Abstract
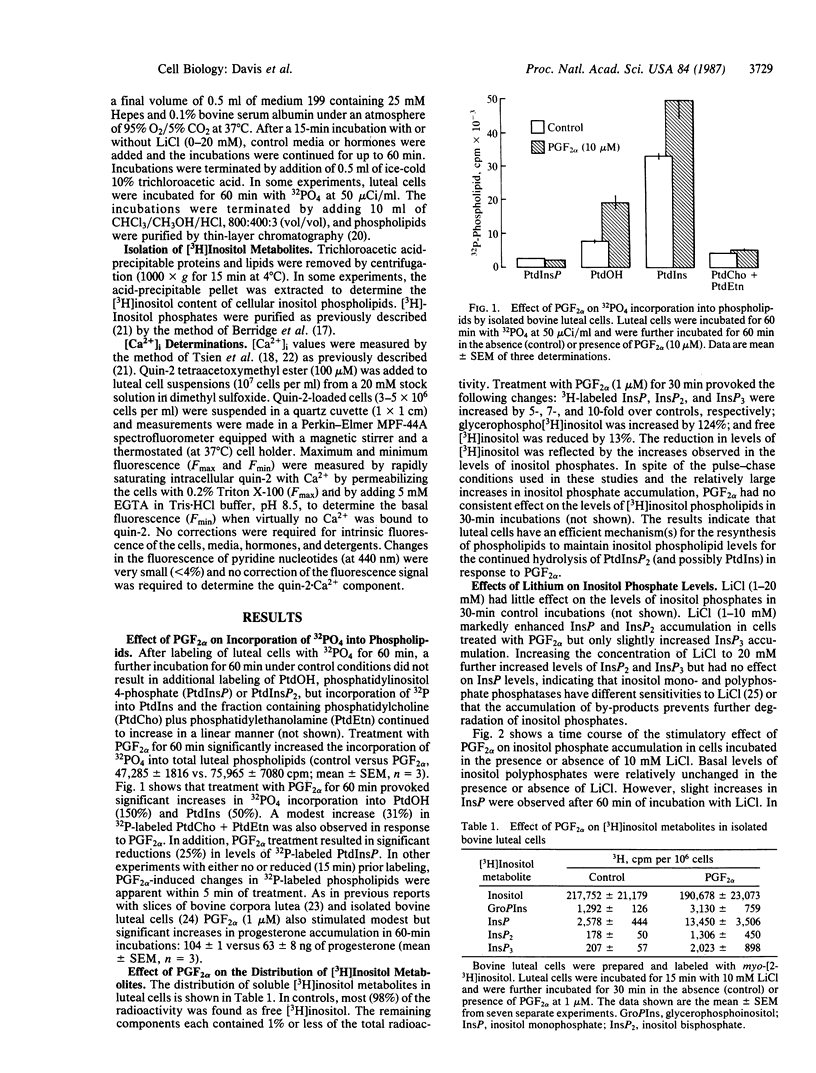
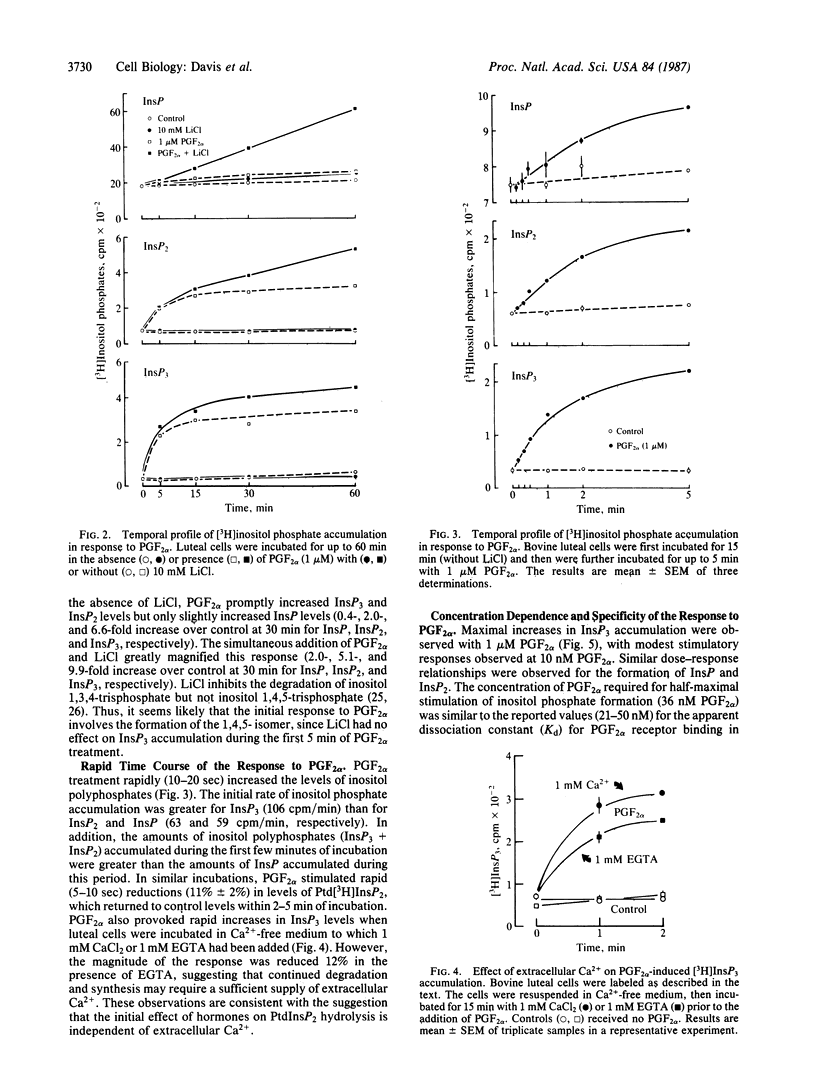
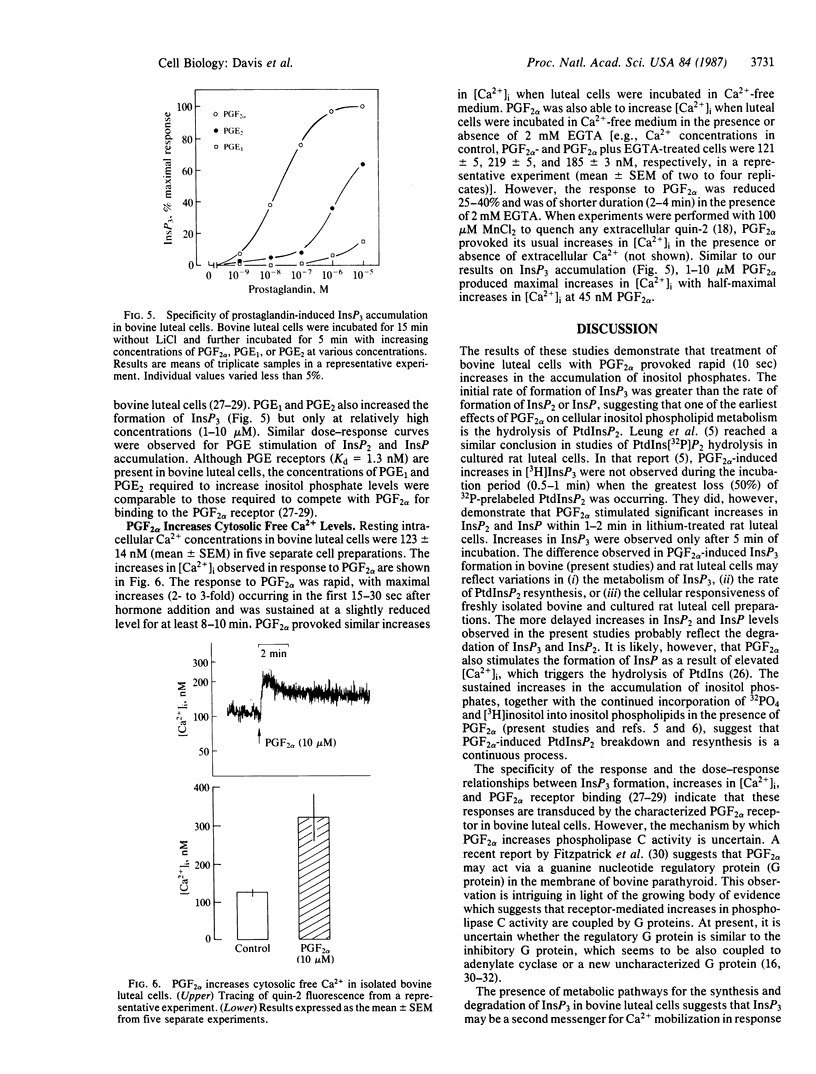
The present studies were conducted to determine whether prostaglandin F2 alpha (PGF2 alpha) stimulates the production of "second messengers" derived from inositol phospholipid hydrolysis and increases intracellular free Ca2+ ([Ca2+]i) in isolated bovine luteal cells. PGF2 alpha provoked rapid (10 sec) and sustained (up to 60 min) increases in the levels of inositol mono-, bis-, and trisphosphates (InsP, InsP2, and InsP3, respectively). InsP3 was formed more rapidly than InsP2 or InsP after PGF2 alpha treatment. In addition, PGF2 alpha increased inositol phospholipid turnover, as evidenced by increased 32PO4 incorporation into phosphatidic acid and phosphatidylinositol. LiCl (1-20 mM) enhanced inositol phosphate accumulation in response to PGF2 alpha. Maximal increases in InsP3 occurred at 1 microM PGF2 alpha, with half-maximal stimulation occurring at 36 nM. The acute effects of PGF2 alpha on InsP3 levels were independent of reductions in extracellular calcium. Prostaglandins E1 and E2 also stimulated increases in inositol phosphate levels, albeit to a lesser extent. PGF2 alpha also induced rapid and concentration-dependent increases in [Ca2+]i as measured by quin-2 fluorescence. The PGF2 alpha-induced increases in [Ca2+]i were maximal within 30 sec (approximately 2- to 3-fold), and [Ca2+]i remained elevated for 8-10 min. The PGF2 alpha-induced increases in [Ca2+]i were also independent of extracellular calcium. These findings demonstrate that the action of PGF2 alpha is coupled to the phospholipase C-InsP3 and diacylglycerol second messenger system in the corpus luteum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blatchley F. R., Donovan B. T. Luteolytic effect of prostaglandin in the guinea-pig. Nature. 1969 Mar 15;221(5185):1065–1066. doi: 10.1038/2211065a0. [DOI] [PubMed] [Google Scholar]
- Brunswig B., Mukhopadhyay A. K., Budnik L. T., Bohnet H. G., Leidenberger F. A. Phorbol ester stimulates progesterone production by isolated bovine luteal cells. Endocrinology. 1986 Feb;118(2):743–749. doi: 10.1210/endo-118-2-743. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. C., Buhr M. M., Riley J. C. Alterations in the cellular membranes of regressing rat corpora lutea. Endocrinology. 1984 Feb;114(2):521–526. doi: 10.1210/endo-114-2-521. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Clark M. R. Activation of protein kinase in the bovine corpus luteum by phospholipid and Ca2+. Biochem J. 1983 Aug 15;214(2):569–574. doi: 10.1042/bj2140569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. S., Farese R. V., Marsh J. M. Stimulation of phospholipid labeling and steroidogenesis by luteinizing hormone in isolated bovine luteal cells. Endocrinology. 1981 Aug;109(2):469–475. doi: 10.1210/endo-109-2-469. [DOI] [PubMed] [Google Scholar]
- Davis J. S., West L. A., Farese R. V. Effects of luteinizing hormone on phosphoinositide metabolism in rat granulosa cells. J Biol Chem. 1984 Dec 25;259(24):15028–15034. [PubMed] [Google Scholar]
- Davis J. S., West L. A., Farese R. V. Gonadotropin-releasing hormone (GnRH) rapidly stimulates the formation of inositol phosphates and diacyglycerol in rat granulosa cells: further evidence for the involvement of Ca2+ and protein kinase C in the action of GnRH. Endocrinology. 1986 Jun;118(6):2561–2571. doi: 10.1210/endo-118-6-2561. [DOI] [PubMed] [Google Scholar]
- Dorflinger L. J., Albert P. J., Williams A. T., Behrman H. R. Calcium is an inhibitor of luteinizing hormone-sensitive adenylate cyclase in the luteal cell. Endocrinology. 1984 Apr;114(4):1208–1215. doi: 10.1210/endo-114-4-1208. [DOI] [PubMed] [Google Scholar]
- Dorflinger L. J., Luborsky J. L., Gore S. D., Behrman H. R. Inhibitory characteristics of prostaglandin F2 alpha in the rat luteal cell. Mol Cell Endocrinol. 1983 Dec;33(2-3):225–241. doi: 10.1016/0303-7207(83)90169-7. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick L. A., Brandi M. L., Aurbach G. D. Prostaglandin F2 alpha and alpha-adrenergic agonists regulate parathyroid cell function via the inhibitory guanine nucleotide regulatory protein. Endocrinology. 1986 May;118(5):2115–2119. doi: 10.1210/endo-118-5-2115. [DOI] [PubMed] [Google Scholar]
- Flint A. P., Sheldrick E. L. Ovarian secretion of oxytocin is stimulated by prostaglandin. Nature. 1982 Jun 17;297(5867):587–588. doi: 10.1038/297587a0. [DOI] [PubMed] [Google Scholar]
- Goodsaid-Zalduondo F., Rintoul D. A., Carlson J. C., Hansel W. Luteolysis-induced changes in phase composition and fluidity of bovine luteal cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4332–4336. doi: 10.1073/pnas.79.14.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore S. D., Behrman H. R. Alteration of transmembrane sodium and potassium gradients inhibits the action of luteinizing hormone in the luteal cell. Endocrinology. 1984 Jun;114(6):2020–2031. doi: 10.1210/endo-114-6-2020. [DOI] [PubMed] [Google Scholar]
- Hansel W., Dowd J. P. Hammond memorial lecture. New concepts of the control of corpus luteum function. J Reprod Fertil. 1986 Nov;78(2):755–768. doi: 10.1530/jrf.0.0780755. [DOI] [PubMed] [Google Scholar]
- Heath E., Weinstein P., Merritt B., Shanks R., Hixon J. Effects of prostaglandins on the bovine corpus luteum: granules, lipid inclusions and progesterone secretion. Biol Reprod. 1983 Nov;29(4):977–985. doi: 10.1095/biolreprod29.4.977. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Codina J., Birnbaumer L. Interaction of the stimulatory and inhibitory regulatory proteins of the adenylyl cyclase system with the catalytic component of cyc-S49 cell membranes. J Biol Chem. 1984 Nov 10;259(21):13178–13185. [PubMed] [Google Scholar]
- Jordan A. W. Effects of prostaglandin F2 alpha treatment on LH and dibutyryl cyclic AMP-stimulated progesterone secretion by isolated rat luteal cells. Biol Reprod. 1981 Sep;25(2):327–331. doi: 10.1095/biolreprod25.2.327. [DOI] [PubMed] [Google Scholar]
- Khan M. I., Rosberg S. Acute suppression by PGF2 alpha on LH, epinephrine and fluoride stimulation of adenylate cyclase in rat luteal tissue. J Cyclic Nucleotide Res. 1979;5(1):55–63. [PubMed] [Google Scholar]
- Kimball F. A., Lauderdale J. W. Prostaglandin E1 and F2alpha specific binding in bovine corpora lutea: comparison with luteolytic effects. Prostaglandins. 1975 Aug;10(2):313–331. doi: 10.1016/0090-6980(75)90051-9. [DOI] [PubMed] [Google Scholar]
- Lahav M., Freud A., Lindner H. R. Abrogation by prostaglandin F2alpha of LH-stimulated cyclic AMP accumulation in isolated rat corpora lutea of pregnancy. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1294–1300. doi: 10.1016/0006-291x(76)90337-5. [DOI] [PubMed] [Google Scholar]
- Lahav M., Weiss E., Rafaeloff R., Barzilai D. The role of calcium ion in luteal function in the rat. J Steroid Biochem. 1983 Jul;19(1C):805–810. doi: 10.1016/0022-4731(83)90015-8. [DOI] [PubMed] [Google Scholar]
- Leung P. C., Minegishi T., Ma F., Zhou F. Z., Ho-Yuen B. Induction of polyphosphoinositide breakdown in rat corpus luteum by prostaglandin F2 alpha. Endocrinology. 1986 Jul;119(1):12–18. doi: 10.1210/endo-119-1-12. [DOI] [PubMed] [Google Scholar]
- Luborsky J. L., Slater W. T., Behrman H. R. Luteinizing hormone (LH) receptor aggregation: modification of ferritin-LH binding and aggregation by prostaglandin F2 alpha and ferritin-LH. Endocrinology. 1984 Dec;115(6):2217–2226. doi: 10.1210/endo-115-6-2217. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Otto A. M., Nilsen-Hamilton M., Boss B. D., Ulrich M. O., Jimenez De Asua L. Prostaglandins E1 and E2 interact with prostaglandin F2alpha to regulate initiation of DNA replication and cell division in swiss 3T3 cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4992–4996. doi: 10.1073/pnas.79.16.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharriss B. B., Wyngarden L. J. The effect of prostaglandin F 2alpha on the progestogen content of ovaries from pseudopregnant rats. Proc Soc Exp Biol Med. 1969 Jan;130(1):92–94. doi: 10.3181/00379727-130-33495. [DOI] [PubMed] [Google Scholar]
- Powell W. S., Hammarström S., Samuelsson B. Occurrence and properties of a prostaglandin F2alpha receptor in bovine corpora lutea. Eur J Biochem. 1975 Aug 1;56(1):73–77. doi: 10.1111/j.1432-1033.1975.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Rao C. V., Ireland J. J., Roche J. F. Decrease of various luteal enzyme activities during prostaglandin F2 alpha-induced luteal regression in bovine. Mol Cell Endocrinol. 1984 Feb;34(2):99–105. doi: 10.1016/0303-7207(84)90060-1. [DOI] [PubMed] [Google Scholar]
- Raymond V., Leung P. C., Labrie F. Stimulation by prostaglandin F2 alpha of phosphatidic acid-phosphatidylinositol turnover in rat luteal cells. Biochem Biophys Res Commun. 1983 Oct 14;116(1):39–46. doi: 10.1016/0006-291x(83)90377-7. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Pozzan T. Using quin2 in cell suspensions. Cell Calcium. 1985 Apr;6(1-2):133–144. doi: 10.1016/0143-4160(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Speroff L., Ramwell P. W. Prostaglandin stimulation of in vitro progesterone synthesis. J Clin Endocrinol Metab. 1970 Mar;30(3):345–350. doi: 10.1210/jcem-30-3-345. [DOI] [PubMed] [Google Scholar]
- Strauss J. F., 3rd, Stambaugh R. L. Induction of 20 alpha-hydroxysteroid dehydrogenase in rat corpora lutea of pregnancy by prostaglandin F-2 alpha. Prostaglandins. 1974 Jan 10;5(1):73–85. doi: 10.1016/s0090-6980(74)80134-6. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Dorflinger L. J., Behrman H. R. Mechanism of the rapid antigonadotropic action of prostaglandins in cultured luteal cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1344–1348. doi: 10.1073/pnas.75.3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]



