001; for F, F3,36=463.92, P<0.0001).
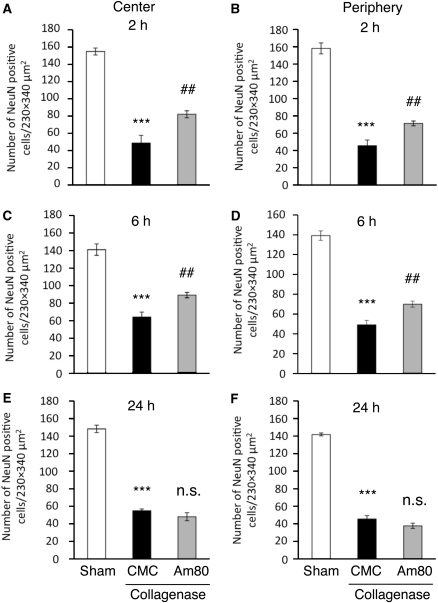
Figure 2.
Effect of posttreatment with Am80 on intracerebral hemorrhage (ICH)-induced neuronal damage. Oral administration of vehicle (carboxymethyl cellulose, CMC) or 5 mg/kg Am80 was performed once daily, starting from 2 hours (A and B), 6 hours (C and D), or 24 hours (E and F) after induction of ICH. The number of NeuN-positive cells in the central (A, C, E) and the peripheral (B, D, F) regions of hematoma was assessed 3 days after induction of ICH. n=4 to 7 for each condition. ***P<0.001 versus sham group; ##P<0.01 versus CMC group; n.s., not significant (analysis of variance results: for A, F2,13=58.847, P<0.0001; for B, F2,13=102.40, P<0.0001; for C, F2,13=54.271, P<0.0001; for D, F2,13=122.18, P<0.0001; for E, F2,14=178.21, P<0.0001; for F, F2,14=347.83, P<0.0001).
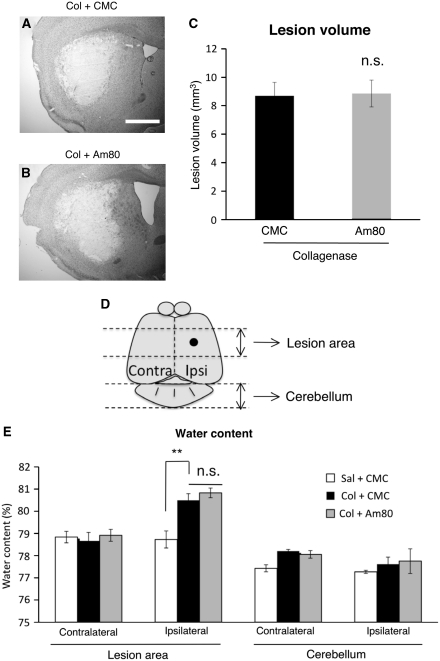
Figure 3.
Effect of Am80 on the lesion volume and the increase in brain water content at 3 days after induction of intracerebral hemorrhage (ICH). Vehicle (carboxymethyl cellulose, CMC) or 5 mg/kg Am80 was administered orally for 4 days, once daily from the day before ICH induction by intrastriatal injection of collagenase (Col). (A and B) Representative images of the area invaded by hematoma in CMC-treated (A) and Am80-treated (B) mice, as revealed by Nissl staining. Scale bar=1 mm. (C) Results of quantification of lesion volume in CMC-treated (n=7) and Am80-treated (n=8) mice. n.s., not significant. (D) The region used for the measurement of water content is shown. The black dot indicates the coordinate of collagenase injection. (E) Results of quantification of brain water content. n=4 to 5 for each condition. **P<0.01; n.s., not significant.
Results
Oral Administration of Am80 Prevents Intracerebral Hemorrhage-Induced Neuron Loss
Three days after induction of ICH in the striatum by collagenase injection, brain sections were obtained and immunostained with an antibody against a neuronal marker NeuN. At low magnification, the striatal region corresponding to the hematoma area was characterized by much lower NeuN-positive signals than the surrounding intact area (Figure 1A). According to the procedures by Ohnishi et al (2007), we counted the number of remaining NeuN-positive cells at the center of hematoma (central region) and at the region adjacent to the intact area (peripheral region). A substantial decrease in the number of NeuN-positive cells was observed both in the central and the peripheral regions of hematoma, compared with the striatum of saline-injected control mice (Figures 1B and 1C). When mice received daily oral administration of 5 mg/kg Am80 for 4 consecutive days starting from the day before collagenase injection, the decrease in the number of striatal neurons was prevented partially but significantly (Figure 1D). A higher dose of 15 mg/kg was also protective, but the degree of the effect did not exceed that of the lower dose (Figures 1E–1G). Accordingly, we used Am80 at a dose of 5 mg/kg in all of the following experiments. The protective effect of Am80 was also observed in the posttreatment regimen, where mice received daily oral administration of 5 mg/kg Am80 for 3 consecutive days starting from 2 or 6 hours after collagenase injection (Figures 2A–2D). When the first administration was performed 24 hours after induction of ICH, Am80 no longer showed a significant effect (Figures 2E and 2F).
Am80 does not Affect Hematoma Expansion and Brain Edema
To clarify whether Am80 treatment affected the amount of bleeding and edema formation, we measured the size of hematoma and the water content of the brain tissue. The size of hematoma estimated by an area with low NeuN immunoreactivity (Ohnishi et al, 2007) was not different between control mice and mice treated with 5 mg/kg Am80 for 4 days starting from the day before collagenase injection (3.18±0.24 mm2, n=15 and 2.92±0.26 mm2, n=10, respectively; P=0.605 by Mann–Whitney U-test). Lesion volume at 3 days after collagenase injection was also assessed by Nissl staining, and obtained values were not different between control mice and Am80-treated mice (Figures 3A and 3B; t13=0.125, P=0.903). In addition, we estimated hemorrhage volume at 6 hours after collagenase injection by spectrophotometrical assay of hemoglobin content (Lee et al, 2006b). Again, the volume was not different between control mice and mice treated with 5 mg/kg Am80 (6.1±0.9 μL, n=6 and 6.6±0.8 μL, n=6, respectively; t10=0.420, P=0.683).
Brain water content, measured at 3 days after collagenase injection, showed a consistent increase in the hemisphere ipisilateral to the hemorrhage, compared with the contralateral side (analysis of variance result: F2,11=11.479, P=0.0020). Am80 treatment did not affect ICH-induced increase in tissue water content (Figures 3D and 3E).
Retinoic Acid Receptor α Is Expressed in Activated Microglia/Macrophages
Am80 exerts agonist activity on RARα and to a lesser extent on RARβ (Umemiya et al, 1997). Accordingly, we performed immunohistochemical localization of RARα and RARβ in the brain after ICH. Moderate to intense immunoreactivity against RARα was observed in the peripheral region of hematoma, whereas RARβ immunoreactivity was not evident in the same region (Figures 4A and 4D). Cells immunoreactive for RARα and RARβ, presumably neurons, were also found scattered in the contralateral striatum and cerebral cortex (Figures 4B, 4C, 4E, and 4F). As RARα-positive cells in the perihematoma region exhibited morphologic characteristics of activated microglia, we next performed double staining of RARα immunoreactivity and isolectin B4 binding. As shown in Figure 4G, RARα immunoreactivity around the hematoma was colocalized with isolectin B4 binding, suggesting that these immunoreactive cells were activated microglia/macrophages. These cells also exhibited immunoreactivity against Iba1, a marker of microglia/macrophages (Figure 4H). On the other hand, we confirmed the presence of neutrophils within the hematoma region, by myeloperoxidase immunohistochemistry (data not shown). Neutrophils are another cell population positive for isolectin B4 binding (Matsumoto et al, 2007), but they were much smaller in cell size and could be easily distinguished from microglia/macrophages.
Figure 4.
Immunohistochemical localization of retinoic acid receptor (RAR)α and RARβ at 3 days after induction of intracerebral hemorrhage (ICH). (A–F) Shown are immunoreactivities against RARα (A–C) and RARβ (D–F) in the peripheral region of hematoma (A and D), in the contralateral striatum (B and E) and in the contralateral cerebral cortex (C and F). Scale bar=50 μm. (G) Double staining of isolectin B4 binding and RARα immunoreactivity in the peripheral region of hematoma. Scale bar=20 μm. (H) Double staining of isolectin B4 binding and Iba1 immunoreactivity. Scale bar=20 μm. Reproducibility of the results shown in this figure was confirmed in four mice.
Am80 Decreases Activated Microglia/Macrophages and Areas Affected by Oxidative Stress Associated with Intracerebral Hemorrhage
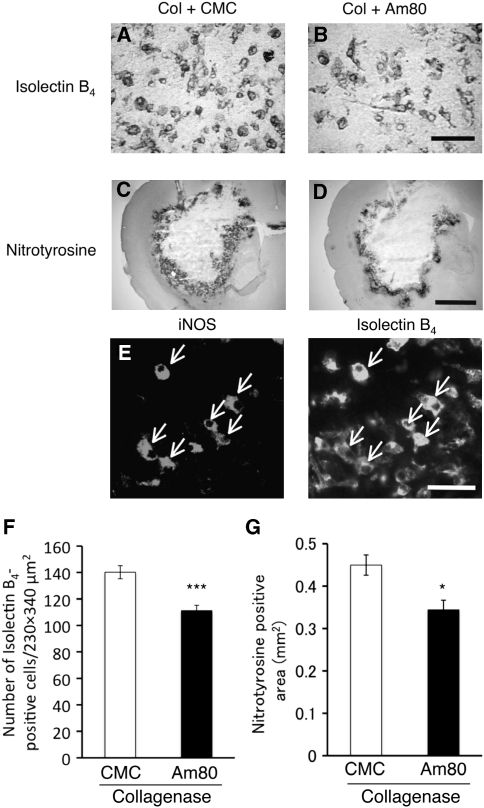
The above results suggest that Am80 could act on activated microglia/macrophages. As microglial activation may contribute to ICH-induced tissue injury, we examined whether Am80 had any effect on activated microglia/macrophages. The number of activated microglia/macrophages, identified by their amoeboid appearance with short, thick processes as well as by isolectin B4 binding, was significantly decreased in mice treated with Am80 for 4 days starting from the day before collagenase injection (Figures 5A, 5B, and 5F; t11=4.486, P=0.0009).
Figure 5.
Effect of Am80 on the number of microglia/macrophages and the level of oxidative stress at 3 days after induction of intracerebral hemorrhage (ICH). Vehicle (carboxymethyl cellulose, CMC) or 5 mg/kg Am80 was administered orally for 4 days, once daily from the day before ICH induction by collagenase (Col). (A and B) Representative images of cells positive for isolectin B4 binding in the peripheral region of hematoma of CMC-treated (A) and Am80-treated (B) mice. Scale bar=50 μm. (C and D) Nitrotyrosine immunoreactivity in the brain of CMC-treated (C) and Am80-treated (D) mice. Scale bar=1 mm. (E) Double staining against inducible nitric oxide synthase (iNOS) immunoreactivity and isolectin B4 binding at 3 days after collagenase injection in control mice. Arrows indicate double-positive cells. Scale bar=50 μm. (F) The number of isolectin B4 binding-positive cells in CMC-treated (n=6) and Am80-treated (n=7) mice. (G) Results of quantification of nitrotyrosine-positive area in CMC-treated (n=5) and Am80-treated (n=7) mice. *P<0.05, ***P<0.001 versus CMC group.
Activated microglia produces nitric oxide and superoxide anion that form peroxynitrite to attack various biomolecules (Brown and Bal-Price, 2003). Indeed, iNOS immunoreactivity overlapped with a subset of isolectin B4-positive cells at 3 days after collagenase injection (Figure 5E), suggesting that microglia/macrophages are a primary source of nitric oxide. Neutrophils, as identified by myeloperoxidase immunoreactivity, did not display iNOS immunoreactivity (data not shown). To clarify whether Am80 treatment affected the level of oxidative stress, we examined immunoreactivity against nitrotyrosine, a marker of peroxynitrite-mediated protein modification. Three days after collagenase injection, intense nitrotyrosine immunoreactivity was observed around the hematoma in control mice (Figure 5C). Increase in nitrotyrosine immunoreactivity was also observed in mice treated with Am80, but immunopositive area was significantly reduced as compared with that in control mice (Figures 5D and 5G; t10=3.122, P=0.0108). Total intensities of nitrotyrosine immunoreactivity obtained by integration of staining intensities were not different between control mice and Am80-treated mice (in arbitrary units: (29.4±3.3) × 105 in control mice, n=5; (29.1±4.0) × 105 in Am80-treated mice, n=7; t10=0.039, P=0.970).
Am80 Decreases HO-1 Expression Associated with Intracerebral Hemorrhage
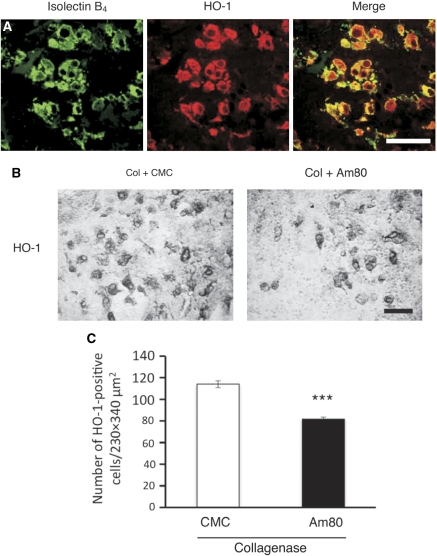
HO-1 is a stress response enzyme whose expression is induced mainly in microglia after ICH (Koeppen et al, 2004; Nakaso et al, 2000). Deletion of HO-1 gene results in improved preservation of tissue integrity after ICH (Wang and Dore, 2007a), suggesting that changes in the level of HO-1 expression affect the consequences of hemorrhagic injury. By double staining of HO-1 immunoreactivity with isolectin B4 binding, we confirmed robust induction of HO-1 in activated microglia/macrophages in the peripheral region of hematoma at 3 days after collagenase injection (Figure 6A). Counting of HO-1-immunoreactive cells revealed that Am80 treatment significantly decreased the number of HO-1-positive cells (Figures 6B and 6C; t11=7.552, P<0.0001).
Figure 6.
Effect of Am80 on the number of HO-1-positive cells in the peripheral region of hematoma. Vehicle (carboxymethyl cellulose, CMC) or 5 mg/kg Am80 was administered orally for 4 days, once daily from the day before intracerebral hemorrhage (ICH) induction by collagenase (Col). (A) Double staining of isolectin B4 binding with HO-1 immunoreactivity at 3 days after ICH. Scale bar=50 μm. (B) HO-1 immunohistochemistry in the peripheral region of hematoma of CMC-treated and Am80-treated mice at 3 days after ICH. Scale bar=50 μm. (C) The number of HO-1-positive cells in CMC-treated (n=5) and Am80-treated (n=8) mice. ***P<0.001 versus CMC group.
Am80 Improves Neurologic Outcome After Intracerebral Hemorrhage
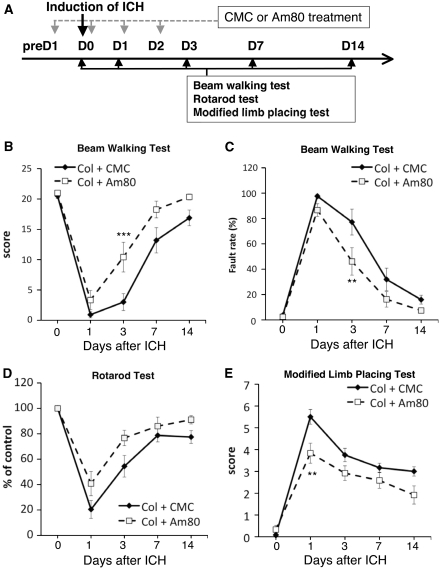
We conducted several sets of behavioral experiments to verify whether Am80 treatment also improved recovery from neurologic deficits. Behavioral assessments were made before, and 1, 3, 7, and 14 days after induction of ICH (Figure 7A). In the beam-walking test, the performance score of control mice with vehicle treatment dropped substantially after collagenase injection, and then gradually recovered during the course of examination for 14 days. Am80 treatment promoted recovery of the performance score, and difference between control mice and Am80-treated mice reached statistical significance (Figure 7B; main effect of treatment: F1,22=7.921, P=0.0101). Foot fault rate in the beam-walking test was also improved by Am80 treatment, and significant difference between control and Am80 groups was detected (Figure 7C; main effect of treatment: F1,22=5.483, P=0.0287).
Figure 7.
Effect of Am80 on neurologic dysfunctions induced by intracerebral hemorrhage (ICH). (A) Schematic representation of the experimental schedule. Vehicle (carboxymethyl cellulose, CMC) or 5 mg/kg Am80 was administered orally for 4 days as indicated. (B and C) Performance in the beam-walking test in CMC-treated and Am80-treated mice, evaluated by performance score (B) and foot fault rate (C). (D and E) Results of performance in the rotarod test (D) and the modified limb-placing test (E). n=12 for each condition. **P<0.01, ***P<0.001 versus Col+CMC group, by Bonferroni's post hoc comparisons.
In the rotarod test, induction of ICH in mice treated with vehicle resulted in substantial disability in performance indicated by a decrease in running time. Am80 treatment partially but significantly prevented the decline in performance (Figure 7D; main effect of treatment: F1,22=5.013, P=0.0356). In the modified limb-placing test, disability in motor performance was evident after ICH as a robust increase in score. Am80 treatment partially prevented the increase in disability score, and the effect reached statistical significance (Figure 7E; main effect of treatment: F1,22=8.583, P=0.0078).
Discussion
Here, we demonstrated for the first time that systemic administration of an RAR agonist prevented neuron loss, lessened inflammatory reactions, and ameliorated neurologic deficits resulting from ICH. The neuroprotective effect of Am80, the RAR agonist used in this study, was evident at 5 mg/kg per day. This dose is comparable to the doses of Am80 previously shown to be effective in ameliorating scopolamine-induced memory deficit in rats (5 to 15 mg/kg per day; Shudo et al, 2004) and in alleviating experimental autoimmune encephalomyelitis in rats and mice (1 to 3 mg/kg per day; Wang et al, 2000; Klemann et al, 2009). In addition, we recently showed that oral administration of Am80 at 10 mg/kg per day prevented dopaminergic cell loss induced by intranigral injection of lipopolysaccharide in mice (Katsuki et al, 2009). Notably, Am80 could rescue neurons from hemorrhagic injury when administered as late as 6 hours after induction of hemorrhage, suggesting that RAR is a promising target for emergency therapy in ICH. In this study, we evaluated neuroprotective effect by an increase in the number of NeuN-immunoreactive cells remaining within the hematoma. In a strict sense, we do not know whether these cells are really alive and how neurons can survive under severe conditions. However, we consider it unlikely that NeuN immunoreactivity is retained for a long time in dead cells. Indeed, acceleration of behavioral recovery by Am80 (as discussed below) provides another line of evidence for neuroprotection by this compound.
In contrast to the significant neuroprotective effect, Am80 showed no effect on hematoma expansion and edema formation after ICH. These profiles of the effect of Am80 are different from those of the effects of several drugs including blood coagulation factor VIIa (Mayer, 2007), statins (Karki et al, 2009), and edaravone (Nakamura et al, 2008) that have been shown to inhibit hematoma expansion and/or edema formation. As formation of hematoma and edema contributes to physical damage of brain tissue, ineffectiveness of Am80 on hematoma and edema may be relevant to the fact that the neuroprotective effect of this compound remained partial. On the other hand, these profiles indicate that combinations of Am80 with other drugs affecting hematoma or edema may provide an additive or synergistic effect on neuronal viability. This possibility deserves further investigations.
Regulation of inflammation is a potential strategy for ICH therapy (Wang and Dore, 2007b). Although inflammatory processes associated with ICH may be intimately linked to edema formation (Wasserman and Schlichter, 2007), edema after ICH can result from multiple mechanisms (Xi et al, 2006), and suppression of inflammation without accompanying inhibition of edema formation has been reported in several cases (Ohnishi et al, 2007; Wang and Dore, 2007a). Here, the neuroprotective effect of Am80 was likely to involve suppression of inflammatory reactions after ICH. This assumption was supported by precedent findings that retinoic acids inhibited expression of iNOS and proinflammatory cytokines in activated microglia in culture (Dheen et al, 2005; Xu and Drew, 2006). Moreover, Am80 suppressed inflammatory events associated with experimental allergic encephalomyelitis in rats (Wang et al, 2000). Indeed, we found in this study that isolectin B4 binding-positive cells accumulating in the perihematoma region expressed RARα, suggesting that Am80 can act on microglia/macrophages in hemorrhagic brain. Isolectin B4 binding can be used as a marker of microglia/macrophages (Lee et al, 2006a) but is also known to label endothelial cells. In this study, intense labeling with isolectin B4 was observed in activated microglia/macrophages but not in microglia with resting morphology. In addition, we could distinguish microglia/macrophages from endothelial cells or neutrophils by their morphologic characteristics. The validity of identification of activated microglia/macrophages by our criteria was confirmed by examinations of immunoreactivities against Iba1 (for microglia/macrophages) and myeloperoxidase (for neutrophils), although utilization of other markers of microglia/macrophages such as CD11b or F4/80 may be also useful. We also showed that Am80 treatment decreased the number of microglia/macrophages in the perihematoma region, and also decreased the area affected by oxidative stress as revealed by nitrotyrosine immunoreactivity. With regard to nitrotyrosine immunoreactivity, integrated values of staining intensity was not different between groups, suggesting that Am80 arrested expansion, but did not affect total amount, of oxidative stress associated with ICH. A plausible mechanism of these effects of Am80 is suppression of microglial proliferation. Intraperitoneal administration of 13-cis-retinoic acid (isotretinoin) in mice has been shown to inhibit lipopolysaccharide-induced microglial proliferation in vivo (Shankaran et al, 2007). If a similar mechanism of action is involved, suppression of microglial proliferation by Am80 can result in a decrease in the number of microglia and in arrested distribution of oxidative stress produced by activated microglia.
The present results on the effect of Am80 relied on the experimental model of ICH induced by collagenase injection. Although this model of ICH is generally thought to mimic human pathology in many features, we should note that collagenase by itself might be able to elicit inflammatory responses (James et al, 2008; Xue and Del Bigio, 2003). Therefore, at present we cannot exclude possible contribution of events unrelated to hemorrhage that result from the action of collagenase. Investigations such as those including autologous blood model of ICH may help further elucidation of the mechanisms of the effect of Am80.
Another issue to be considered is a change in HO-1 expression levels. HO-1 is a stress response protein whose expression increases after ICH. The principal cell population expressing HO-1 is microglia at 24 to 48 hours after injection of autologous blood in rats and rabbits (Nakaso et al, 2000; Koeppen et al, 2004) and at 24 hours after collagenase injection in mice (Wang and Dore, 2007a). We confirmed that HO-1 was robustly expressed in microglia/macrophages in the perihematoma region at 3 days after collagenase injection. In addition, we showed that Am80 treatment decreased the number of HO-1-positive cells. Importantly, Wang and Dore (2007a) have shown that HO-1-deficient mice displayed smaller neuronal injury, reduced microglia/macrophages activation, and reduced free radical levels after ICH than wild-type littermates, whereas ICH-associated edema formation was not affected by HO-1 deficiency. Most of these neuropathological features of HO-1-deficient mice are consistent with those of Am80-treated animals in this study. Therefore, the decrease in HO-1 expression might have a fundamental function in the effect of Am80 on ICH-induced injury. Although the precise mechanisms of the decrease in HO-1 expression by Am80 are unclear at present, RARα stimulation in a mammary cell line has been shown to suppress the activity of Nrf2 (Wang et al, 2007), a transcription factor regulating the expression of various genes including those encoding HO-1 (Syapin, 2008).
We do not exclude the possibility that direct effects on neurons are also involved in the protective effect of Am80, as RARs are also expressed in neurons (Katsuki et al, 2009; Zetterstrom et al, 1999). In fact, our earlier study on midbrain slice cultures has shown that Am80 protected dopaminergic neurons from inflammatory degeneration, presumably through upregulation of brain-derived neurotrophic factor (Katsuki et al, 2009). Whether neurotrophin expression contributes to the protective effect of Am80 in ICH model is an interesting point to be addressed.
We also showed that neurologic deficits after induction of ICH were significantly attenuated by Am80. Remarkably, administration of Am80 was discontinued at 2 days, whereas the significant effect of Am80 on various behavioral parameters was observed as late as 14 days, after induction of ICH. The results suggest that Am80 effectively inhibited neuropathological events occurring at acute phase of ICH, which consequently resulted in better recovery of neurologic functions after a long period. Along with the fact that Am80 is already available for therapy of acute promyelocytic leukemia (Ohnishi, 2007), the present findings indicate that RAR agonists including Am80 may constitute a novel class of therapeutic drugs for ICH.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from Itsuu Laboratory, Takeda Science Foundation, and Mitsubishi Pharma Research Foundation, by a Grant-in-Aid for Scientific Research (20390026) from The Japan Society for the Promotion of Science, and by ‘Research for Promoting Technological Seeds A' program of Japan Science and Technology Agency.
References
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J, Jeong SW, Kim SU, Lee SK, Shin HS, Roh JK. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023:213–221. doi: 10.1016/j.brainres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA. Retinoic acid inhibits expression of TNF-α and iNOS in activated rat microglia. Glia. 2005;50:21–31. doi: 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2008;9:139–152. doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- Karki K, Knight RA, Han Y, Yang D, Zhang J, Ledbetter KA, Chopp M, Seyfried DM. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke. 2009;40:3384–3389. doi: 10.1161/STROKEAHA.108.544395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Kurimoto E, Takemori S, Kurauchi Y, Hisatsune A, Isohama Y, Izumi Y, Kume T, Shudo K, Akaike A. Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling. J Neurochem. 2009;110:707–718. doi: 10.1111/j.1471-4159.2009.06171.x. [DOI] [PubMed] [Google Scholar]
- Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Horsten S, Shudo K, Oki S, Yamamura T. Synthetic retinoid Am80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol. 2009;174:2234–2245. doi: 10.2353/ajpath.2009.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen AH, Dickson AC, Smith J. Heme oxygenase in experimental intracerebral hemorrhage: the benefit of tin-mesoporphyrin. J Neuropathol Exp Neurol. 2004;63:587–597. doi: 10.1093/jnen/63.6.587. [DOI] [PubMed] [Google Scholar]
- Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–1300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- Lee JC, Cho GS, Choi BO, Kim HC, Kim YS, Kim WK. Intracerebral hemorrhage-induced brain injury is aggravated in senescence-accelerated prone mice. Stroke. 2006a;37:216–222. doi: 10.1161/01.STR.0000195151.46926.7b. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Sinn DI, Jung KH, Kim EH, Kim SJ, Kim JM, Ko SY, Kim M, Roh JK. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J Neurochem. 2006b;96:1728–1739. doi: 10.1111/j.1471-4159.2006.03697.x. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Malaspina A, Michael-Titus A. Is the modulation of retinoid and retinoid-associated signaling a future therapeutic strategy in neurological trauma and neurodegeneration. J Neurochem. 2008;104:584–595. doi: 10.1111/j.1471-4159.2007.05071.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Ii C, Takahashi H, Imai Y, Tanaka J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J Neurosci Res. 2007;85:994–1009. doi: 10.1002/jnr.21198. [DOI] [PubMed] [Google Scholar]
- Mayer SA. Recombinant activated factor VII for acute intracerebral hemorrhage. Stroke. 2007;38 (2 Suppl:763–767. doi: 10.1161/01.STR.0000254499.46122.22. [DOI] [PubMed] [Google Scholar]
- Mey J. New therapeutic target for CNS injury? The role of retinoic acid signaling after nerve lesions. J Neurobiol. 2006;66:757–779. doi: 10.1002/neu.20238. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, Nagao S, Xi G, Keep RF, Itano T. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39:463–469. doi: 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Kitayama M, Mizuta E, Fukuda H, Ishii T, Nakashima K, Yamada K. Co-induction of heme oxygenase-1 and peroxiredoxin I in astrocytes and microglia around hemorrhagic region in the rat brain. Neurosci Lett. 2000;293:49–52. doi: 10.1016/s0304-3940(00)01491-9. [DOI] [PubMed] [Google Scholar]
- Ohnishi K. PML-RARα inhibitors (ATRA, tamibaroten, arsenic troxide) for acute promyelocytic leukemia. Int J Clin Oncol. 2007;12:313–317. doi: 10.1007/s10147-007-0694-6. [DOI] [PubMed] [Google Scholar]
- Ohnishi M, Katsuki H, Fujimoto S, Takagi M, Kume T, Akaike A. Involvement of thrombin and mitogen-activated protein kinase pathways in hemorrhagic brain injury. Exp Neurol. 2007;206:43–52. doi: 10.1016/j.expneurol.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Qureshi AI. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkowski MA, Kim GH, Garrett MC, Zacharia BE, Otten ML, Sosunov SA, Komotar RJ, Hassid BG, Ducruet AF, Lambris JD, Connolly ES. C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:98–107. doi: 10.1038/jcbfm.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran M, Marino ME, Busch R, Keim C, King C, Lee J, Killion S, Awada M, Hellerstein MK. Measurement of brain microglial proliferation rates in vivo in response to neuroinflammatory stimuli: application to drug discovery. J Neurosci Res. 2007;85:2374–2384. doi: 10.1002/jnr.21389. [DOI] [PubMed] [Google Scholar]
- Shudo K, Kagechika H, Yamazaki N, Igarashi M, Tateda C. A synthetic retinoid Am80 (tamibarotene) rescues the memory deficit caused by scopolamine in a passive avoidance paradigm. Biol Pharm Bull. 2004;27:1887–1889. doi: 10.1248/bpb.27.1887. [DOI] [PubMed] [Google Scholar]
- Syapin PJ. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya H, Fukasawa H, Ebisawa M, Eyrolles L, Kawachi E, Eisenmann G, Gronemeyer H, Hashimoto Y, Shudo K, Kagechika H. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J Med Chem. 1997;40:4222–4234. doi: 10.1021/jm9704309. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007a;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dore S. Inflammation after intracerebral haemorrhage. J Cereb Blood Flow Metab. 2007b;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1–3 in an animal model of intracerebral hemorrhage. Ann Neurol. 2003;54:655–664. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- Wang T, Niwa S, Bouda K, Matsuura S, Homma T, Shudo K, Nagai H. The effect of Am-80, one of retinoids derivatives on experimental allergic encephalomyelitis in rats. Life Sci. 2000;67:1869–1879. doi: 10.1016/s0024-3205(00)00776-1. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci USA. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol. 2007;207:227–237. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xu J, Drew PD. 9-cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J Neuroimmunol. 2006;171:135–144. doi: 10.1016/j.jneuroim.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Comparison of brain cell death and inflammatory reaction in three models of intracerebral hemorrhage in adult rats. J Stroke Cerebrovasc Dis. 2003;12:152–159. doi: 10.1016/S1052-3057(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Lindqvist E, Mata de Urquiza A, Tomac A, Eriksson U, Perlmann T, Olson L. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 1999;11:407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]