Abstract
Positron emission tomography studies of 5-hydroxytryptamine (5-HT)1A receptors have hitherto been limited to antagonist radiotracers. Antagonists do not distinguish high/low-affinity conformations of G protein-coupled receptors and are less likely to be sensitive to intrasynaptic serotonin levels. We developed a novel 5-HT1A agonist radiotracer [11C]CUMI-101. This study evaluates the sensitivity of [11C]CUMI-101 binding to increases in intrasynaptic serotonin induced by intravenous citalopram and fenfluramine. Two Papio anubis were scanned, using [11C]CUMI-101 intravenous bolus of 4.5±1.5 mCi. Binding potential (BPF=Bavail/KD) was measured before (n=10) and 20 minutes after elevation of intrasynaptic serotonin by intravenous citalopram (2 mg/kg, n=3; 4 mg/kg, n=3) and fenfluramine (2.5 mg/kg, n=3) using a metabolite-corrected arterial input function. Occupancy was also estimated by the Lassen graphical approach. Both citalopram and fenfluramine effects were significant for BPF (P=0.031, P=0.049, respectively). The Lassen approach estimated 15.0, 30.4, and 23.7% average occupancy after citalopram 2 mg/kg, 4 mg/kg, and fenfluramine 2.5 mg/kg, respectively. [11C]CUMI-101 binding is sensitive to a large increase in intrasynaptic serotonin in response to robust pharmacological challenges. These modest changes in BPF may make it unlikely that this ligand will detect changes in intrasynaptic 5-HT under physiologic conditions; future work will focus on evaluating its utility in measuring the responsiveness of the 5-HT system to pharmacological challenges.
Keywords: graphical approach, in vivo, kinetic modeling, Lassen plot, positron emission tomography, VT/fp
Introduction
Serotonin 1A (5-hydroxytryptamine, 5-HT1A) receptor binding measured with in vivo imaging has been found to be altered in mood disorders (Parsey et al, 2006), anxiety disorders (Judd et al, 1994), and some (Abi-Dargham et al, 1997), but not all (Frankle et al, 2006), schizophrenia studies. All published human imaging studies to date have used an antagonist as the imaging ligand, most commonly [11C]WAY100635. Although initial efforts to develop an agonist tracer for the 5HT1A receptor have not succeeded in primates (Kumar and Mann, 2007), we have recently reported success with a novel agonist 5-HT1A radioligand, [11C]CUMI-101 (Milak et al, 2008). A G protein-coupled transmembrane receptor, the 5-HT1A receptor exists in an active or high-affinity state, which is coupled to the G protein, and in an inactive or low-affinity state, which is not coupled to the G protein (Cheney et al, 1982). As antagonists bind with equal affinity to both states of the receptor (Lahti et al, 1992), antagonists alone cannot detect the proportion of high-affinity or active receptors.
Theory predicts that agonist radiotracers are more likely to be sensitive to changes in intrasynaptic concentrations of the neurotransmitter. This claim has been substantiated in studies of dopamine D2 receptors, which found that binding of an agonist ligand proved to be more sensitive than that of an antagonist to pharmacologically evoked changes in dopamine release in awake mice (Cumming et al, 2002) and anesthetized monkeys (Narendran et al, 2004).
Both citalopram (Hjorth, 1993; Sharp et al, 1989) and fenfluramine (Hoebel et al, 1989; Laferrere and Wurtman, 1989) are known to increase endogenous 5-HT release extracellularly. To evaluate the sensitivity of [11C]CUMI-101 to displacement by endogenous serotonin from 5-HT1A receptors, we measured the binding potential (BPF=Bavail/KD) of [11C]CUMI-101 in Papio anubis before and after intravenous citalopram or fenfluramine. We hypothesized that the transient increase in intrasynaptic serotonin, in response to citalopram and fenfluramine, will increase occupancy measurable by [11C]CUMI-101 positron emission tomography (PET).
Materials and methods
All animal experiments were carried out with the approval of the Institutional Animal Care and Use Committees from Columbia University Medical Center and New York State Psychiatric Institute.
Chemistry and Radiochemistry
[11C]CUMI-101 was synthesized as described by our laboratory (Kumar et al, 2007). In brief, the radiotracer was prepared by radiomethylation of the corresponding desmethyl analog using [11C]CH3OTf. The final product was purified by high-performance liquid chromatography and a C-18 SepPak (Milford, MA, USA). The radioproduct eluted from the C-18 SepPak (in 1 mL ethanol) was diluted with 9 mL of normal saline, filtered through an aseptically prepared 0.22 μm filter, and used for further studies. A small portion of the product was analyzed with high-performance liquid chromatography for chemical and radiochemical purities, specific activity, and other quality control indices. The average radiochemical yield of [11C]CUMI-101 was 25% end of synthesis (EOS) with a specific activity of 2,600±500 Ci/μmol.
Positron Emission Tomography Studies
A series of [11C]CUMI-101 PET scans were performed in two male P. anubis using an ECAT EXACT HR+ scanner (Siemens, Knoxville, TN, USA). Animal anesthesia and preparation were as described previously (Milak et al, 2005). Overall, 4.48±1.47 mCi (specific activity: 1.8±0.9 Ci/μmol) of [11C]CUMI-101 was injected as an intravenous bolus over 30 seconds and emission data were collected for 120 minutes in a three-dimensional mode. Plasma samples were collected every 10 seconds for the first 2 minutes using an automated system, and thereafter manually for a total of 34 samples. The challenge experiments used the high-affinity, selective serotonin transporter inhibitor citalopram (3 scans at 2 mg/kg, intravenous and 3 scans at 4 mg/kg, intravenous) or fenfluramine (3 scans at 2.5 mg/kg intravenous) 30 minutes before the second injection of [11C]CUMI-101. Citalopram (Ki>10,000) and fenfluramine (Ki=673) or their metabolites have no significant binding affinity to the 5-HT1A receptors (PDSP Database—UNC %U; http://kidb.cwru.edu/pdsp.php). For comparison, seven baseline experiments were used from each animal. For the graphical analysis of occupancy by an antagonist, one scan was used from each animal wherein WAY100635 (a potent 5-HT1A antagonist synthesized in house) was administered intravenously (0.5 mg/kg) 30 minutes before scanning. It must be noted that there is a fundamental difference between occupancy by an antagonist injected intravenously such as WAY100635 and increasing occupancy (relative to baseline) by the endogenous serotonin in response to a pharmacological challenge. Nevertheless, the radiotracer [11C]CUMI-101 may be displaced from receptor binding by either intervention.
Metabolite and Free Fraction Analysis
Six plasma samples were obtained at 2, 4, 12, 30, 60, and 90 minutes during each scan to measure the percentage of unmetabolized [11C]CUMI-101. Free fraction was determined using an ultracentrifugation method as described elsewhere (Gandelman et al, 1994; Ginovart et al, 2001). (High-performance liquid chromatography column: Phenomenex (Torrance, CA, USA) Prodigy ODS(3) 4.6°—250 mm, 5 μm; mobile phase: acetonitrile: 0.25 mol/L sodium phosphate solution (40:60); flow rate: 2 mL/min; retention time: 6 minutes). The metabolites and free fractions were assayed using a Perkin-Elmer 3′′ NaI gamma detector (Perkin-Elmer, Waltham, MA, USA). Data were corrected for background radioactivity and decay. The six metabolite points were fitted with the Hill function (1−AtB/(tB+C)) and weighted using the delta method (Wu et al, 2007). This metabolite fit was then used to correct the plasma radioactivity (34 samples), and a 3-exponential function was fit to the metabolite-corrected plasma curve.
Image Processing and Analysis
Positron emission tomography data were reconstructed after transmission-based attenuation correction and model-based scatter correction (Watson et al, 1996). The reconstruction filter and estimated image filter were Shepp 0.5 (2.5 full-width half maximum; Siemens/CTI), the Z filter was All Pass 0.4 (2.0 full-width half maximum; Siemens/CTI), and the zoom factor was 4.0, leading to a final image resolution of 5.1 mm full-width half maximum at the center of the field of view (Mawlawi et al, 2001).
A T1-weighted magnetic resonance image of the head of both animals was acquired on a GE 1.5-T Signa Advantage system (GE Healthcare Biosciences, Pittsburgh, PA, USA). Regions of interest (ROIs) included the anterior cingulate, amygdala, hippocampus, insular cortex, prefrontal cortex, and temporal cortex and were drawn on coregistered magnetic resonance images; the dorsal raphe nucleus was delineated on baseline PET scans. A part of the cerebellar hemispheres, excluding areas adjacent to the occipital cortex and the vermis, was the reference region.
VT values were derived using likelihood estimation in graphical analysis (Ogden, 2003; Parsey et al, 2003) of the time–activity curve data and metabolite-corrected arterial input functions (Milak et al, 2008). Outcome measures were total volume of distribution (VT) and binding potential (BPF=difference in VT values of an ROI and the reference region divided by the free fraction, fp). Test–retest variability was calculated as the absolute value of the difference between test and retest values, divided by the mean of the two measurements as reported elsewhere (Milak et al, 2008).
Statistical Analysis
Statistical analysis was performed on the BPF data at the ROI level using a linear mixed-effects model with ROI and dose as fixed effects (separately for citalopram and fenfluramine). The random effects are animal, scan date (nested within animal), and scan (nested within scan date). Response variables were taken to be log-transformed BPF values. This is to stabilize the variance between regions, to correct for some slight skewness in the measurements, and because our primary hypothesis specifies a proportional change in each ROI.
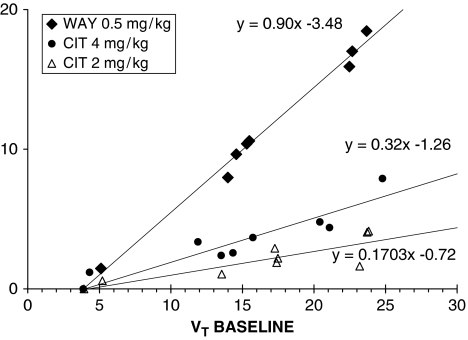
As we have previously shown, there is no ideal reference region for 5-HT1A scans in baboons (Milak et al, 2008). Therefore, occupancy was also evaluated using a graphical approach first described by Lassen et al (1995) and recently modified and expanded by Cunningham et al (2010). Lassen et al showed that graphical representation of x=VTBase and y=VTBase−VTDrug produces a linear relationship with the x intercept equal to VND and slope of the linear fit equal to the occupancy. As this approach yields a linear relationship between ‘x' and ‘y' regardless of whether any of the ROIs plotted is a ‘true' reference region, it follows that this approach does not require a reference region. This approach provides a reference region independent of the verification of occupancy and an estimate of VND (x intercept), assuming it is uniform across ROIs. Cunningham et al (2010) showed that this linear relationship holds true even when two different drug concentrations are used in lieu of a baseline scan (x=VTDrug1 and y=VTDrug1−VTDrug2). They also suggested that ‘when more than one occupancy scan is available within the same individual, it may be better to constrain all x axis intercepts to be equal as part of the fitting process.' Accordingly, we estimated the ‘true' VND using the near complete block produced by nonradioactive WAY100635 0.5 mg/kg intravenous (published elsewhere (Milak et al, 2008); see Figure 1) and used this value to constrain the x axis intercepts of the linear fit in the graphical approach for estimating occupancy.
Figure 1.
Graphical analysis applied to PET occupancy studies. Data are collected from a single [11C]CUMI-101 study in baboons at baseline and after intravenous administration of a 5-HT1A antagonist WAY100635 at 0.5 mg/kg (diamonds); black circles and triangles represent data at baseline and after intravenous administration of citalopram at 4 mg/kg and 2 mg/kg, respectively (single studies). In all cases, the estimated VND values (or x axis intercept of the linear fit), were not included in the regression analysis. PET, positron emission tomography; 5-HT1A, 5-hydroxytryptamine1A.
Results
Citalopram
The citalopram dose response (main effect) was significant for BPF (df=1, 5; F=8. 97; P=0.031). The interaction terms between citalopram dose and ROI remained nonsignificant (df= 6, 84; F=0.42; P=0.87).
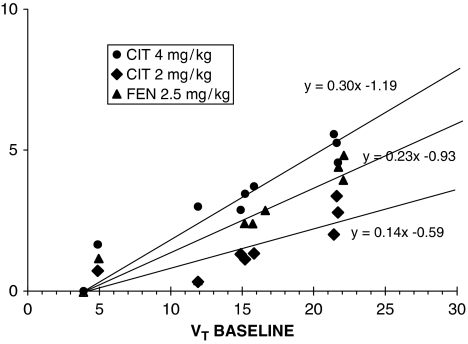
Figure 1 shows the application of the Lassen graphical approach to representative single experiments, whereas Figure 2 shows the application of this approach to the average of all the experiments at each dose level. The Lassen graphical approach based on VT yielded an average occupancy of 14.98% (see Figure 2) in response to 2 mg/kg citalopram and an average occupancy of 30.39% (see Figure 2) in response to 4 mg/kg citalopram (relative to the unknown basal occupancy).
Figure 2.
Graphical analysis applied to PET occupancy studies. Data are collected from [11C]CUMI-101 study in baboons at baseline and after intravenous administration of citalopram 4 mg/kg (circles); citalopram 2 mg/kg (diamonds), and fenfluramine 2 mg/kg (triangles), respectively (each data points representing means from three experiments). In all cases, the estimated VND values were constrained to estimates from the blocks achieved using cold 0.5 mg/kg WAY100635 (see Figure 1). PET, positron emission tomography.
Fenfluramine
The effect of fenfluramine administration was significant (df=1, 2; F=18.8; P=0.049). There was no significant interaction between fenfluramine dose and ROI (df=6, 24; F=1.37; P=0.27). The Lassen graphical approach based on VT yielded an average occupancy of 23.74% in response to 2.5 mg/kg fenfluramine.
Clearance of [11C]CUMI-101 from the plasma after citalopram or fenfluramine pretreatment, estimated as the area under the fitted metabolite-corrected plasma input function divided by the injected dose, was unchanged (df=1, 5; F=3.68; P=0.11; df=1, 2; F=0.006; P=0.95, respectively).
The plasma free fraction (fP) was also unaffected by citalopram or fenfluramine pretreatment (df=1, 5; F=0.03; P=0.86; df=1, 2; F=1.34; P=0.37, respectively).
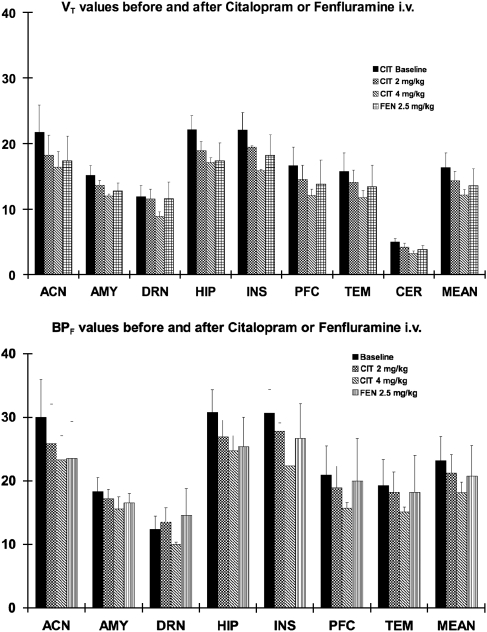
Although cerebellar VT values were small (see Figure 3, top panel), reduction was also seen in the cerebellum in response to citalopram: VT was reduced 15%±12% by 2 mg/kg and 34%±8% by 4 mg/kg citalopram pretreatment. Therefore, any measurements of change in BPF, which is dependent on a ‘true' reference tissue, are underestimates. Nevertheless, the dose response of BPF to citalopram is significant and well outside the range of test–retest variability of 11%±5% (for measures of BPF) (Milak et al, 2008).
Figure 3.
Mean VT (top) and BPF (bottom) of [11C]CUMI-101 after citalopram (2 or 4 mg/kg) and fenfluramine (2.5 mg/kg) intravenously. These values were derived from the LEGA model with scan duration of 100 minutes. Error bars represent the s.d. LEGA, likelihood estimation in graphical analysis.
Discussion
Pretreatment with either citalopram or fenfluramine intravenous resulted in a decrease in [11C]CUMI-101 binding (BPF) in 5-HT1A-rich regions of the brain, without any observable changes in the metabolite-corrected arterial input function or free fraction.
[11C]CUMI-101 Displacement by Endogenous 5-Hydroxytryptamine
BPF as the Outcome Measure
Changes in BPF values after citalopram pretreatment show a significant dose–response effect. Changes are modest in response to 2 mg/kg intravenous citalopram pretreatment when averaged across all ROIs. However, the 4 mg/kg dose produces a greater reduction in both VT and BPF across all ROIs, including the cerebellum. This is consistent with our earlier finding that the cerebellum is not completely devoid of 5-HT1A receptors (Parsey et al, 2005). Although the cerebellar VT is small, this decrease in VT of the reference region introduces a bias in the estimation of BPF that causes an underestimation of the change in BPF in ROIs. The average cerebellar VT before WAY100635 block across animals was 5.35±0.3; the average cerebellar VT across animals after WAY100635 block was 3.89±0.36; the average VND estimated by the Lassen graphical approach across animals was 3.91±0.03. This suggests that there is a ∼27.3% difference between cerebellar VT and VND, as estimated by the Lassen plot, in baboons.
VT/fp as the Outcome Measure
To provide an outcome measure free of this bias, we repeated the analysis using VT/fp, which is the closest approximation of Bavail/KD that can be calculated in the absence of a ‘true' reference region (Theodore et al, 2007). Using VT/fp, both citalopram and fenfluramine dose effects remained significant (df=1, 5, F=36.10, P=0.002; df=1, 2, F=25.83, P=0.037, respectively). Therefore, we conclude that administration of both citalopram and fenfluramine reduces 5-HT1A binding in P. anubis, despite both a possible anesthesia effect (Whittington and Virag, 2006) and the reduction of VT in the reference region, which acts to diminish the percentage change measured in BPF (see limitations below). The corresponding, but greater reduction in VT/fp provides convincing support to this conclusion.
Lassen Graphical Approach
In addition, these results are supported by occupancy estimates obtained by the Lassen graphical approach in which we found a doubling of the receptor occupancy by serotonin after 4 mg/kg compared with 2 mg/kg of citalopram. This approach is less likely to be affected by bias and measurement noise, as it requires far fewer assumptions than using either one of the previous outcome measures (i.e., the method does not require a measurement of VND or fp). The Lassen graphical approach provides a reliable estimate of occupancy and VND (x intercept), even if there is no ‘true' reference region devoid of the receptor being investigated.
Previous Attempts to Measure Endogenous 5-Hydroxytryptamine Release
Similar studies using antagonist ligands [11C]WAY100635 or [18F]MPPF report no reliably measurable effects on binding in response to pharmacological challenges known to stimulate the release of endogenous serotonin. In baboons, using 1.5 mg/kg of amphetamine intravenously or fenfluramine per os, we have previously reported no decrease in [11C]WAY100635 binding (Parsey et al, 1998). These data as well as data reported in the current study may be confounded by an anesthetic affect which has been described previously (Whittington and Virag, 2006). Isoflurane is reported to reduce serotonin release and may mitigate the effects of citalopram or fenfluramine.
It is likely that only a robust increase in intrasynaptic serotonin can be detected by such methods. In vivo studies in rats using microdialysis (Hume et al, 2001b) report that doses of fenfluramine (10 mg/kg intravenous), which resulted in an ∼15-fold increase in extracellular serotonin in the hippocampus, produce a modest (<20%) decrease in hippocampal [11C]WAY100635 binding. In these same experiments, no changes in cortex and raphe binding were found, despite an approximately fivefold increase in extracellular serotonin measured by microdialysis.
Serotonin releasers and depleters, such as reserpine, do not affect [11C]WAY100635 binding in the hippocampus or cortex of male Sprague–Dawley rats (Maeda et al, 2001). Similarly, in the human brain, [11C]WAY100635 binding was unaffected by tryptophan depletion and augmentation (Rabiner et al, 2002).
Few studies have been carried out with other radioligands. One study (Mathis et al, 1995) has shown that fenfluramine decreases the specific binding of [11C]WAY100635 in rats and rhesus monkeys. However, it has been argued (Zimmer et al, 2002) that these results were of little practical value as the dose of fenfluramine was high (8 mg/kg, intravenous) and the resulting displacement low (<20%).
In the past, we found that administration of fenfluramine failed to decrease [11C]WAY100635 binding (Parsey et al, 1999). In contrast, others report 10% to 20% reduction in the specific binding of [11C]WAY100635 in the hippocampus of rats, but only after large doses of fenfluramine treatment (10 mg/kg intraperitoneally) (Hume et al, 2001a). No changes in BPF in either the prefrontal cortex or midbrain raphe were observed. These authors argue that these minimal effects are consistent with a low baseline occupancy of the 5-HT1A receptor by 5-HT in vivo, suggesting that only a large change in endogenous agonist concentration would affect radioligand binding. However, the validity of this claim has since been refuted (Zimmer et al, 2002). In contrast, our results show that [11C]CUMI-101 binding (BPF) is reduced by an average of 23.74% in response to a much lower dose of fenfluramine (2.5 mg/kg). The studies showing direct competition between endogenous 5-HT and [18F]-MPPF were carried out mostly in rats and radioactivity was measured by a β-sensitive probe, not PET. Unfortunately, when these paradigms were tested in nonhuman primates (Udo de Haes et al, 2006) and humans using PET, the antagonist [18F]-MPPF did not show measurable binding changes (Udo de Haes et al, 2002; Udo de Haes et al, 2005; Udo de Haes et al, 2006).
[11C]CUMI-101 appears more vulnerable to displacement by endogenous serotonin than several antagonists ligands tested to date (but perhaps not as much as would have been expected on the basis of the agonist competition model alone).
Limitations
This study has been carried out in animals under general anesthesia using isoflurane which, as mentioned above, is reported to reduce serotonin release. This study is also limited by small sample size (only two animals were used). However, this design allowed us to show that the findings were consistently reproduced in each animal more than once and that the occupancy results show a convincing dose–response effect.
Future Directions
Future work will adapt these studies to humans to evaluate levels of serotonin release in health and disorders involving the serotonin system. In addition to testing the sensitivity of this ligand to increases in serotonin, the approach can evaluate effects of acute tryptophan depletion that reduces serotonin levels (and is considerably more tolerable to humans than reserpine). Pharmacokinetic studies of citalopram in humans (Fredricson Overo, 1982) showed that the steady-state plasma levels in patients range from 95 to 720 nmol/L (∼40 to 300 ng/mL) at doses of 30 to 60 mg per day. The mean level was 245 nmol/L (∼100 ng/mL) at the standard dose of 40 mg daily (Fredricson Overo, 1982). In comparison, in the current study we found that citalopram plasma levels after pretreatment with 4 mg/kg were ∼238 ng/mL (∼590 nmol/L) when averaged throughout the PET scans. This suggests that comparable citalopram plasma levels are readily achievable and therefore these experiments may be reproducible in human subjects.
Summary
This study is the first report of a 5-HT1A agonist PET radioligand in primates that can measure robust increases in intrasynaptic endogenous serotonin. If replicated in humans, this radioligand may open the door to studying dynamic changes in intrasynaptic 5-HT levels in a wide range of experimental conditions and psychiatric disorders, as well as studies related to drug development and pharmacotherapy.
Acknowledgments
We thank the staff of the Molecular Imaging and Neuropathology division of the department of Psychiatry at Columbia University, the Kreitchman PET Center, and the Radioligand Laboratory for expert help.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by PHS grants MH076258, MH062185, MH077161, and MH040695.
References
- Abi-Dargham A, Laruelle M, Aghajanian GK, Charney D, Krystal J. The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- Cheney BV, Lahti RA, Barsuhn C, Gay DD. An analysis of binding at the opioid receptor based upon an agonist/antagonist two-state model. Mol Pharmacol. 1982;22:349–359. [PubMed] [Google Scholar]
- Cumming P, Wong DF, Gillings N, Hilton J, Scheffel U, Gjedde A. Specific binding of [(11)C]raclopride and N-[(3)H]propyl-norapomorphine to dopamine receptors in living mouse striatum: occupancy by endogenous dopamine and guanosine triphosphate-free G protein. J Cereb Blood Flow Metab. 2002;22:596–604. doi: 10.1097/00004647-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab. 2010;30:46–50. doi: 10.1038/jcbfm.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham VJ, Gunn RN, Matthews JC. Quantification in positron emission tomography for research in pharmacology and drug development. Nucl Med Commun. 2004;25:643–646. doi: 10.1097/01.mnm.0000134330.38536.bc. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, Kegeles LS, Slifstein M, Martin JH, Huang Y, Hwang DR, Reich E, Cangiano C, Gil R, Laruelle M, Abi-Dargham A. Serotonin 1A receptor availability in patients with schizophrenia and schizo-affective disorder: a positron emission tomography imaging study with [11C]WAY 100635. Psychopharmacology (Berl) 2006;189:155–164. doi: 10.1007/s00213-006-0543-8. [DOI] [PubMed] [Google Scholar]
- Fredricson Overo K. Kinetics of citalopram in man; plasma levels in patients. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:311–318. doi: 10.1016/s0278-5846(82)80181-4. [DOI] [PubMed] [Google Scholar]
- Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free-fraction determination of single photon emission computed tomography (SPECT) radiotracers: beta-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83:1014–1019. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. Positron emission tomography quantification of [(11)C]-DASB binding to the human serotonin transporter: modeling strategies. J Cereb Blood Flow Metab. 2001;21:1342–1353. doi: 10.1097/00004647-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Hjorth S. Serotonin 5-HT1A autoreceptor blockade potentiates the ability of the 5-HT reuptake inhibitor citalopram to increase nerve terminal output of 5-HT in vivo: a microdialysis study. J Neurochem. 1993;60:776–779. doi: 10.1111/j.1471-4159.1993.tb03217.x. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA.1989Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications Ann N Y Acad Sci 575171–191.discussion 92–3 [DOI] [PubMed] [Google Scholar]
- Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V, Grasby P. Effect of 5-HT on binding of [(11)C] WAY 100635 to 5-HT(IA) receptors in rat brain, assessed using in vivo microdialysis nd PET after fenfluramine. Synapse (New York, NY) 2001a;41:150–159. doi: 10.1002/syn.1069. [DOI] [PubMed] [Google Scholar]
- Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V, Grasby P. Effect of 5-HT on binding of [11C]WAY 100635 i 5-HT1A receptors in rat brain, assessed using in vivo microdialysis and PET after fenfluramine. Synapse. 2001b;41:150–159. doi: 10.1002/syn.1069. [DOI] [PubMed] [Google Scholar]
- Judd FK, Apostolopoulos M, Burrows GD, Norman TR. Serotonergic function in panic disorder: endocrine responses to D-fenfluramine. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:329–337. doi: 10.1016/0278-5846(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Kumar JS, Mann JJ. PET tracers for 5-HT(1A) receptors and uses thereof. Drug Discov Today. 2007;12:748–756. doi: 10.1016/j.drudis.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kumar JS, Prabhakaran J, Majo VJ, Milak MS, Hsiung SC, Tamir H, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. Synthesis and in vivo evaluation of a novel 5-HT(1A) receptor agonist radioligand [O-methyl- (11)C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazi ne-3,5(2H,4H)dione in nonhuman primates. Eur J Nucl Med Mol Imaging. 2007;34:1050–1060. doi: 10.1007/s00259-006-0324-y. [DOI] [PubMed] [Google Scholar]
- Laferrere B, Wurtman RJ. Effect of D-fenfluramine on serotonin release in brains of anaesthetized rats. Brain Res. 1989;504:258–263. doi: 10.1016/0006-8993(89)91365-6. [DOI] [PubMed] [Google Scholar]
- Lahti RA, Figur LM, Piercey MF, Ruppel PL, Evans DL. Intrinsic activity determinations at the dopamine D2 guanine nucleotide-binding protein-coupled receptor: utilization of receptor state binding affinities. Mol Pharmacol. 1992;42:432–438. [PubMed] [Google Scholar]
- Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, Osman S, Bloomfield PM, Jones T, Patsalos PN, Oconnell MT, Duncan JS, Andersen JV. Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J Cereb Blood Flow Metab. 1995;15:152–165. doi: 10.1038/jcbfm.1995.17. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Ogawa M, Okauchi T, Kawabe K, Zhang MR, Semba J, Suzuki K. In vivo binding properties of [carbonyl-11C]WAY-100635: effect of endogenous serotonin. Synapse. 2001;40:122–129. doi: 10.1002/syn.1033. [DOI] [PubMed] [Google Scholar]
- Mathis C, Simpson N, Price J, Mahmood K, Bowman K, Mintun M.1995Evaluation of [11C1]WAY 100635: a potent serotonin 5-HT1A receptor antagonist J Nucl Med 36162Abstract [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Milak MS, Ogden RT, Vinocur DN, Van Heertum RL, Cooper TB, Mann JJ, Parsey RV. Effects of tryptophan depletion on the binding of [(11)C]-DASB to the serotonin transporter in baboons: response to acute serotonin deficiency. Biol Psychiatry. 2005;57:102–106. doi: 10.1016/j.biopsych.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Milak MS, Severance AJ, Ogden RT, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV. Modeling considerations for 11C-CUMI-101, an agonist radiotracer for imaging serotonin 1A receptor in vivo with PET. J Nucl Med. 2008;49:587–596. doi: 10.2967/jnumed.107.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, Cooper TB, Martinez D, Kegeles LS, Abi-Dargham A, Laruelle M. In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (-)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse. 2004;52:188–208. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- Ogden RT. Estimation of kinetic parameters in graphical analysis of PET imaging data. Stat Med. 2003;22:3557–3568. doi: 10.1002/sim.1562. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hwang DR, Simpson N, Kegeles L, Anjivel S, Zea-Ponce Y, Lombardo I, Popilskis S, Van Heertum R, Mann JJ, Laruelle M. Kinetic derivation of serotonin 5-HT-1A receptor binding potential with [C-11]carbonyl-WAY 100635 and competition studies with endogenous serotonin. J Nucl Med. 1998;39:167P. [Google Scholar]
- Parsey RV, Hwang DR, Simpson N, Guo NN, Mawlawi O, Kegeles LS, Bergert B, Montoya J, Popilskis S, Mann JJ, Laruelle M. PET studies of competition between [C-11]carbonyl-way 100635 and endogenous serotonin. J Nucl Med. 1999;40:29P. [Google Scholar]
- Parsey RV, Ogden RT, Mann JJ. Determination of volume of distribution using likelihood estimation in graphical analysis: elimination of estimation bias. J Cereb Blood Flow Metab. 2003;23:1471–1478. doi: 10.1097/01.WCB.0000099460.85708.E1. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- PDSP Database—UNC %U 2010 ) http://kidb.cwru.edu/pdsp.php
- Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, Bench CJ, Gunn RN, Cowen P, Grasby PM. A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage. 2002;15:620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Clark D, Grahame-Smith DG. In vivo measurement of extracellular 5-hydroxytryptamine in hippocampus of the anaesthetized rat using microdialysis: changes in relation to 5-hydroxytryptaminergic neuronal activity. J Neurochem. 1989;53:234–240. doi: 10.1111/j.1471-4159.1989.tb07319.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Hasler G, Giovacchini G, Kelley K, Reeves-Tyer P, Herscovitch P, Drevets W. Reduced hippocampal 5HT1A PET receptor binding and depression in temporal lobe epilepsy. Epilepsia. 2007;48:1526–1530. doi: 10.1111/j.1528-1167.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Bosker FJ, Van Waarde A, Pruim J, Willemsen AT, Vaalburg W, Den Boer JA. 5-HT(1A) receptor imaging in the human brain: effect of tryptophan depletion and infusion on [(18)F]MPPF binding. Synapse. 2002;46:108–115. doi: 10.1002/syn.10134. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Cremers TI, Bosker FJ, Postema F, Tiemersma-Wegman TD, den Boer JA. Effect of increased serotonin levels on [18F]MPPF binding in rat brain: fenfluramine vs the combination of citalopram and ketanserin. Neuropsychopharmacology. 2005;30:1624–1631. doi: 10.1038/sj.npp.1300721. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse (New York, NY) 2006;59:18–26. doi: 10.1002/syn.20209. [DOI] [PubMed] [Google Scholar]
- Watson C, Newport D, Casey M.1996A single scatter simulation technique for scatter correction in 3D PET. In Three-dimensional image reconstruction in radiology and nuclear medicineGrangeat P, Amans J-L, eds), Dordrecht; Boston: Kluwer Academic Publishers; pp 255–68 ISBN: 0792341295 [Google Scholar]
- Whittington RA, Virag L.2006Isoflurane decreases extracellular serotonin in the mouse hippocampus Anesth Analg 10392–98.table of contents [DOI] [PubMed] [Google Scholar]
- Wu S, Ogden RT, Mann JJ, Parsey RV. Optimal metabolite curve fitting for kinetic modeling of 11C-WAY-100635. J Nucl Med. 2007;48:926–931. doi: 10.2967/jnumed.106.038075. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Mauger G, Le Bars D, Bonmarchand G, Luxen A, Pujol JF. Effect of endogenous serotonin on the binding of the 5-hT1A PET ligand 18F-MPPF in the rat hippocampus: kinetic beta measurements combined with microdialysis. J Neurochem. 2002;80:278–286. doi: 10.1046/j.0022-3042.2001.00696.x. [DOI] [PubMed] [Google Scholar]