Abstract
Moyamoya disease is characterized by the progressive stenosis and often occlusion of the terminal internal carotid arteries, which leads to ischemic and hemorrhagic injuries. The etiology is unknown and surgical revascularization remains the mainstay treatment. We analyzed various hemodynamic factors in 292 patients with moyamoya disease, representing 496 revascularization procedures, including vessel dimension and intraoperative blood flow, using a perivascular ultrasonic flowprobe. Mean middle cerebral artery (MCA) flow rate was 4.4±0.26 mL/min. After superficial temporal artery (STA)–MCA bypass surgery, flows at the microanastomosis were increased fivefold to a mean of 22.2±0.8 mL/min. The MCA flows were significantly lower in the pediatric (16.2±1.3 mL/min) compared with the adult (23.9±1.0 mL/min; P<0.0001) population. Increased local flow rates were associated with clinical improvement. Permanent postoperative complications were low (<5%), but very high postanastomosis MCA flow was associated with postoperative stroke (31.2±6.8 mL/min; P=0.045), hemorrhage (32.1±10.2 mL/min; P=0.045), and transient neurologic deficits (28.6±5.6 mL/min; P=0.047) compared with controls. Other flow and vessel dimension data are presented to elucidate the hemodynamic changes related to the vasculopathy and subsequent to surgical intervention.
Keywords: cerebral blood flow, extracranial to intracrania bypass, hemodynamic, moyamoya, STA–MCA, vessel diameter
Introduction
Moyamoya disease is a chronic cerebrovascular disease characterized by progressive steno-occlusion of the terminal internal carotid arteries that may also involve the proximal anterior and middle cerebral arteries. The name is derived from the Japanese word meaning ‘puff of smoke,' which characterizes the angiographic display of collateral vessels around the basal ganglia that are commonly seen in this condition (Suzuki and Takaku, 1969).
The pathogenesis is unknown, and it can affect both children and adults. The clinical features of both groups may differ and include ischemic strokes, transient ischemic attacks (TIAs), intracerebral hemorrhages, seizures, and debilitating headaches. Although first described in the Japanese population, it can occur in other ethnic groups, albeit with less frequency. Left untreated, a progressive clinical deterioration usually occurs and medical treatment with antithrombotics or vasodilators is ineffective. Current treatment options involve surgical revascularization of the affected hemisphere, either directly with extracranial to intracranial bypass surgery, or indirectly through different techniques, including encephalo-duro-arterio-synangiosis, encephalo-duro-arterio-myo-synangiosis, encephalo-myo-synangiosis, and placement of multiple burr holes (Sainte-Rose et al, 2006).
There has been considerable interest and knowledge gained in the evaluation of regional blood flow in this group of patients (Lee et al, 2009). In contrast, there have been limited reports on intraoperative cerebral blood flow before and after bypass surgery, especially in moyamoya disease (Nakayama et al, 2001). We present here our analysis of intraoperative flow rates on 256 patients with moyamoya disease and its variants in a single institution, undergoing direct superficial temporal artery branch to middle cerebral artery branch anastomoses (STA–MCA). The variations in size of the STA and MCA vessels are also described, together with their relationship with blood flow. In addition, the relationship of flow with age, gender, ethnicity, disease laterality, and postoperative complications are described.
Materials and methods
Patient Population
In all, 292 patients with moyamoya disease or syndrome admitted to Stanford University Medical Center between 1991 and 2008 were analyzed. A total of 496 cerebral revascularization procedures were performed in this group by the senior author (GKS). A retrospective and prospective review of these patients was undertaken. Each patient underwent preoperative clinical and radiological evaluation, which included at least brain magnetic resonance imaging and cerebral angiography. The diagnosis and treatment of moyamoya disease or syndrome were based on clinical findings of stroke, hemorrhage, TIAs or debilitating headaches, and the presence of steno-occlusive disease in at least one terminal internal carotid artery (ICA) or proximal middle cerebral artery, with or without moyamoya vessels, as seen on cerebral angiograms.
Informed consent was obtained from each patient before enrollment in this institutional review board-approved clinical study. Complete intraoperative flow studies were analyzed only in patients with moyamoya disease and syndrome undergoing direct STA–MCA bypass procedure.
Intraoperative Blood Flow Measurement
All patients underwent STA–MCA bypass operation with neuromonitoring, under mild hypothermia, and thiopental. End-tidal CO2 and mean arterial pressures (MAPs) were kept constant throughout the perianastomotic period during which flow measurements were performed. Intraoperative papaverine was used after STA and MCA dissection. The diameters of the distal STA and MCA M4 branch were measured under the microscope. A flexible perivascular (Charbel) flowprobe (HQN-1.5 MB (1.5 mm), HQN-2 MB (2.0 mm); Transonic Systems, Ithaca, NY, USA) was positioned over the freely dissected target vessel. The flowmeter uses an ultrasonic transit-time principle to directly measure volumetric flow in mL/min. The signal was relayed to an electronic flow detection unit (Transonic HT331 Neurosurgery Flow-QC Meter). An analog to digital converter (PowerLab 8/30; ADInstruments, Colorado Springs, CO, USA) then allowed data acquisition and analysis using the proprietary Chart and Scope software. After stabilization of the flow signal, a 15-second recording was commenced. The maximum value during this recording period was noted, and all subsequent blood flow analysis was applied to this maximum value. The recording also generated an average of the minimum, mean, and maximum flow values for the 15-second cycle. The direction of flow was also noted. In this study, distal flow was referred to as flow measured at the MCA segment distal to the microanastomosis, and proximal flow was referred to as flow measured along the MCA segment just proximal to the microanastomosis. Flow may be positive, running in a proximal to distal direction along the MCA branch, or negative, in a distal to proximal direction, back towards the terminal ICA. Intraoperative MAP and end-tidal CO2 concentration were also recorded during each blood flow measurement.
Postoperative Assessment
All patients underwent clinical and radiological evaluation in the immediate postoperative period and again at 6 months.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism (version 4.00 for Windows, GraphPad Software, San Diego, CA, USA). Univariate and multivariate analyses were performed to compare vessel dimensions and blood flow between patient groups. The Pearson correlation coefficient was used to analyze correlations between size, flow, occlusion time, and other parameters. Diameters and flow rates are presented as means±s.e. Probability values <0.05 were considered significant.
Results
Patient Demography
Of the 292 patients analyzed, 6-month follow-up evaluations were complete and available in 256 patients. Pediatric patients were defined as anyone below 18 years old. The mean age was 31 years (range 1 to 68 years), and there were 183 (71.5%) females and 73 (28.5%) males. Similarly, 183 (71.5%) patients were adults and 73 (28.5%) patients were children or adolescents (<18 years old). In all, 149 (58.2%) patients were ethnically Caucasians, 80 (31.3%) Asians, and 27 (10.5%) others, including African-Americans, Hispanics, and Native Americans. In all, 384 direct STA–MCA bypass and 49 indirect revascularization procedures were performed in these patients. In all, 287 (74.7%) direct procedures were performed in adults and 97 (25.3%) in the pediatric group. All patients had angiographic evidence of varying degrees of collateralization. Patients with bilateral terminal ICA, proximal anterior cerebral artery (ACA) or MCA stenosis, or occlusion were classed as moyamoya disease and those with unilateral arteriopathy as variant or probable moyamoya disease as defined by the Research Committee on Spontaneous Occlusion of the Circle of Willis (in English) (Fukui, 1997). In all, 184 (71.9%) patients underwent bilateral and 72 (28.1%) unilateral revascularization procedures at the time of initial presentation. Two patients with unilateral bypass proceeded to a contralateral bypass within the period of the study. In all, 25 (9.7%) patients had another significant disease in conjunction with the moyamoya disease, such as neurofibromatosis, Down's syndrome, and primordial dwarfism. These patients were classified as having moyamoya syndrome.
Before surgical revascularization, the mean modified Rankin score (mRS) was 1.6±0.06 (range 0 to 5). Overall, 174 (70%) patients presented with a clinical stroke and 42 (16.4%) had a hemorrhage. In all, 176 (68.8%) patients had TIAs, 49 (19.1%) had seizures, and 153 (59.8%) had persistent headaches despite medical therapy. In all, 149 (58.2%) patients had evidence of a neurologic deficit at presentation.
Superficial Temporal Artery and Middle Cerebral Artery Size
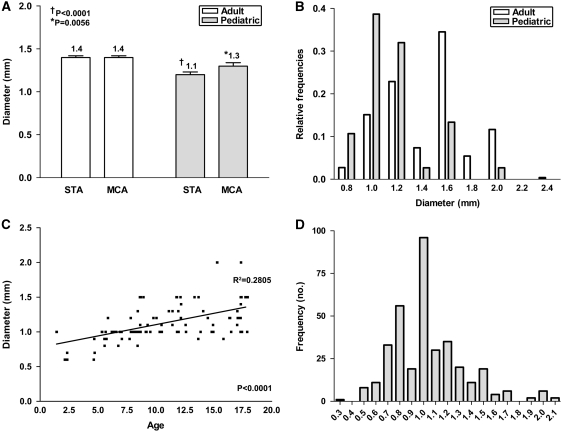
Table 1 summarizes the main comparisons of mean STA and MCA diameters. The mean diameter of the STA and MCA was 1.3 and 1.4 mm, respectively. The STA diameter was significantly smaller in the pediatric population compared with adults and in the indirect bypass group compared with the direct bypass group (Figure 1). Although statistically significant, the mean MCA diameter was only slightly smaller in the pediatric population compared with adults. The most frequent STA and MCA size encountered were both 1.5 mm in the adult and 1.0 mm in the pediatric group (Figure 1). The STA size increased with age, especially in the pediatric population (Figure 1), and MCA size also increased with age, but this was significant only in the pediatric population (P=0.0008; R=0.348; R2=0.12). There was no difference in mean STA or MCA diameter among the five main ethnic groups.
Table 1. STA and MCA diameter (mm).
| Mean STA | Median | Range | Mean MCA | Median | Range | |
|---|---|---|---|---|---|---|
| Overall | 1.3±0.02 | 1.3 | 0.5–2.4 | 1.4±0.02 | 1.3 | 0.7–3.0 |
| Male | 1.4±0.03 | 1.4 | 1.0–2.0 | 1.3±0.03 | 1.2 | 0.8–2.0 |
| Female | 1.3±0.02 | 1.3 | 0.5–2.4 | 1.4±0.02 | 1.3 | 0.7–3.0 |
| Adult | 1.4±0.02* | 1.5 | 0.5–2.4 | 1.4±0.02* | 1.3 | 0.8–3.0 |
| Pediatric | 1.1±0.03* | 1.0 | 0.6–2.0 | 1.3±0.03* | 1.2 | 0.7–2.0 |
| Direct | 1.3±0.02† | 1.3 | 0.7–2.4 | 1.4±0.02 | 1.3 | 0.8–3.0 |
| Indirect | 1.1±0.14† | 1.0 | 0.5–2.0 | 1.3±0.20 | 1.2 | 0.7–2.0 |
| Unilateral | 1.3±0.04 | 1.3 | 0.6–2.0 | 1.4±0.04 | 1.3 | 0.8–2.0 |
| Bilateral | 1.3±0.02 | 1.3 | 0.5–2.4 | 1.4±0.02 | 1.3 | 0.7–3.0 |
MCA, middle cerebral artery; STA, superficial temporal artery.
STA: *P<0.0001; †P=0.0046; MCA: *P=0.0056.
Figure 1.
Mean superficial temporal artery (STA) and middle cerebral artery (MCA) diameter at site of anastomosis: (A) in adults and pediatric patients; (B) STA size distribution; (C) correlation of STA diameter and age in pediatric patients; and (D) ratio of STA to MCA diameter.
Intraoperative Mean Arterial Pressure, CO2, and Hematocrit
Flow measurements are susceptible to various physiological variations, including arterial pressure, blood CO2 concentration, and hematocrit, which may affect blood viscocity. To ensure uniformity of these physiological parameters during blood flow measurements, MAP and CO2 measurements were taken during each blood flow recording. The mean MAP was 83±0.59 mmHg and mean end-tidal CO2 was 31±0.22 mmHg. During the anastomosis, MAP was raised by about 10 to 15 mmHg. However, during blood flow measurements, the MAP was brought back down to preanastomosis levels. The difference in MAP before (83±0.6 mmHg) and after (85.4±0.6 mmHg) anastomosis was only slight. Similarly, the CO2 concentration remained nearly the same as before (31.5±0.2 mmHg) and after (32.4±0.2 mmHg) the anastomosis. The mean hematocrit was 42.2%±0.6%.
Superficial Temporal Artery to Middle Cerebral Artery Size Match
An ideal anastomosis would involve graft and recipient sizes that are well matched. There was no significant difference in the mean STA and MCA diameter in adults, both at 1.4 mm. There was a significant difference between the two vessels in the pediatric group, with a mean STA diameter of 1.1 mm and mean MCA diameter of 1.3 mm (P=0.0051). Most graft to recipient vessels were well matched, with a ratio of 1.0 (Figure 1).
Occlusion Time
The mean occlusion time was 32±0.6 minutes (range 15 to 91 minutes), with a mode of 27 minutes. Occlusion time was not significantly different between the adult (32.6 minutes) and pediatric (30.1 minutes) groups (P=0.23). There was no correlation between occlusion time and the size of the STA or MCA vessels in all patients. Similarly, there was no correlation of occlusion time and the ratio of STA to MCA diameter. However, there was a trend for the occlusion time to increase with STA size in the pediatric group (P=0.0022; R=0.355; R2=0.13).
Preanastomosis Middle Cerebral Artery Flow
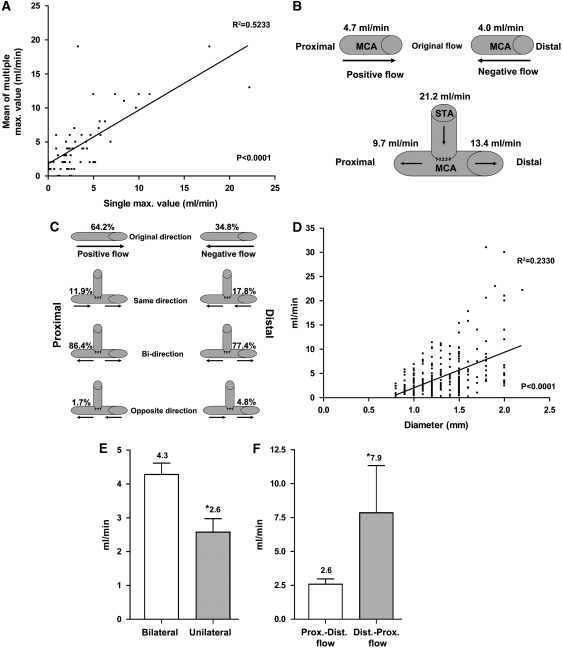
The pulsatile flow recorded by the flow probe generated several cycles of flow data over each 15 seconds period. The single maximum value generated during this period was used for subsequent analysis. To validate our methodology, we analyzed how this single maximal value correlated with the mean of the multiple maximum values. As shown in Figure 2, there was good correlation to all values measured, and there was no significant difference between the means of the single (4.4±0.26 mL/min) and multiple (4.9±0.56 mL/min) maximum flow values (P=0.40). All other flow rates were similarly validated.
Figure 2.
Preanastomosis mean middle cerebral artery (MCA) flow rate: (A) correlation of different measurement techniques; (B) mean MCA flow rates; (C) direction before and after microanastomosis; (D) correlation of MCA size and flow rate; (E) comparison of positive MCA flow rates and laterality of disease; and (F) positive and negative MCA flow in unilateral disease before microanastomosis. *Statistically significant P<0.05.
The recipient MCA branch was usually an M4 branch over the inferior frontal or superior temporal gyri. The mean MCA flow at the recipient vessel was 4.4 mL/min regardless of the direction of flow (Table 2). In all, 193 out of 296 (64.2%) MCA flows recorded were flowing in a proximal to distal direction, named here as positive flow (Figure 2). In all, 103 out of 296 (34.8%) flowed from a distal to proximal direction, termed negative flow. There was a positive relationship between mean MCA flow and MCA diameter (Figure 2). Table 2 summarizes the MCA flow rates between different groups.
Table 2. Intraoperative STA and MCA blood flow in direct STA–MCA bypass (mL/min).
| Mean | Median | Range | P-value | |
|---|---|---|---|---|
| Preanastomosis MCA | ||||
| Overall | 4.4±0.26 | 3.0 | 0–31 | |
| Male | 4.0±0.48 | 3.0 | 0.1–31 | 0.3732 |
| Female | 4.5±0.31 | 3.1 | 0–30 | |
| Adult | 4.2±0.27 | 3.0 | 0–23 | 0.2098 |
| Pediatric | 5.0±0.68 | 3.2 | 0.1–31 | |
| Unilateral | 4.4±0.68 | 2.5 | 0–31 | 0.9925 |
| Bilateral | 4.4±0.28 | 3.1 | 0.1–30 | |
| Postanastomosis STA | ||||
| Overall | 21.2±0.84 | 18.6 | 0.5–77 | |
| Male | 21.8±1.7 | 19.6 | 1.6–77 | 0.7046 |
| Female | 21.1±1.0 | 18 | 0.5–68 | |
| Adult | 23.2±1.0* | 21 | 0.5–77 | <0.0001* |
| Pediatric | 14.5±1.2* | 12.6 | 1.6–42.5 | |
| Unilateral | 18.6±1.63 | 15.8 | 2.0–47.5 | 0.1367 |
| Bilateral | 21.8±0.96 | 19.6 | 0.5–77 | |
| Postanastomosis distal MCA | ||||
| Overall | 9.7±0.47 | 8.0 | 0.2–42.5 | |
| Male | 10.1±1.0 | 8.0 | 0.4–42.5 | 0.6541 |
| Female | 9.6±0.5 | 8.0 | 0.2–38 | |
| Adult | 10.1±0.6 | 8.0 | 0.4–42.5 | 0.1242 |
| Pediatric | 8.3±0.8 | 8.0 | 0.2–29 | |
| Unilateral | 9.0±1.0 | 6.8 | 0.2–31 | 0.4879 |
| Bilateral | 9.9±0.5 | 8.3 | 0.4–42.5 | |
| Postanastomosis proximal MCA | ||||
| Overall | 13.4±0.7 | 10.5 | 0.3–70 | |
| Male | 15.2±1.4 | 11.8 | 0.3–54 | 0.1230 |
| Female | 12.8±0.8 | 10 | 0.4–70 | |
| Adult | 14.7±0.8* | 11.4 | 0.4–70 | 0.0006* |
| Pediatric | 8.9±1.2* | 5.6 | 0.3–35 | |
| Unilateral | 10.3±1.1* | 8.5 | 0.4–39 | 0.0254* |
| Bilateral | 14.2±0.8* | 11 | 0.3–70 | |
| Postanastomosis total MCA | ||||
| Overall | 22.2±0.8 | 20 | 0.8–88 | |
| Male | 24.2±1.7 | 20 | 3.5–63.6 | 0.1528 |
| Female | 21.5±1.0 | 19.9 | 0.8–88 | |
| Adult | 23.9±1.0* | 21 | 2.0–88 | <0.0001* |
| Pediatric | 16.2±1.3* | 13.3 | 0.8–48 | |
| Unilateral | 19.1±1.4 | 18.3 | 0.8–48 | 0.0771 |
| Bilateral | 22.9±1.0 | 20.1 | 1.8–88 | |
| Postanastomosis change in MCA | ||||
| Overall | 21.3±0.9 | 18.1 | 0–84 | |
| Male | 23.3±1.8 | 22 | 0–66.7 | 0.1736 |
| Female | 20.5±1.0 | 17.8 | 0–84 | |
| Adult | 22.9±1.0* | 20.5 | 0–84 | 0.0004* |
| Pediatric | 15.4±1.6* | 12.2 | 0–54 | |
| Unilateral | 22.1±1.0* | 15.9 | 0–54 | 0.0489* |
| Bilateral | 17.6±1.5* | 20.3 | 0–84 | |
MCA, middle cerebral artery; STA, superficial temporal artery.
*Statistically significant P<0.05.
Interestingly, although there was no significant difference in the mean preanastomosis MCA flow between unilateral and bilateral disease groups, when MCA flow was examined according to the laterality of the disease and direction of flow, significant flow differences could be seen. The positive flow was significantly lower in patients with unilateral disease (2.6±0.4 mL/min) compared with bilateral disease (4.3±0.33 mL/min; P=0.039; Figure 2). In addition, within the group of patients with unilateral disease, the positive flow was also significantly lower (2.6±0.4 mL/min) compared with the negative flow (7.9±3.5 mL/min; P=0.013; Figure 2).
We examined the relationship of preanastomosis MCA flows with presenting symptoms and mRSs. No significant correlation was found between flow and patients who presented with strokes, hemorrhage, TIAs, or seizures. Furthermore, there was no correlation of preanastomosis MCA flow and mRS.
Postanastomosis Superficial Temporal Artery and Middle Cerebral Artery Flows
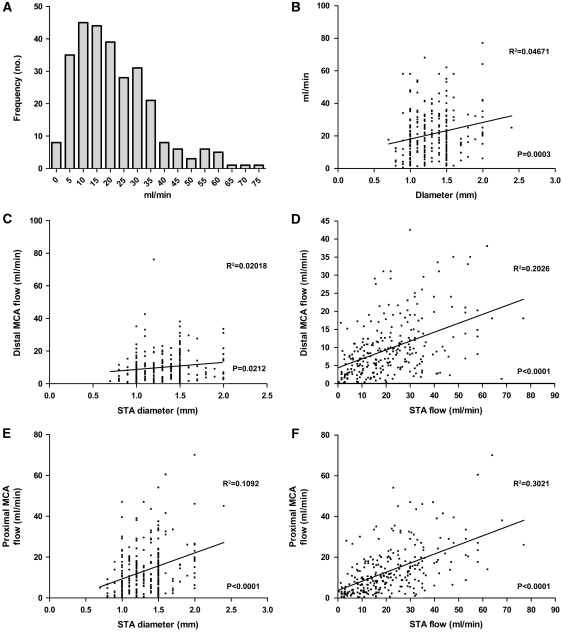
The mean postanastomosis flow rates measured at the distal STA, distal MCA, and proximal MCA were 21.2, 9.7, and 13.4 mL/min, respectively. Table 2 summarizes the results of the various groups compared, and Figure 3 shows the distribution of STA flow recorded. The STA and proximal MCA flow was significantly lower in the pediatric group. In addition, proximal MCA flow was lower in patients with unilateral disease. There was a positive relationship between STA size and flow, and a positive correlation of both STA size and flow with all MCA flow (Figure 3). No difference in STA or MCA flow was found among the five ethnic groups.
Figure 3.
Relationship of superficial temporal artery (STA) and middle cerebral artery (MCA) diameter and postanastomosis blood flow: (A) distribution of STA flow; (B) correlation of distal MCA flow with STA size (C) and STA flow (D); correlation of proximal MCA flow with STA size (E) and STA flow (F).
Change and Balance of Blood Flow
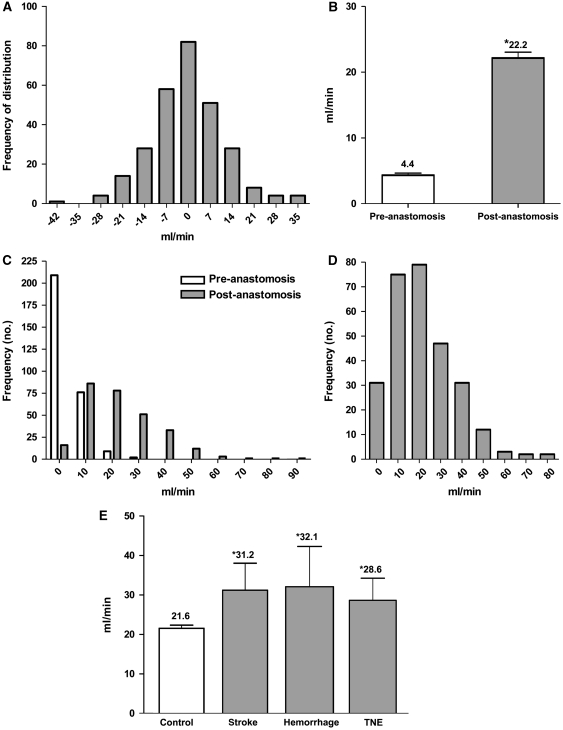
Ideally, all of the recorded postanastomotic STA flow should be distributed to the distal and proximal MCA segments. The existing preanastomosis MCA flow is usually small and its contribution to the total flow and direction of flow is likely to be small as well. Inevitably, some imbalance of flow because of the limitations of the measuring technique occurs. Overall, the balance of STA to total MCA flow is mostly maintained (Figure 4). The mean total MCA flow is 22.2 mL/min, an average fivefold increase of flow from the preanastomotic flow rate (Figure 4).
Figure 4.
Blood flow characteristics after superficial temporal artery-middle cerebral artery (STA–MCA) bypass: (A) STA and MCA flow balance; (B) total MCA flow; (C) distribution of MCA flow before and after microanastomosis; (D) distribution of change in MCA flow after microanastomosis; and (E) association of high MCA flow rates with postoperative complications. *Statistically significant P<0.05.
Clearly, a lower total MCA flow rate in the pediatric group (16.2±1.3 mL/min) exists compared with the adult group (23.9±1.0 mL/min; P<0.0001; Table 2). Most of the increase in the total MCA flow was between 10 and 20 mL/min (Figure 4), and the increase seen was significantly greater in adults and in patients with bilateral disease.
Blood Flow Direction
In moyamoya disease, steno-occlusion of the terminal carotid often means that blood may flow retrograde from collateral branches. The pattern of blood flow directions before and after microanastomosis is depicted in Figure 2. Direction of flow after microanastomosis showed that overall, 51.8% of flow was positive and 48.2% was negative.
Postanastomosis Blood Flows and Postoperative Complications
Postoperative complications were defined as new neurologic events occurring within 30 days of each bypass operation. In this series of 292 patients, 7 (2.4%) patients in 7 procedures (1.8% of direct bypasses) developed a new ischemic stroke (new neurologic deficit with new diffusion-weighted imaging (DWI) lesion on magnetic resonance (MR)), 7 (2.4%) patients in 8 procedures (2.1% of direct bypasses) developed a postoperative hemorrhage (new neurologic deficit and hemorrhage on computed tomography (CT) and/or MR), and 10 (3.4%) patients in 10 (2.7% of direct bypasses) procedures developed a transient neurologic deficit, usually in the form of a dysphasia with or without focal weakness or paresthesia and demonstrating no new DWI lesions or edema on postop MRs. All transient neurologic deficits resolved between a few hours to 14 days.
We examined the flow rates within the group of patients with postoperative complications and compared them with the control group of patients who recovered from surgery uneventfully. The results are summarized in Table 3 and revealed that higher postanastomosis STA and distal MCA flow rates were associated with higher postoperative complications. Analysis of total MCA flow also showed significantly higher flow rates in all three postoperative complication groups when compared with controls.
Table 3. Postanastomosis blood flow and postoperative complications (mL/min).
| Mean | P-value | |
|---|---|---|
| STA | ||
| Control | 20.7±0.85 | |
| Stroke | 34.1±5.3 | 0.0074* |
| Hemorrhage | 29.5±7 | 0.1005 |
| TNE | 28.8±4.8 | 0.0363* |
| Distal MCA | ||
| Control | 9.4±0.5 | |
| Stroke | 15.5±3.1 | 0.0267* |
| Hemorrhage | 17.1±3.4 | 0.0096* |
| TNE | 12.1±4.0 | 0.2407 |
| Proximal MCA | ||
| Control | 13.1±0.7 | |
| Stroke | 15.7±4.3 | 0.5034 |
| Hemorrhage | 17.5±10.6 | 0.3412 |
| TNE | 14.5±5.5 | 0.6580 |
| Total MCA | ||
| Control | 21.6±0.8 | |
| Stroke | 31.2±6.8 | 0.0451* |
| Hemorrhage | 32.1±10.2 | 0.0448* |
| TNE | 28.6±5.6 | 0.0469* |
| Increase in MCA | ||
| Control | 20.7±0.9 | |
| Stroke | 33.3±5.9 | 0.0049* |
| Hemorrhage | 28.2±10.3 | 0.1829 |
| TNE | 26.6±5.7 | 0.1229 |
MCA, middle cerebral artery; STA, superficial temporal artery; TNE, transient neurological event.
*Statistically significant P<0.05.
There was no correlation between other parameters, such as vessel size and occlusion time, and postoperative complications. Analysis of other clinical factors also did not show any associations with postoperative complications, as reported in detail elsewhere (Guzman et al, 2009).
Discussion
We present here the results from the largest single center revascularization series for moyamoya disease and syndrome to date, both in the adult and pediatric population, defined as patients who were under 18 years at the time of surgery. The current series, which reflects the practice of the senior author in a single North American institution during the past 18 years, consisted mainly of a Caucasian population (60%), although Asians comprised the second largest population (30%). The spectrum of clinical presentation was similar to that published earlier, and a detailed analysis of the clinical features and outcomes of this series after revascularization surgery was presented recently (Guzman et al, 2009). Here, we have focused on presenting data on STA and MCA dimensions, blood flow measurements through the graft and recipient vessels, their hemodynamic relationship with each other and their relationship with clinical presentation, outcome, and postoperative complications.
Surgical Treatment
With our current limited knowledge of the pathogenesis of moyamoya disease, no proven primary drug treatment exists for this condition. Numerous studies have shown improved clinical outcomes after revascularization surgery, with either prevention or reduction of further strokes, hemorrhages, and TIAs (Houkin et al, 1996; Kawaguchi et al, 2000; Veeravagu et al, 2008). Surgical treatment, in the form of cerebral blood flow augmentation, remains the primary therapeutic modality to prevent clinical deterioration from this condition. However, there are as yet no prospective randomized studies to compare surgery versus best medical treatment.
In our practice, we favor direct revascularization surgery, most commonly in the form of an STA–MCA bypass. Indirect procedures are also effective in providing cerebral blood flow augmentation (Matsushima et al, 1998; Scott et al, 2004). However, the time taken for revascularization is longer, and the benefits are therefore delayed compared with direct bypass graft procedures.
Hemodynamic Factors
The main hemodynamic factors that govern blood flow throughout the circulatory system consist of the pressure gradient, resistance, and viscosity of the blood. Cerebral autoregulation ensures a relatively constant blood flow, but it is not known whether autoregulatory mechanisms are significantly impaired in moyamoya disease (Ogawa et al, 1990). Of note, interpretation of any flow data during open surgery must be interpreted in light of the pervading cerebrovascular physiology (Kikuta et al, 2007), especially when comparing intraoperative flow data with subsequent postoperative blood flow measurements.
During the intraoperative flow measurement, it was important to keep MAP and CO2 conditions tightly controlled and this was achieved. Blood flow through the graft and recipient vessels also depends on resistance. With the pressure gradient largely controlled, it is the resistance in the individual vessels and vascular network that largely determines the blood flow measured locally. This is of even greater importance in the context of a steno-occlusive disease with variable amounts of collateral network. In contrast, numerous other cerebral hemodynamic factors such as cerebral vascular reactivity, extent of collateral supply, and disease severity will influence regional blood flow, in addition to the blood flow through the graft.
A linear relationship exists between vessel length and resistance, which can often be exploited in bypass surgery to alter the resistance and therefore flow through the graft, thereby tailoring the amount of flow going into the recipient vessels. In our series, the STA length was typically about 8 cm, and ranged between 7 and 9 cm, as measured between the superior border of the zygomatic arch and the site of the microanastomosis.
Blood viscosity is mainly affected by the hematocrit and body temperature. All the patients in our series underwent preoperative and intraoperative blood work to ensure normal hematocrit levels were achieved before and during surgery. In addition, all revascularization surgery was performed under mild hypothermia, with temperatures of about 33°C achieved at the time of microanastomosis. It has been shown that decreasing blood temperature increases blood viscosity by about 2% per °C (Cinar et al, 2001). Postoperative measurements should take this into account, as the normothermic patient would be expected to have a blood viscosity of about 10% lower than during surgery.
In our study, vessel diameter is the most important factor determining resistance to local flow, especially when the driving pressure, viscosity, and vessel length are held effectively constant in this study. A change in radius alters resistance and therefore flow to the fourth power of the radius. Given the importance of this relationship, we systematically measured the diameter of each STA and MCA branch at the site of flow measurement in all patients.
Vessel Dimension and Clinical Disease
Almost all large anatomical studies of STA and MCA dimensions have been performed in cadavers or at autopsy, with the inherent inaccuracy that fixatives and the use of latex-injected vessels may cause in the vessels studied (Kadri et al, 2007; Kawashima et al, 2005; Marano et al, 1985; Pinar and Govsa, 2006; Stock et al, 1980; Waddington, 1979). In addition, STA sizes are usually reported at the level of the zygoma or bifurcation and not at the site of potential microanastomosis. To our knowledge, we present here the first detailed and large study of STA and MCA vessel dimensions at the site of the anastomosis intraoperatively.
Histopathological studies of moyamoya disease demonstrate a hyperplasia of smooth muscle cells and luminal thrombosis that contributes to the progressive stenosis and eventual occlusion seen in the terminal ICA (Fukui et al, 2000; Takagi et al, 2007). There have also been reports of the hyperplastic etiology affecting distal MCA branches and extracranial vessels, such as the STA (Aoyagi et al, 1996; Takagi et al, 2007). However, we could not detect any significant relationship between the size of the MCA branch and clinical presentation, severity of the disease, or ethnicity. Important limitations of this analysis include the fact that only vessels at the site of the anastomosis were analyzed and assessment of external size does not necessarily reflect internal diameter.
Moyamoya disease is a chronic condition and many patients develop marked collateral blood supply. Serial angiographic studies demonstrate that these collateral vessels often increase in size with time, as the demand from the ischemic brain is met. The embryological development of the external carotid artery and ICA systems are closely related and anastomosis of the two systems at the level of the cavernous ICA segment constitutes the most common pathway of reestablishing blood supply in the presence of ICA occlusion. Indeed, it is frequent to see collaterals developing from branches of the ophthalmic and ethmoidal arteries in moyamoya disease. In addition, dilation of meningeal and leptomeningeal collateral vessels derived from the external carotid artery system are frequently seen. Despite the early involvement of these external carotid artery collaterals, the size of the STA does not seem to be affected. Again, the mean STA diameters we found compared similarly with those recorded in nonmoyamoya patients. We also did not see any relationship between STA size and clinical features.
Vessel Dimension and Surgery
Although vessel size is clearly associated with the intraoperative flow rates, a definite relationship also exists between vessel size and the success of a microanastomosis. One of the limitations to performing a direct bypass procedure was the size, and therefore, fragility of the graft or recipient vessel. Size was also intimately associated with age, and this was most obvious in the pediatric population, especially the prepubertal population.
We found that the technical limitation in our series was any vessel, STA or MCA that had a diameter of ⩽0.6 mm. This was usually the case for infants and young children. The youngest patient who underwent a successful direct bypass surgery was 4 years old, and this patient had an STA and MCA diameter of 0.9 mm. In contrast, the smallest STA–MCA bypass occurred with an STA diameter of 0.7 mm in a 34-year-old adult.
We found that almost inevitably, it was the STA diameter that determined the feasibility of the direct procedure. This is supported by our finding that the pediatric STAs were 21.4% smaller than adults and that patients who underwent indirect bypasses had STAs that were 15.4% smaller compared with STAs in direct bypass. Rarely was the MCA size, usually an M4 branch, the limiting factor for microanastomosis. In fact, the only statistically significant difference in MCA size between all the groups studied (Table 1) were adult and pediatric MCA size and this only showed a 7.1% difference in their diameters. Similarly, a size mismatch between STA and MCA that would limit a successful microanastomosis was also rare. As can be seen in Figure 4, the majority of microanastomosis occurred with well-matched STA and MCA diameters, and the less favorable matches occurred in the pediatric group.
Occlusion times during bypass surgery have obvious importance to clinical outcome. However, this may be less critical in moyamoya because of the presence of well established, albeit inadequate, collaterals. Certainly, nonocclusive anastomosis techniques such as ELANA have the advantage of not requiring temporary occlusion during the microanastomosis (Streefkerk et al, 2005). However, currently the technology is limited to vessels that are ⩾2.6 mm. We looked at occlusion time to see whether this correlated with size and therefore the technical difficulty of the microanastomosis. We found no significant correlation between size and occlusion time in the overall population. A larger, potentially more robust vessel did not mean a faster microanastomosis. As all our microanastomoses were performed with between 8 and 12 interrupted 10/0 sutures, the potentially easier and faster stitching may be prolonged by having to apply a few more sutures. This may explain why we saw a positive correlation of time and size in the pediatric group. There was also no correlation between occlusion time and postoperative clinical complications.
Preanastomosis Middle Cerebral Artery Flow
As predicted by Poiseuille's equation, the MCA flow was directly proportional to the MCA diameter. It is not surprising that the relationship was unlike that seen in a rigid tube as Poiseuille had described, with flow α radius4, as the vasculature is a dynamic and pulsatile system. Nevertheless, the correlation holds true.
Scanty data exist on flow rates in M3 or M4 branches, either in normal or moyamoya patients regardless of the method of measurement. Most of our knowledge on cerebral blood flow in moyamoya disease is based on regional blood flow as determined by PET, SPECT, or Xenon CT. The data presented here are unique as they demonstrate the blood flow across one MCA branch distal to the steno-occlusive vessel in a large population of patients with moyamoya disease. The degree of stenosis or presence of occlusion on a vessel would be predicted to influence the flow rate measured more distally. In addition, both the quality and quantity of collateral network will also influence the flow rate and direction at this distal branch.
The mean flow rate was only 4.4 mL/min, which is significantly less than that observed in other groups, using the transonic flow probe as a method of measurement. Flow rates along M3 or M4 branches of the MCA in patients undergoing bypass procedures for nonmoyamoya conditions, such as tumors or aneurysms, have been reported to be around 10 to 20 mL/min (Nakayama et al, 2001). Validation of the Transonics Charbel flow probe for accurately measuring quantitative cerebral blood flow has been presented earlier (Charbel et al, 1998).
We could not demonstrate any correlation between the flow rate and clinical presentation, severity of the disease, and ethnicity. This is not surprising as clinical presentation and severity are likely to be influenced by a multitude of factors, including age, severity of the vasculopathy as seen on angiogram, degree of collateral supply, and vascular reserve in the ischemic brain. To rely on flow rate from one distal branch as a predictor of clinical severity or presentation would be an oversimplification of the complex physiological interplay between the above factors. However, the value of evaluating flow rates at a distal branch is that we can begin to understand how local flow rates may change the disease process, both clinically and angiographically. This would help develop a better cerebral blood flow model in the normal and ischemic brain that may one day predict how interventions, such as revascularization surgery, can change local and regional blood flow, and how this change may influence the clinical condition of the patient.
Unexpectedly, MCA flow was about 40% lower in patients with unilateral disease compared with bilateral disease. A possible explanation may be that unilateral moyamoya disease represents the early stages of a bilateral disease process and collaterals, which contribute to the flow in the distal MCA branches, may not be as well established as in bilateral disease. Another possible explanation is that they are distinct pathological entities and the lower flow rate in unilateral disease reflects the severity of the ipsilateral vasculopathy. Mechanisms, such as the steal phenomenon, may be more marked in unilateral disease where intrinsic physiological mechanisms may overcompensate to redirect blood away from ischemic areas to supply the unaffected contralateral side. In addition, no obvious explanation exists for why distal flow in unilateral disease is on average 67% lower than proximal flow. We have therefore embarked on a detailed study between the angiographic findings in both unilateral and bilateral disease and MCA flow, which will hopefully elucidate the reasons behind these findings.
Postanastomosis Superficial Temporal Artery and Middle Cerebral Artery Flow
As expected, the STA flow correlated positively with STA size. As the pediatric STA size is significantly smaller, this would explain why the mean STA flow in the pediatric population was about 38% lower than in adults. The STA flow and size correlated positively with postanastomosis MCA flow. Conversely, MCA size did not correlate with any of the postanastomostic MCA flow measurements, further emphasizing the importance of STA size and flow rate as the main determinant of MCA flow postanastomosis.
A simple way of depicting the change in MCA flow rates after the microanastomosis would be to analyze the total flow through the MCA branch. However, this would not give any information on directional flow rates. As the STA–MCA bypass is an end-to-side anastomosis, a unidirectional system was converted into a potentially bidirectional system. In fact, regardless of the original direction of flow, about 82% of anastomoses led to bidirectional flow. More unexpected, however, was to see flow solely along the original direction in about 15% of cases, and more rarely, in the opposite direction in about 3% of cases. It is uncertain whether the unidirectional flow could be due to a pressure gradient favoring flow only in one direction, perhaps because of a high pressure system contributed by collaterals, or the existence of a large difference in resistance that favors flow in the direction of least resistance, or indeed a combination of these effects.
Consequences of Local Blood Flow After Microanastomosis
As we have seen, there is a four- to fivefold increase in blood flow through the MCA branch after microanastomosis. This is associated with an improvement in clinical outcomes as seen from improved mRS scores at 6 months and decreased frequencies of presenting symptoms. There was a very low rate (<1%) of graft failure as seen on cerebral angiogram at 6 months. Adults had about 50% greater change in MCA flow than in the pediatric population, a consistent finding throughout our analysis. In addition, unilateral disease seemed to be associated with about 25% greater change in the MCA flow compared with bilateral disease. Why this occurs is unclear. It is also not clear as to how the additional blood flow is distributed regionally.
The overall ischemic or hemorrhagic complication rate per procedure has been <3%. We found that those who had an ischemic stroke, hemorrhage, or transient neurologic deficit within the 30 days of revascularization surgery tended to have significantly higher postanastomosis flow rates. More specifically, total MCA flow was ∼40% higher than in patients who had uneventful surgeries.
The reason for the association of high MCA flows and postoperative complications is uncertain but may involve a hyperperfusion or intracerebral steal syndrome, which have been reported after revascularization surgery, such as carotid endarterectomy (Ogasawara et al, 2003; Symon, 1969). Hyperperfusion injury has also been reported after bypass surgery in moyamoya disease (Fujimura et al, 2007, 2009a; Ogasawara et al, 2005; Uno et al, 1998). After revascularization surgery, a rapid and significant increase in ipsilateral cerebral blood flow beyond the metabolic demand of the brain tissue may occur. Hemorrhage may result from the excessive hemodynamic stress on fragile collateral vessels (Fujimura et al, 2009b). In addition, impairment of autoregulatory functions in the regions of chronic ischemia may contribute to a transient or permanent ischemic injury to an already susceptible area. Patients with poorer cerebrovascular reactivity are known to have potentially higher risk for hyperperfusion syndrome (Fujimura et al, 2009a).
Intracerebral steal syndrome, another overlapping but distinct phenomenon, is seen in chronic cerebrovascular disorders (Lim et al, 2007; Mosmans and Jonkman, 1980; Oshima et al, 2000). Vessels within a region of chronic ischemia may be maximally dilated such that a vasodilatory stimulus, like a rise in CO2, may cause vasodilation in other parts of the brain, creating a steal phenomenon (Kawaguchi et al, 1999). The establishment of a new blood supply also introduces a new flow and pressure pattern. It is conceivable that in certain situations, competing flows can cause an unfavorable flow pattern to occur, resulting in a hypoperfused area and permanent or transient deficits.
The observed association of high MCA flows and postoperative complications has led us to modify our postoperative management. Mean arterial blood pressures are reduced by about 10 to 20 mmHg and more tightly controlled in the intensive care unit environment when we encounter such high flows. These patients are also monitored 1 to 2 days longer in the intensive care unit. With time, we will assess whether this will have any effect in further lowering the complication rates.
Cerebral Blood Flow Measurements
It is not possible to compare directly individual flow rates within a vessel and regional blood flow as measured by Xenon CT or SPECT. Apart from the intrinsically different quantitative method, the measurements are not performed under intraoperative conditions. However, the relationship between intraoperative flow rates and other cerebral hemodynamic parameters as measured by Xenon CT and SPECT will be of obvious importance and is currently being analyzed. With the recent development of noninvasive techniques of blood flow measurements, such as quantitative MR angiography, we can now begin to make comparisons between intraoperative findings and postoperative measurements of blood flow in individual blood vessels. We have recently applied quantitative MR angiography in the form of Noninvasive Optimal Vessel Analysis (VasSol, River Forest, IL, USA), a commercially available software, to patients undergoing evaluation for moyamoya disease and postoperative analysis of blood flow after revascularization procedures.
Conclusion
Much effort is needed to further elucidate the pathogenesis and genetic susceptibility of moyamoya disease, which will hopefully lead to the development of effective pharmaceutical therapy in the future. Currently, surgery remains the mainstay for the treatment of moyamoya disease. If technically possible, we favor direct revascularization surgery, believing that this promotes clinical benefits more promptly, with low morbidity. We have shown that this results in about a four- to fivefold increase in blood flow through the anastomosis, and that the STA diameter and flow is the main determinant of this blood flow augmentation. Although this increase is associated with improved clinical outcome, our preliminary data show that postoperative cerebrovascular reactivity, as demonstrated with Xenon CT, is also improved.
Postoperative complications in this series were low, and several risk factors are likely to have an important function. We demonstrate that significantly higher postanastomosis MCA flow rates may be one such risk factor, but clearly cannot be the only factor. Undoubtedly, other cerebral hemodynamic factors, such as cerebrovascular reserve, degree of collaterals, and disease severity are also important.
The intraoperative flow data presented here will help us begin to better understand the complex hemodynamic pathophysiology of this vasculopathy and how STA–MCA bypass surgery changes this hemodynamic system. By combining different approaches to cerebral blood flow analysis, including intraoperative flows, Xenon CT, SPECT, quantitative MR angiography, MR perfusion, and CT perfusion, we may be able to develop a cerebrovascular model to better predict how changes in the cerebrovascular tree, both from underlying pathology and from intervention, may affect the patient.
Acknowledgments
The authors thank Beth Hoyte for help in preparing the figures, and Cindy H. Samos for assistance with the manuscript.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by Russell and Elizabeth Siegelman, Bernard and Ronni Lacroute, the William Randolph Hearst Foundation, and the Huber Family Moyamoya Fund.
References
- Aoyagi M, Fukai N, Yamamoto M, Nakagawa K, Matsushima Y, Yamamoto K. Early development of intimal thickening in superficial temporal arteries in patients with moyamoya disease. Stroke. 1996;27:1750–1754. doi: 10.1161/01.str.27.10.1750. [DOI] [PubMed] [Google Scholar]
- Charbel FT, Hoffman WE, Misra M, Ostergren L. Ultrasonic perivascular flow probe: technique and application in neurosurgery. Neurol Res. 1998;20:439–442. doi: 10.1080/01616412.1998.11740545. [DOI] [PubMed] [Google Scholar]
- Cinar Y, Senyol AM, Duman K. Blood viscosity and blood pressure: role of temperature and hyperglycemia. Am J Hypertens. 2001;14:433–438. doi: 10.1016/s0895-7061(00)01260-7. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Kaneta T, Shimizu H, Tominaga T. Symptomatic hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a child with moyamoya disease. Childs Nerv Syst. 2007;23:1195–1198. doi: 10.1007/s00381-007-0361-2. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T. Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol. 2009a;71:442–447. doi: 10.1016/j.surneu.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Shimizu H, Mugikura S, Tominaga T. Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol. 2009b;71:223–227. doi: 10.1016/j.surneu.2007.07.077. [DOI] [PubMed] [Google Scholar]
- Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya' disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997;99 (Suppl 2:S238–S240. [PubMed] [Google Scholar]
- Fukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology. 2000;20 (Suppl:S61–S64. doi: 10.1046/j.1440-1789.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Guzman G, Lee M, Achrol A, Bell-Stephens TE, Kelly M, Do H, Marks M, Steinberg GK. Clinical outcome after 450 revascularization procedures for moyamoya disease. J Neurosurg. 2009;111:927–935. doi: 10.3171/2009.4.JNS081649. [DOI] [PubMed] [Google Scholar]
- Houkin K, Kamiyama H, Abe H, Takahashi A, Kuroda S. Surgical therapy for adult moyamoya disease. Can surgical revascularization prevent the recurrence of intracerebral hemorrhage. Stroke. 1996;27:1342–1346. doi: 10.1161/01.str.27.8.1342. [DOI] [PubMed] [Google Scholar]
- Kadri PA, Krisht AF, Gandhi GK.2007An anatomic mathematical measurement to find an adequate recipient M4 branch for superficial temporal artery to middle cerebral artery bypass surgery Neurosurgery 6174–78.discussion 78 [DOI] [PubMed] [Google Scholar]
- Kawaguchi S, Okuno S, Sakaki T. Effect of direct arterial bypass on the prevention of future stroke in patients with the hemorrhagic variety of moyamoya disease. J Neurosurg. 2000;93:397–401. doi: 10.3171/jns.2000.93.3.0397. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S, Sakaki T, Uranishi R.1999Effects of bypass on CO2 cerebrovascular reactivity in ischaemic cerebrovascular diseases—based on the intra-operative LCBF and CO2 cerebrovascular reactivity studies Acta Neurochir (Wien) 141369–374.discussion 374–365 [DOI] [PubMed] [Google Scholar]
- Kawashima M, Rhoton AL, Jr, Tanriover N, Ulm AJ, Yasuda A, Fujii K. Microsurgical anatomy of cerebral revascularization. Part I: anterior circulation. J Neurosurg. 2005;102:116–131. doi: 10.3171/jns.2005.102.1.0116. [DOI] [PubMed] [Google Scholar]
- Kikuta K, Takagi Y, Nozaki K, Yamada K, Miyamoto S, Kataoka H, Arai T, Hashimoto N. Effects of intravenous anesthesia with propofol on regional cortical blood flow and intracranial pressure in surgery for moyamoya disease. Surg Neurol. 2007;68:421–424. doi: 10.1016/j.surneu.2006.11.064. [DOI] [PubMed] [Google Scholar]
- Lee M, Zarharchuk G, Guzman R, Achrol A, Bell-Stephens TE, Steinberg GK. Quantitative hemodynamic studies in moyamoya disease. Neurosurg Focus. 2009;26:E5. doi: 10.3171/2009.1.FOCUS08300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Chae EJ, Kim MY, Kim JK, Kim SJ, Choi CG, Ahn JS, Ra YS, Kim JU, Hahm KD, Pyun HW, Suh DC. Steal phenomenon through the anterior communicating artery in Moyamoya disease. Eur Radiol. 2007;17:61–66. doi: 10.1007/s00330-006-0239-9. [DOI] [PubMed] [Google Scholar]
- Marano SR, Fischer DW, Gaines C, Sonntag VK. Anatomical study of the superficial temporal artery. Neurosurgery. 1985;16:786–790. doi: 10.1227/00006123-198506000-00008. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Inoue T, Ikezaki K, Matsukado K, Natori Y, Inamura T, Fukui M. Multiple combined indirect procedure for the surgical treatment of children with moyamoya disease. A comparison with single indirect anastomosis and direct anastomosis. Neurosurg Focus. 1998;5:e4. doi: 10.3171/foc.1998.5.5.7. [DOI] [PubMed] [Google Scholar]
- Mosmans PC, Jonkman EJ. The significance of the collateral vascular system of the brain in shunt and steal syndromes. Clin Neurol Neurosurg. 1980;82:145–156. doi: 10.1016/0303-8467(80)90032-3. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Kuroda S, Houkin K, Takikawa S, Abe H. Intraoperative measurement of arterial blood flow using a transit time flowmeter: monitoring of hemodynamic changes during cerebrovascular surgery. Acta Neurochir (Wien) 2001;143:17–24. doi: 10.1007/s007010170133. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Komoribayashi N, Kobayashi M, Fukuda T, Inoue T, Yamadate K, Ogawa A.2005Neural damage caused by cerebral hyperperfusion after arterial bypass surgery in a patient with moyamoya disease: case report Neurosurgery 56E1380discussion E1380 [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, Inoue T, Ogawa A. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg. 2003;99:504–510. doi: 10.3171/jns.2003.99.3.0504. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Nakamura N, Yoshimoto T, Suzuki J. Cerebral blood flow in moyamoya disease. Part 2: autoregulation and CO2 response. Acta Neurochir (Wien) 1990;105:107–111. doi: 10.1007/BF01669991. [DOI] [PubMed] [Google Scholar]
- Oshima H, Katayama Y, Hirayama T. Intracerebral steal phenomenon associated with global hyperemia in moyamoya disease during revascularization surgery. J Neurosurg. 2000;92:949–954. doi: 10.3171/jns.2000.92.6.0949. [DOI] [PubMed] [Google Scholar]
- Pinar YA, Govsa F. Anatomy of the superficial temporal artery and its branches: its importance for surgery. Surg Radiol Anat. 2006;28:248–253. doi: 10.1007/s00276-006-0094-z. [DOI] [PubMed] [Google Scholar]
- Sainte-Rose C, Oliveira R, Puget S, Beni-Adani L, Boddaert N, Thorne J, Wray A, Zerah M, Bourgeois M. Multiple bur hole surgery for the treatment of moyamoya disease in children. J Neurosurg. 2006;105:437–443. doi: 10.3171/ped.2006.105.6.437. [DOI] [PubMed] [Google Scholar]
- Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100:142–149. doi: 10.3171/ped.2004.100.2.0142. [DOI] [PubMed] [Google Scholar]
- Stock AL, Collins HP, Davidson TM. Anatomy of the superficial temporal artery. Head Neck Surg. 1980;2:466–469. doi: 10.1002/hed.2890020604. [DOI] [PubMed] [Google Scholar]
- Streefkerk HJ, Bremmer JP, Tulleken CA. The ELANA technique: high flow revascularization of the brain. Acta Neurochir Suppl. 2005;94:143–148. doi: 10.1007/3-211-27911-3_23. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Takaku A. Cerebrovascular ‘moyamoya' disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- Symon L. The concept of intracerebral steal. Int Anesthesiol Clin. 1969;7:597–615. doi: 10.1097/00004311-196907030-00009. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol Med Chir (Tokyo) 2007;47:1–4. doi: 10.2176/nmc.47.1. [DOI] [PubMed] [Google Scholar]
- Uno M, Nakajima N, Nishi K, Shinno K, Nagahiro S. Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease—case report. Neurol Med Chir (Tokyo) 1998;38:420–424. doi: 10.2176/nmc.38.420. [DOI] [PubMed] [Google Scholar]
- Veeravagu A, Guzman R, Patil CG, Hou LC, Lee M, Steinberg GK. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurg Focus. 2008;24:E16. doi: 10.3171/FOC/2008/24/2/E16. [DOI] [PubMed] [Google Scholar]
- Waddington MM. Intraluminal diameters of middle cerebral branches for microanastomoses. Neurol Res. 1979;1:65–76. doi: 10.1080/01616412.1979.11739542. [DOI] [PubMed] [Google Scholar]