Abstract
Neural stem cells (NSCs) derived from human fetal striatum and transplanted as neurospheres survive in stroke-damaged striatum, migrate from the implantation site, and differentiate into mature neurons. Here, we investigated how various steps of neurogenesis are affected by intrastriatal transplantation of human NSCs at different time points after stroke and with different numbers of cells in each implant. Rats were subjected to middle cerebral artery occlusion and then received intrastriatal transplants of NSCs. Transplantation shortly after stroke (48 hours) resulted in better cell survival than did transplantation 6 weeks after stroke, but the delayed transplantation did not influence the magnitude of migration, neuronal differentiation, and cell proliferation in the grafts. Transplanting greater numbers of grafted NSCs did not result in a greater number of surviving cells or increased neuronal differentiation. A substantial number of activated microglia was observed at 48 hours after the insult in the injured striatum, but reached maximum levels 1 to 6 weeks after stroke. Our findings show that the best survival of grafted human NSCs in stroke-damaged brain requires optimum numbers of cells to be transplanted in the early poststroke phase, before the inflammatory response is established. These findings, therefore, have direct clinical implications.
Keywords: cerebral ischemia, inflammation, neurogenesis, rat
Introduction
Stroke caused by the occlusion of a cerebral artery leads to disability or death in most instances (Meairs et al, 2006). Effective methods to promote recovery in the chronic phase after the insult are lacking. Stem cell-based approaches have been suggested as potential new treatments for stroke (Bacigaluppi et al, 2008; Locatelli et al, 2009). Different types of human-derived stem cells induce behavioral recovery in animal models of stroke (Bacigaluppi et al, 2008; Locatelli et al, 2009), probably through several mechanisms. First, the replacement of functional neurons by human embryonic stem cell-derived and fetal brain-derived neural stem cell (NSC) grafts has been shown in the stroke-damaged brain (Darsalia et al, 2007; Daadi et al, 2008). Second, stem cells can ameliorate poststroke impairments in the rodent brain also by modulating inflammation, inducing angiogenesis, and by providing neuroprotection (Lee et al, 2007; Bacigaluppi et al, 2009; Liu et al, 2009). Finally, recovery could potentially be induced by the stimulation of neurogenesis from endogenous NSCs (for references see Lindvall and Kokaia, 2008). Before stem cell-based approaches can be used in clinical trials, it will be necessary to understand how the proliferation, survival, migration, differentiation, and functional integration of stem cells into the stroke-damaged brain can be controlled. Successful clinical translation also requires knowledge about transplantation parameters such as optimum time for surgery after the insult.
We have earlier shown that human fetal striatum-derived NSCs, transplanted as neurospheres, survive in stroke-damaged rat striatum, migrate toward the site of the injury, and differentiate into mature neurons in the absence of tumor formation (Darsalia et al, 2007). Here, we have assessed how various steps in neurogenesis are affected by transplantation at different time points after stroke and the effects of implanting different cell numbers. To explore the possible mechanisms influencing neurogenesis from grafted NSCs, we also analyzed the microglia response after transplantation.
Materials and methods
Animals and Experimental Groups
Fifty-seven male Wistar rats were used. All experiments (Table 1) were conducted according to the policies on the use of animals of the Society for Neuroscience and approved by Malmö/Lund ethical committee. Forty-two rats were subjected to 30 minutes of middle cerebral artery occlusion as described earlier (Kokaia et al, 1995) and received human striatal neurosphere grafts into their striatum either 48 hours (n=28) or 6 weeks (n=14) afterwards. Ten intact animals were also grafted. Rats were killed for immunocytochemical analyses at 4 hours, 1, or 5 weeks after transplantation. All animals received 10 mg/kg Cyclosporine-A subcutaneously every second day from the day of transplantation.
Table 1. Animals and experimental groups.
| Number of animals | Time of grafting after MCAO | Number of deposits | Density of neurosphere suspension | Number of grafted cells | Time of killing after grafting |
|---|---|---|---|---|---|
| 3 | 48 hours | 2 | 100,000 cells/μL | 300,000 | 4 hours |
| 4 | 48 hours | 2 | 100,000 cells/μL | 300,000 | 1 week |
| 8 | 48 hours | 2 | 100,000 cells/μL | 300,000 | 5 weeks |
| 8 | 48 hours | 2 | 250,000 cells/μL | 750,000 | 5 weeks |
| 5 | 48 hours | 4 | 250,000 cells/μL | 1,500,000 | 5 weeks |
| 4 | 6 weeks | 2 | 100,000 cells/μL | 300,000 | 4 hours |
| 5 | 6 weeks | 2 | 100,000 cells/μL | 300,000 | 1 week |
| 5 | 6 weeks | 2 | 100,000 cells/μL | 300,000 | 5 weeks |
| 4 | Intact | 2 | 100,000 cells/μL | 300,000 | 1 week |
| 6 | Intact | 2 | 100,000 cells/μL | 300,000 | 5 weeks |
| 5 | Intact | — | — | — | — |
MACO, middle cerebral artery occlusion.
Transplantation
The derivation of NSCs from human fetal striatum and expansion as neurospheres has been described earlier (Kallur et al, 2006). Stem cell cultures were passaged 18 to 21 times (over ∼2 years) before implantation. On the day of transplantation, neurospheres (diameter≈100 μm) were centrifuged and resuspended in Hank's Balanced Salt Solution (Gibco, Carlsbad, CA, USA). The neurosphere suspension had a concentration of either ∼100,000 or ∼250,000 viable cells/μL and was kept on ice throughout the transplantation procedure. The neurosphere suspension (1.5 μL) was delivered to the right striatum of each rat ipsilateral to the site of injury at either two sites (0.5 mm anterior and 3 mm lateral from bregma and 5 mm from brain surface; 0.5 mm posterior and 3 mm lateral from bregma and 5 mm from brain surface) (Paxinos and Watson, 1997) or at four sites (0.5 mm anterior and 3 mm lateral from bregma and 4 or 6 mm from brain surface; 0.5 mm posterior and 3 mm lateral from bregma and 4 or 6 mm from brain surface) (Table 1), by use of a Hamilton syringe at a speed of 0.5 μL/min. Thus, a total of 300,000, 750,000, or 1,500,000 cells were grafted.
Immunocytochemistry
Animals were anesthetized and perfused transcardially with 4% paraformaldehyde. A measure of 30 μm coronal sections were cut and processed for immunocytochemistry as described earlier (Darsalia et al, 2007). The following primary antibodies were used: mouse anti-Ki67 (1:200; Novocastra, Newcastle, UK), a marker of cell proliferation, goat anti-doublecortin (DCX; 1:400; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), a market for migrating neuroblasts, mouse anti-human nucleus (HuNu; 1:100; Chemicon, Billerica, MA, USA), mouse anti-NeuN (1:100; Chemicon), a neuronal marker, rabbit anti-HuD (1:100; Millipore, Billerica, MA, USA) a neuronal marker, rabbit anti-Iba1 (1:1000; Wako Chemicals, Osaka, Japan), a marker for microglia, mouse anti-ED1 (1:200; Serotec, Oxford, UK), a marker of activated microglia, and mouse anti-major histocompatibility complex class II (1:100; Serotec) and, a marker for antigen-presenting cells.
Primary antibodies were detected by use of appropriate fluorescent Cy3 (Jackson ImmunoResearch, West Grove, PA, USA) or biotin-conjugated (Vector Laboratories Inc., Burlingame, CA, USA) secondary antibodies (1:200), which were detected with Alexa 488-conjugated streptavidin (1:200; Molecular Probes, Carlsbad, CA, USA). For double labeling, only one biotinylated secondary antibody was used at a time. For chromogenic visualization, biotinylated secondary antibodies, avidin–boitin complex (Elite ABC kit, Vector), and diaminobenzidine were used. Tyramide Signal Amplification (TSA, Perkin-Elmer, Waltham, MA, USA) was used for HuD staining.
Cell Quantification and Statistical Analysis
Cell counting was performed by blinded investigator using either a computerized setup for stereology, driven by C.A.S.T-Grid software (Olympus, Olympus Europa GmbH, Hamburg, Germany), or by using an Olympus BX61 epifluorescence/light microscope. Number of HuNu+cells was quantified using the optical fractionator method. Briefly, grafts were displayed live on the computer monitor and delineated at low magnification. Quantifications were performed using × 100 oil immersion lens with high numeric aperture. Every section in parallel-cut series that contained grafts in striatum was included. Random sampling was performed using the counting frame, which systematically moved at predefined intervals, so that between 100 and 200 immunoreactive cells were counted. Total number of cells was estimated according to the optical fractionator formula (West et al, 1991). Double staining was verified with a laser scanning confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany).
The magnitude of migration was assessed as the number of HuNu+ cells outside the graft core. Cell differentiation is presented as the number of HuNu+ cells coexpressing DCX or HuD. In addition, to describe differentiation qualitatively, numbers of DCX/HuNu− and HuD/HuNu-double-positive cells are presented as percentage of the total number of HuNu+ cells outside the graft core.
Statistical analyses were performed using one-way analysis of variance followed by Bonferroni post hoc test. Data are given as means±s.e.m. and differences significant at P<0.05.
Results
Effect of Poststroke Delay and Cell Numbers on Survival and Migration of Grafted Cells
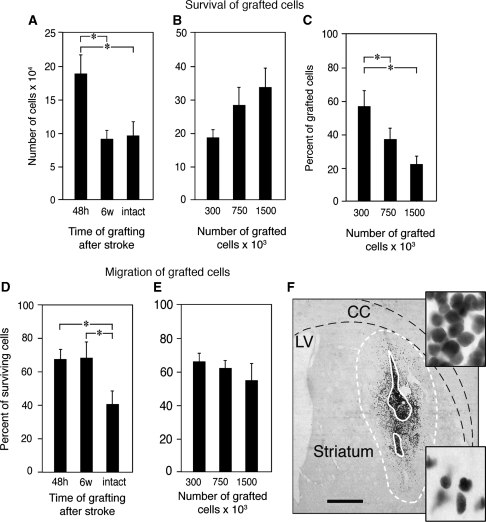
Human NSCs were implanted into intact and stroke-damaged striatum 48 hours or 6 weeks after the insult. Five weeks after transplantation, regardless of when the cells were implanted, the transplanted cells (identified by staining with the human-specific HuNu antibody) had formed a dense core surrounded by migrating cells (Figure 1F). Survival was highest (58%) with transplantation 48 hours after stroke (Figure 1A) and only 27% and 31% of transplanted cells survived after implantation at 6 weeks or in the intact striatum, respectively. Transplantation of higher number of implanted NSCs resulted only in a slight, but nonsignificant, increase in the number of surviving cells after 5 weeks (Figure 1B) and, thus, was associated with decreased cell survival (Figure 1C).
Figure 1.
Influence of poststroke delay and cell numbers on the survival and migration of grafted neural stem cells (NSCs). (A and B) Number of surviving cells in NSC grafts at 5 weeks after implantation in stroke-damaged (grafted at 48 hours (A and B) and 6 weeks (A) after the insult) or intact brain. (C) Percentage surviving cells of total number of grafted NSCs at 5 weeks after transplantation performed 48 hours after stroke. (D and E) Migration of grafted NSCs as evaluated outside the transplant core in stroke-damaged or intact brain. Means±s.e.m. *P<0.05, one-way analysis of variance followed by Bonferroni post hoc test. (F) Typical appearance of NSC graft. Solid white line depicts core of the graft; dashed white line marks border of migration. Upper inset—high magnification of the core of the graft; lower inset—high magnification of the area close to the border of the graft. Scale bar=1 mm. CC, corpus callosum; LV, lateral ventricle.
Owing to the stroke-induced tissue loss and resulting gradual shrinkage of the striatum, it was impossible to compare the direction and distance of cell migration between the groups transplanted at different time points after the insult (48 hours versus 6 weeks). Therefore, to assess the migration of the grafted NSCs, we quantified the number of HuNu+ cells located outside the graft core. The NSCs migrated outside the core of the implant to comparable extent regardless of whether they were implanted 48 hours or 6 weeks after stroke; by comparison, the migratory capacity of cells implanted into the intact brain was reduced (Figure 1D). Cell migration was not influenced by the number of implanted NSCs (Figure 1E).
Effect of Poststroke Delay and Cell Numbers on Proliferation and Differentiation of Grafted Cells
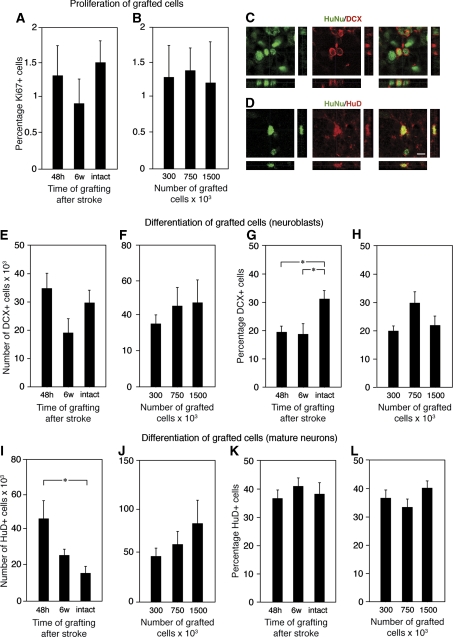
At 4 hours after implantation, ∼43% of cells in the grafts were Ki67+ proliferating cells, which at 5 weeks had decreased to about 1%, irrespective of the timing of transplantation or number of implanted NSCs (Figures 2A and 2B). These parameters did not influence the number of DCX+ neuroblasts (Figures 2C, 2E, and 2F) located outside the core. Although the total number of surviving HuNu+ cells was lower, the percentage of DCX+ cells was higher in grafts in the intact compared with the stroke-damaged striatum (34% versus 25%, respectively) (Figure 2G), but was unaffected by number of NSCs that were grafted (Figure 2H).
Figure 2.
Influence of poststroke delay and cell numbers on the proliferation and differentiation of grafted neural stem cells (NSCs). (A and B) Percentage Ki67+ cells of total number of cells in the graft at 5 weeks after transplantation in the stroke-damaged and intact brain. (C and D) Confocal photomicrographs with orthogonal reconstruction of grafted HuNu+ NSCs coexpressing doublecortin (DCX) and HuD, respectively. Number of DCX+ cells (E and F). Percentage DCX+ cells (G and H) or HuD+ cells (I–L) of total number of migrated, HuNu+ cells in the graft at 5 weeks after implantation in the stroke-damaged or intact brain. Scale bar=15 μm. Means±s.e.m. *P<0.05, one-way analysis of variance followed by Bonferroni post hoc test.
The highest number of HuD+ mature neurons in the grafts (Figure 2D) was observed when transplantation was performed 48 hours after stroke (Figure 2I). Increasing the number of grafted NSCs did not result in significantly higher numbers of mature neurons (Figure 2J). The proportion of cells, which differentiated into HuD+ neurons, was similar in all groups (Figures 2K and 2L).
Characterization of the Environment Surrounding Grafted Cells
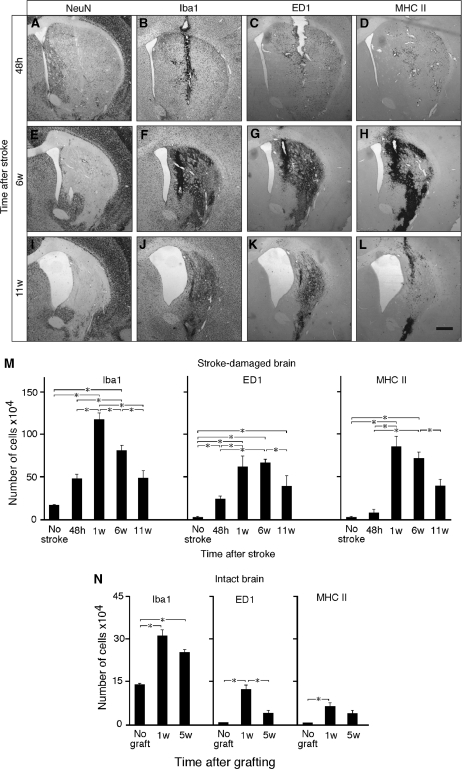
We hypothesized that inflammatory changes could influence neurogenesis by the grafted cells. At 48 hours after stroke, the lesion occupied three quarters of the striatum, predominantly its dorso-lateral part (Figure 3). At 11 weeks, the striatum had undergone shrinkage to ∼50% of its original volume, the lesion sparing only a small, ventro-medial part (Figure 3I). At 48 hours and 1 week after transplantation, the number of cells immunoreactive for Iba1, a general marker for microglia, increased 2.5- and 8.5-fold from baseline levels, respectively, but had declined by 6 and 11 weeks after transplantation (Figures 3B, 3F, 3J, and 3M). Virtually no ED1+activated microglia were detected in the intact striatum (Figure 3M). A substantial number of activated microglia were observed in the damaged striatum 48 hours after stroke. At 1 week, numbers of ED1+-activated microglia had increased further and remained at this level until 6 weeks (Figures 3C, 3G, and 3M). The number of ED1+ cells had declined at 11 weeks (Figures 3K and 3M). ED1+ microglia constituted ∼50% of Iba1+ cells at 1 week and ∼80% at 6 and 11 weeks (Figure 3M). Few antigen-presenting cells expressing major histocompatibility complex class II were present at 48 hours after stroke, but their numbers followed a similar time course to that of activated microglia (Figures 3D, 3H, 3L, and 3M). It is likely that the majority of these cells were themselves microglial cells (Perry, 1998). When human NSCs were implanted in the intact striatum, the number of Iba1+ cells increased 2.3-fold from the baseline levels at 1 week. About 40% of microglia were ED1+. At 5 weeks, both the number of Iba1+ and ED1+ cells had declined (Figure 3N). The number of major histocompatibility complex class II+ cells followed a similar time course (Figure 3N). The microglia response to graft alone without stroke was much smaller compared with that after stroke (Figure 3M). Therefore, the contribution of graft-induced inflammation to the overall inflammatory response after stroke is most likely only marginal.
Figure 3.
Expression of inflammatory markers at different time points after stroke and transplantation of neural stem cells (NSCs). (A, E, and I) Typical appearance of striatal damage after 30 minutes of middle cerebral artery occlusion in the NeuN-stained sections. Photomicrographs showing immunoreactivity for Iba1 (B, F, and J), ED1 (C, G, and K), and major histocompatibility complex (MHC) class II (D, H, and L) in stroke-damaged brain. (M and N) Time course of number of cells expressing Iba1, ED1, and MHC class II in the stroke-damaged (M) and intact (N) striatum. Animals transplanted at 48 hours (A–D) and 6 weeks (E–L) after stroke were killed 4 hours (A–H) or 5 weeks (I–L) thereafter. All animals in stroke groups had received grafts, and all groups (‘stroke-damaged brain' (M) and ‘intact brain' (N)) were given immunosuppressive treatment. Scale bar=1 mm (F) and 15 μm (insets). Means±s.e.m. *P<0.05, one-way analysis of variance followed by Bonferroni post hoc test.
Discussion
We show here that better cell survival is achieved by transplantation of the cells shortly after stroke (48 hours) as compared with a later time point (6 weeks). Increasing the number of grafted NSCs beyond a certain number does not result in a greater number of surviving cells. This observation suggests an optimal threshold of cell numbers for graft survival, which, if saturated in the damaged striatum, may result in insufficient amounts of nutrients reaching the grafted cells, resulting in a progressively decreasing survival rate. Although we found here the maximum survival of human fetal striatal NSCs when implanted in stroke-damaged striatum at 48 hours after the insult, it is possible that cells of other origin subjected to other treatment or delivered in other model of stroke may survive better when grafted during another time period after ischemia. Several earlier studies have implanted different types of NSCs into striatum or cortex of rats subjected to stroke (Grabowski et al, 1994; Borlongan et al, 1998; Saporta et al, 1999; Chu et al, 2004; Kelly et al, 2004; Bliss et al, 2006; Darsalia et al, 2007). The time after insult (from 1 day to 1 month) and the number of transplanted cells (from 5000 to 400,000) have varied between studies, and results from these studies are, therefore, difficult to compare.
Grabowski et al (1994) transplanted primary fetal rat cortical tissue into stroke-damaged rat cortex and found the biggest graft volume when cells were implanted at 3 weeks after the insult. However, cellular composition or proliferation was not assessed and just volume measurement is, most likely, not the optimal way of evaluating survival of grafted cells. Moreover, primary fetal cortical cells transplanted allogeneically into the damaged rat cortex probably behave differently when compared with human striatal NSCs expanded in culture and grafted in the stroke-damaged rat striatum. Differences in the time course of inflammatory responses and use of immunosuppressant could also have contributed to the differences between our study and that of Grabowski et al (1994). It is conceivable that when transplanting bone marrow or other cells, the time window for best graft survival may be different. The optimal time window could also depend on the stroke model and route of cell delivery.
Our study is the first direct evaluation of how numbers of implanted cells and the timing of transplantation after stroke affect various steps of neurogenesis by grafted human NSCs.
We provide further evidence that survival and migration of grafted NSCs are markedly influenced by the inflammation associated with stroke (Friling et al, 2009). The patterns of microglia activation in our experiments agree well with earlier observations (Morioka et al, 1993; Lehrmann et al, 1997). Increased numbers of microglia after stroke are caused by the proliferation of local microglia followed by the infiltration of blood-derived macrophages (Schroeter et al, 1997; Thored et al, 2009). Microglia can have both negative and positive effects on neurogenesis depending on their molecular phenotype (Biscaro et al, 2009). Microglia activation was relatively mild at 48 hours after stroke, increased after 1 week, remained unchanged at 6 weeks, and declined at 11 weeks. Thus, NSCs implanted early after stroke (48 hours) were exposed to less hostile conditions than those transplanted at 6 weeks, when the NCSs encountered well-established inflammation. We have earlier reported that ∼30% of cell survive when the same NSCs were grafted into rat striatum 1 to 2 weeks after stroke (Darsalia et al, 2007), which is similar to that observed in this study when cells were implanted 6 weeks after insult.
Stem cell preconditioning by exposure to a variety of factors is beneficial for cell survival (Haider and Ashraf, 2008). The first weeks after stroke are characterized by upregulation of several cytokines and growth factors, which are downregulated at later time points. These factors promote cell migration and survival (Newman et al, 2005; Cho et al, 2008; Jiang et al, 2008; Thored et al, 2009). We hypothesize that differences in the environment surrounding the graft, including degree of preconditioning with growth factors and cytokines and of microglia activation, account for the better survival of early compared with late transplants.
Migration of grafted NSCs did not differ between stroke-subjected groups, but was reduced in intact brain. These findings are consistent with earlier reports showing that the injured brain is more favorable for stem cell migration than is intact tissue (Lu et al, 1991; Coyne et al, 2006). Guzman et al (2008) showed that implanted neural progenitor cells become activated after brain injury and migrate toward the damaged parenchyma. Stroke induces upregulation of factors stimulating migration, such as stromal cell-derived factor (SDF), whose receptor CXCR4 is present on a variety of stem cells (Bohl et al, 2008).
Regardless of the poststroke delay and numbers of NSC that were implanted, the percentage proliferating cells in the grafts decreased to minute values by 5 weeks after transplantation. Neuronal differentiation of grafted NSCs was also not influenced by these parameters. The grafts in the stroke-subjected groups exhibited a similar percentage of DCX+ and HuD+ cells. Thus, both proliferative activity and neuronal differentiation of grafted NSCs are determined predominantly by intrinsic properties rather than by the characteristics of the pathological tissue environment.
In summary, we report here the two major findings with direct implications for defining the parameters for transplantation of human NSCs in a clinical setting: first, a time window for transplantation exists early after stroke (before maximal activation of microglia) that is optimal for cell survival within the graft. In stroke patients, the corresponding time window could be before days 17 to 18 after the insult, which is when the maximum accumulation of macrophages has been observed (Lindsberg et al, 1996). Second, an optimum number of NSCs that can be implanted at each site for maximum survival exists and that further increases will not lead to higher numbers of surviving cells in the grafts. Consideration of these parameters will be important when human NSC transplantation procedures are scaled up from rodents to clinical trials in patients with stroke.
Acknowledgments
The authors thank Camilla Ekenstierna for excellent technical support.
The authors declare no conflict of interest.
Footnotes
This study was supported by the Swedish Research Council, Juvenile Diabetes Research Foundation, Swedish Diabetes Foundation, EU project LSHB-CT-2006-037526 (STEMSTROKE), and the Swedish Foundation for Strategic Research.
References
- Bacigaluppi M, Pluchino S, Jametti LP, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–77. doi: 10.1016/j.jns.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Biscaro B, Lindvall O, Hock C, Ekdahl C, Nitsch R. Aβ immunotherapy protects morphology and survival of adult-born neurons in doubly transgenic APP/PS1 mice. J Neurosci. 2009;29:14108–14119. doi: 10.1523/JNEUROSCI.2055-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TM, Kelly S, Shah AK, Foo WC, Kohli P, Stokes C, Sun GH, Ma M, Masel J, Kleppner SR, Schallert T, Palmer T, Steinberg GK. Transplantation of hNT neurons into the ischemic cortex: cell survival and effect on sensorimotor behavior. J Neurosci Res. 2006;83:1004–1014. doi: 10.1002/jnr.20800. [DOI] [PubMed] [Google Scholar]
- Bohl D, Liu S, Blanchard S, Hocquemiller M, Haase G, Heard JM. Directed evolution of motor neurons from genetically engineered neural precursors. Stem Cells. 2008;26:2564–2575. doi: 10.1634/stemcells.2008-0371. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport. 1998;9:3703–3709. doi: 10.1097/00001756-199811160-00025. [DOI] [PubMed] [Google Scholar]
- Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, Kim SU, Roh JK. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One. 2008;3:e1644. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26:605–614. doi: 10.1111/j.1460-9568.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- Friling S, Andersson E, Thompson LH, Jonsson ME, Hebsgaard JB, Nanou E, Alekseenko Z, Marklund U, Kjellander S, Volakakis N, Hovatta O, El Manira A, Bjorklund A, Perlmann T, Ericson J. Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc Natl Acad Sci USA. 2009;106:7613–7618. doi: 10.1073/pnas.0902396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski M, Johansson BB, Brundin P. Survival of fetal neocortical grafts implanted in brain infarcts of adult rats: the influence of postlesion time and age of donor tissue. Exp Neurol. 1994;127:126–136. doi: 10.1006/exnr.1994.1086. [DOI] [PubMed] [Google Scholar]
- Guzman R, Bliss T, De Los Angeles A, Moseley M, Palmer T, Steinberg G. Neural progenitor cells transplanted into the uninjured brain undergo targeted migration after stroke onset. J Neurosci Res. 2008;86:873–882. doi: 10.1002/jnr.21542. [DOI] [PubMed] [Google Scholar]
- Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Newman M, Saporta S, Chen N, Sanberg C, Sanberg PR, Willing AE. MIP-1alpha and MCP-1 induce migration of human umbilical cord blood cells in models of stroke. Curr Neurovasc Res. 2008;5:118–124. doi: 10.2174/156720208784310259. [DOI] [PubMed] [Google Scholar]
- Kallur T, Darsalia V, Lindvall O, Kokaia Z. Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. J Neurosci Res. 2006;84:1630–1644. doi: 10.1002/jnr.21066. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Zhao Q, Kokaia M, Elmer E, Metsis M, Smith ML, Siesjo BK, Lindvall O. Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol. 1995;136:73–88. doi: 10.1006/exnr.1995.1085. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrmann E, Christensen T, Zimmer J, Diemer NH, Finsen B. Microglial and macrophage reactions mark progressive changes and define the penumbra in the rat neocortex and striatum after transient middle cerebral artery occlusion. J Comp Neurol. 1997;386:461–476. [PubMed] [Google Scholar]
- Lindsberg PJ, Carpen O, Paetau A, Karjalainen-Lindsberg ML, Kaste M. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. 1996;94:939–945. doi: 10.1161/01.cir.94.5.939. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z.2008Neurogenesis following stroke affecting the adult brain Adult Neurogenesis(Gage F, Kempermann G, Song H, eds)Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA; 549–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Seckin H, Izci Y, Du ZW, Yan YP, Baskaya MK. Neuroprotective effects of mesenchymal stem cells derived from human embryonic stem cells in transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2009;29:780–791. doi: 10.1038/jcbfm.2009.1. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, Bresolin N, Comi GP, Corti S. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66:757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SY, Shipley MT, Norman AB, Sanberg PR. Striatal, ventral mesencephalic and cortical transplants into the intact rat striatum: a neuroanatomical study. Exp Neurol. 1991;113:109–130. doi: 10.1016/0014-4886(91)90168-c. [DOI] [PubMed] [Google Scholar]
- Meairs S, Wahlgren N, Dirnagl U, Lindvall O, Rothwell P, Baron JC, Hossmann K, Engelhardt B, Ferro J, McCulloch J, Kaste M, Endres M, Koistinaho J, Planas A, Vivien D, Dijkhuizen R, Czlonkowska A, Hagen A, Evans A, De Libero G, Nagy Z, Rastenyte D, Reess J, Davalos A, Lenzi GL, Amarenco P, Hennerici M. Stroke research priorities for the next decade--a representative view of the European scientific community. Cerebrovasc Dis. 2006;22:75–82. doi: 10.1159/000093098. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol. 1993;327:123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- Newman MB, Willing AE, Manresa JJ, Davis-Sanberg C, Sanberg PR. Stroke-induced migration of human umbilical cord blood cells: time course and cytokines. Stem Cells Dev. 2005;14:576–586. doi: 10.1089/scd.2005.14.576. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.1997The Rat Brain in Stereotaxic Coordinates3rd ed.San Diego: Academic Press [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Saporta S, Borlongan CV, Sanberg PR. Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative dose-response analysis of cell survival and behavioral recovery. Neuroscience. 1999;91:519–525. doi: 10.1016/s0306-4522(98)00610-1. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28:382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]