Abstract
The Prader–Willi syndrome (PWS), a rare multisystem genetic disease, leads to severe disabilities, such as morbid obesity, endocrine dysfunctions, psychiatric disorders, and social disturbances. We explored the whole brain of patients with PWS to detect abnormalities that might explain the behavioral and social disturbances, as well as the psychiatric disorders of these patients. Nine patients with PWS (six males, three females; mean age 16.4 years) underwent a positron emission tomography (PET) scan with H215O as a tracer to measure regional cerebral blood flow (rCBF). The images were compared with those acquired from nine controls (six males, three females; mean age 21.2 years). A morphologic magnetic resonance imaging (MRI) was also performed in PWS patients, and their cognitive and behavioral skills were assessed with Wechsler Intelligence Scale for Children III and the Child Behavior Check List (CBCL). The MRI images showed no evident anatomic abnormalities, whereas PET scans revealed hypoperfused brain regions in PWS patients compared with controls, particularly in the anterior cingulum and superior temporal regions. We observed a significant relationship (P<0.05) between rCBF in the hypoperfused regions and CBCL scores. The functional consequences of these perfusion abnormalities in specific brain regions might explain the behavioral and social problems observed in these individuals.
Keywords: brain imaging, positron emission tomography, Prader–Willi syndrome, psychiatric troubles, social disturbances
Introduction
The Prader–Willi syndrome (PWS) is a complex multisystem genetic disorder caused by the lack of expression of paternally inherited imprinted genes on chromosome 15q11–q13. In this rare disease, the lack of expression of the paternal allele is caused by a deletion of chromosome 15q11–q13 (type I or type II, depending on the proximal chromosomal break point) (Chai et al, 2003) in 75% of the cases, caused by maternal uniparental disomy in 24% of the cases, caused by imprinting errors in 1%, and caused by a chromosomal translocation in <1%. Recent epidemiologic surveys have estimated birth incidence at 1 in 20,000 and population prevalence at 1 in 50,000 (Goldstone et al, 2008). The syndrome has characteristic phenotypes, including severe neonatal hypotonia, facial dysmorphia with acromicria, early onset of hyperphagia, and the development of morbid obesity, short stature, hypogonadism, learning disabilities, behavioral problems, social disturbances, and psychiatric disorders, with severe consequences, and difficult management issues for patients, families, and caregivers (Clarke et al, 2002; Goldstone et al, 2008; Whittington et al, 2004). Children with PWS greatly benefit from early diagnosis, appropriate multidisciplinary intervention, and growth hormone treatment (Bachere et al, 2008), but many of the social and psychiatric issues remain unresolved with no effective treatment.
Psychiatric Disorders and Social Disturbances in Patients With the Prader–Willi Syndrome
In addition to the universal propensity to overeating and compulsive and ritualistic behaviors (Clarke et al, 1996, 2002), patients with PWS show pronounced emotional lability and a striking inability to control their emotions, which results in frequent temper outbursts. Anger often seems to be an expression of frustration and the feeling of not being understood, but it may also be attributed to the impaired capacity to understand the motivations of others in the social milieu (Koenig et al, 2004).
The difficulties of patients with PWS in understanding social codes have long been described in the literature. These individuals display poor social adjustment, with poor peer relationships and tendencies of social withdrawal (Koenig et al, 2004). Patients with PWS show few attributions of feeling in social relationships, which may indicate a deficit in ‘theory of mind' (TOM) and empathy. Theory of mind is a concept describing the cognitive attribute that allows us to understand the thinking of others and to take into account their views (Baron-Cohen et al, 2000), whereas empathy is part of the interpersonal processes that are crucial for healthy social and moral development (Eisenberg and Fabes, 2006). Besides social disturbances, individuals with PWS had more behavioral and psychiatric problems than did age-matched mentally retarded patients but comparable scores with patients with psychiatric disorders (Jauregi et al, 2007).
Frontal Syndrome and Prader–Willi Syndrome
The reported clinical difficulties in PWS are similar to those observed in the frontal syndrome and include eating disorders, distractibility, inflexibility of operation, digressions in speech, and executive function disorders (difficulties in planning or solving problems in complex situations) (Ogura et al, 2008). In fact, PWS is essentially a complex neurodevelopmental disease, with hypothalamic dysfunction, cognitive, and behavioral problems causing disturbed social relationships, and specific psychiatric phenotypes that may contribute to compulsive hyperphagia leading to early morbid obesity.
Brain Imaging in the Prader–Willi Syndrome
Brain imaging and particularly activation studies have been performed in patients with PWS for few years. Until now, the few functional imaging studies that were performed in patients with PWS have particularly focused on obesity and/or eating disorders and most of them have used functional magnetic resonance imaging (MRI) techniques. One of the first study (Dimitropoulos et al, 2006) showed increased activation by MRI in the hypothalamus and orbitofrontal cortex in response to high- versus low-calorie foods in patients with PWS in comparison with controls. In another study based on viewing pictures of food after an oral glucose load (Miller et al, 2007) in patients with PWS and normal weight controls, the authors suggest that an increased reward value for food may underlie the excessive hunger in PWS, and support the importance of the frontal cortex in modulating the response to food in humans. In addition, patients with PWS were shown to exhibit a significant delay in activation at the hypothalamus, the insula, ventromedial prefrontal cortex, and nucleus accumbens compared with controls after glucose administration (Shapira et al, 2005), The most recent MRI study in patients with PWS (Holsen et al, 2009) suggests that divergent neural mechanisms are associated with behavioral phenotypes in genetic subtypes of PWS. The deletion subtype showed increased food motivation network activation both before meal and after meal, especially in the medial prefrontal cortex and amygdala.
Given the overlap between PWS and autism reported elsewhere (Koenig et al, 2004), the brain imaging data obtained in autism may well be relevant to PWS. Studies of autistic patients have emphasized that specific brain regions, linked to empathy and TOM deficits, showed reduced activity at rest (Kennedy and Courchesne, 2008) and under activation condition (Kennedy and Courchesne, 2008; Redcay, 2008; Thakkar et al, 2008).
At present, multidisciplinary intervention and growth hormone treatment can prevent obesity and improve the height of children with PWS, but cognitive dysfunctions, and behavioral and psychiatric disorders remain poorly understood and inadequately managed. The objective in this study was to use positron emission tomography (PET) imaging of relative regional cerebral blood flow (rCBF) to search for brain areas in which reduced perfusion might be functionally linked to social disturbances, and behavioral and psychiatric disorders in patients with PWS, thereby opening new perspectives for therapeutic approaches. To our knowledge, there is no published study in patients with PWS designed to correlate cerebral perfusion or metabolic defects with psychiatric disorders or social disturbances.
Materials and methods
Subject Selection
Nine patients (six males, three females) followed up in the French Reference Center for PWS were included in this study. The mean age of these patients was 16.4 years (two children were under 16 years of age). The diagnosis of PWS was genetically confirmed using the standard DNA methylation test, and subsequent molecular analyses showed a classic genotype distribution with six patients having a deletion (four type I, two type II), two cases of uniparental disomy, and one an imprinting defect. Four patients were receiving psychotropic medication during the study (see Table 1). These patients underwent brain imaging as part of the clinical evaluation of their cognitive dysfunction and psychiatric problems.
Table 1. Clinical characteristics of PWS patients.
| Subjects | Age (years) | Sex | BMI (kg/m2) | BMI (Z-score)a | Genetic abnormality | Psychotropic medication | Tests |
|---|---|---|---|---|---|---|---|
| P1 | 14.4 | M | 16.5 | −1.1 | UPD | Pipemperone Fluoxetine Pericyazine | CBCL WISC-III |
| P2 | 16.9 | F | 46 | 11.3 | Type I deletion | — | CBCL WISC-III |
| P3 | 16.7 | M | 33 | 6.5 | Imprinting defect | Haloperidol Clonazepam | NA WISC-III |
| P4 | 16.3 | M | 29.3 | 4.8 | Type II deletion | — | CBCL WISC-III |
| P5 | 18 | F | 37.7 | 7.7 | Type II deletion | — | NA NA |
| P6 | 18.6 | F | 36.3 | 7.1 | UPD | Carbamazepine Risperidone | NA NA |
| P7 | 16.1 | M | 45.5 | 12.4 | Type I deletion | Fluoxetine | NA WISC-III |
| P8 | 12.7 | M | 18.5 | 0.6 | Type I deletion | — | CBCL NA |
| P9 | 16.1 | M | 24.2 | 2.4 | Type I deletion | — | CBCL WISC-III |
BMI, body mass index; CBCL, Child Behavior Check List; NA, not applicable; P, patient; PWS, Prader–Willi syndrome; UPD, uniparental disomy; WISC-III, Wechsler Intelligence Scale for Children III.
P8 had been previously evaluated and the results were not available; P5 and P6 were too old for the WISC-III test; CBCL questionnaires were not completed by the family for P3, P5, P6, and P7.
Obesity is defined as a Z-score BMI >2.
The control group was composed of young adults (six males and three females), with a mean age of 21.2 years. The mean age of patients with PWS was 16.4 years; it was not possible to age match controls with patients. These nine individuals were part of a control group (carefully selected for being without behavioral or psychiatric problems) of an ongoing research program conducted in the brain research unit and approved by the local ethics committee. Control subjects had given written informed consent, whereas parents or caregivers gave consent on behalf of PWS patients.
Evaluation of Intelligence Quotient and Behavioral Problems
The cognitive skills of patients were assessed using the Wechsler Intelligence Scale for Children III (Wechsler, 1993) (WISC-III) and their behavior by the Child Behavior Checklist (Achenbach and Rescorla, 2001) (CBCL) as part of routine follow-up. The clinical characteristics of PWS patients are summarized in Table 1.
Cerebral Imaging Procedures
Cerebral imaging was performed without sedation. We used voxel-wise statistical methods to conduct an undirected search and explored the entire brain rather than focusing on a particular region.
In addition, we compared each subgroup (deletion and uniparental disomy) with controls at the same statistical threshold (P<0.05). The objectives were to describe any abnormalities observed in the basal state of patients with PWS and to look for a relationship between the imaging results and clinical scores. The PET acquisition was performed in a semi-obscure place with no noise but ventilation. A nurse well known by the patient remained in the room to reassure him and survey his compliance.
Positron Emission Tomography Procedures and Data Analysis
We performed relative rCBF evaluation at rest. A venous canula was inserted to administer tracer in the antecubital fossa vein. Positron emission tomography measurements were performed using an EXACT HR+ tomograph (CTI/Siemens, Knoxville, TN, USA) allowing the simultaneous three-dimensional acquisition of 63 transaxial slices. Spatial resolution after reconstruction reached 4.5 and 4.1 mm in the transaxial and axial directions, respectively (Bendriem et al, 1996). To measure relative rCBF, 150 MBq of H215O was administered for each 80-second emission scan. To allow for complete decay of the injected tracer activity, measure of auto-attenuation correction was obtained by transmission scan with rotating Ge-68 source before PET H2015. Triplicate perfusion images were carried out for each subject to avoid artifacts owing to movements.
Image analysis was performed on a personal computer station (Dell, Round Rock, TX, USA) using a ‘statistical parametric mapping' package (SPM2, Wellcome Department of Cognitive Neurology, London, UK) (Friston, 1994). Images for each subject were realigned to the first volume and normalized to the Montreal Neurological Institute standard proportional stereotaxic space, which is based on that of Talairach and Tournoux (1988). Images were corecorded on a template and spatially smoothed with a Gaussian kernel of 8 mm full-width at half maximum to take into account variations in gyral anatomy and individual variability in structure–function relationships and to improve the signal-to-noise ratio. The nine patients and nine control subjects were included in the same statistical analysis on a voxel-by-voxel basis. Statistical parametric maps were then generated using an ANCOVA (analysis of covariance) model implemented through the general linear model formulation of SPM (Friston et al, 1994) after normalization for the global effect by proportional scaling. The multigroup SPM tool was used for analysis for intergroup comparisons, as well as for finding a link between hypoperfusion and clinical scores; the statistical threshold was set at P<0.05 (uncorrected).
Morphologic Magnetic Resonance Imaging (Volumetric T1)
For the MRI procedure, a Magnetom Vision 1.5-T Siemens was used. All subjects underwent T2-weighted axial imaging turbo spin-echo sequence (repetition time: 4,000 milliseconds, echo time: 102 milliseconds, flip angle: 180°) with a 5-mm slice thickness and three-dimensional volume acquisition (repetition time: 9.70 milliseconds, echo time: 4.0 milliseconds, flip angle: 15°, 1.16 mm isotropic voxel). The scan was also focused on the sella turcica in the sagital and coronal planes (T1 spin echo, repetition time: 600 milliseconds, echo time: 12 milliseconds, α: 90°, 3-mm slice thickness). The MRI examination lasted 30 minutes and no sedation was used.
Results
Magnetic Resonance Imaging Results
There were no evident anatomic abnormalities on MRI images, except a possible increased gyrification in the posterior part of the left superior temporal gyrus in one patient.
Positron Emission Tomography Results
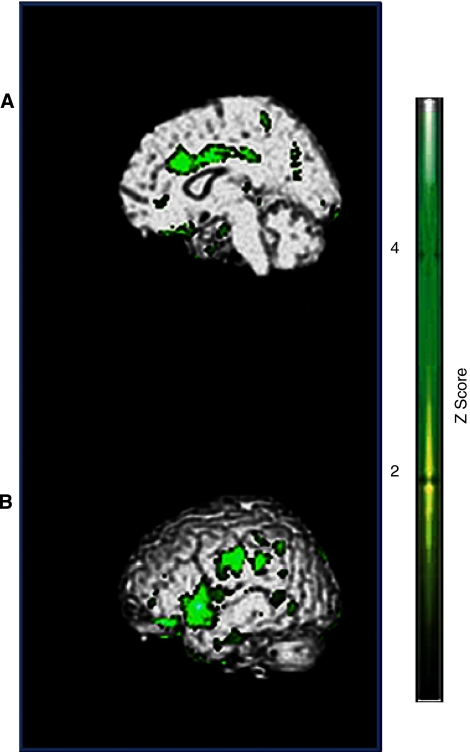
A significant hypoperfusion was observed in the limbic lobe (Figure 1A) and in the superior temporal and parietal lobes (Figure 1B) in patients with PWS compared with controls (see Table 2). We found comparable results in the two genetic subgroups of patients, deletion, and uniparental disomy (data not shown).
Figure 1.
(A) Limbic lobe, anterior cingulum. (B) Superior temporal gyrus, parietal lobe, postcentral gyrus. Hypoperfused brain regions in PET scan study: green areas correspond to hypoperfusion in patients with PWS versus controls (P<0.05 uncorrected). PET, positron emission tomography; PWS, Prader–Willi syndrome.
Table 2. Hypoperfused brain regions in patients with PWS.
| Brain region | Brodmann's area | x, y, za | Z-score |
|---|---|---|---|
| Limbic lobe, anterior cingulum | BA32 | 6, 30, 30 | 3.42 |
| Cingulate gyrus | BA24 | 2, −2, 34 | 4.71 |
| Superior temporal gyrus | BA38 | −18, 2, −42 | 3.98 |
| BA22 | −60, 8, 2 | 3.98 | |
| Frontal lobe, right orbitofrontal gyrus | BA11 | −22, 8, −44 | 3.56 |
| Parietal lobe, postcentral gyrus | BA 2 | −50, −22, 34 | 3.56 |
MNI, Montreal Neurological Institute; PWS, Prader–Willi syndrome.
x, y, z are coordinates in MNI space at height threshold, (−) left hemisphere, (+) right hemisphere.
Simple Regression of Child Behavioral Check List Against Relative Regional Cerebral Blood Flow
The results of the CBCL test in patients with PWS are presented in Table 3.
Table 3. Summary of CBCL results for patients with PWS.
| CBCL | Scores | Clinical interpretation |
|---|---|---|
| Skills | ||
| Activity | 33 | P |
| Social | 27.8 | P |
| Syndrome scores | ||
| Anxious/depressed | 67.4 | N |
| Withdrawn/depressed | 64 | N |
| Somatic complains | 63.4 | N |
| Social problems | 71 | P |
| Thought problems | 71.6 | P |
| Attention problems | 63.2 | N |
| Rule-breaking behavior | 61 | N |
| Aggressive behavior | 64 | N |
| Mean syndrome scores | ||
| Internalizing | 68.4 | P |
| Externalizing | 63.2 | P |
| Total problems | 69.2 | P |
| DSM-oriented scores | ||
| Affective problems | 73.2 | P |
| Anxiety problems | 71.8 | P |
| Somatic problems | 61.2 | N |
| Attention deficit/ hyperactivity problems | 58.6 | N |
| Oppositional defiant problems | 63.4 | N |
| Conduct problems | 61 | N |
CBCL, Child Behavior Check List; DSM, Diagnostic and Statistical Manual, Revision IV; N, normal; P, pathologic; PWS, Prader–Willi syndrome.
We observed a significant relationship (P<0.05) between clinical scores and relative rCBF in the hypoperfused regions. There was a positive relationship between relative rCBF in the anterior cingulum and CBCL activity, social and attention scores, and a negative relationship with the CBCL-depressed score. We found a positive relationship between relative rCBF in the superior temporal gyrus and CBCL attention and social scores. The relative rCBF in the superior frontal gyrus BA11 was positively associated with the CBCL activity score. The results are shown in Table 4.
Table 4. Relationships between CBCL scores and relative rCBF in patients with PWS.
| CBCL | Positive relationships | Brodmann's area | x, y, za | Z-score |
|---|---|---|---|---|
| Anterior cingulum | BA 32 | 16, 36, 4 | 3.81 | |
| Activity | Primary motor cortex | BA 6 | 16, −6, 56 | 3.69 |
| Superior frontal gyrus | BA 11 | 28, 58, −20 | 3.55 | |
| Attention problems | Anterior cingulum | BA 24 | 0, −10, 28 | 4.51 |
| Superior temporal gyrus | BA 39 | 40, −52, 30 | 4.10 | |
| Social | Superior temporal gyrus | BA 39 | 36, −52, 32 | 3.57 |
| |
Anterior cingulum |
BA 23 |
0, −24, 24 |
3.54 |
|
CBCL |
Negative relationships |
Brodmann's area |
x, y, za |
Z-score |
| Premotor cortex | BA 6 | 22, 24, 64 | −6.14 | |
| Depressed | Anterior cingulum | BA 32 | 20, 42, −2 | −5.85 |
| Superior parietal cortex | BA 7 | 14, −50, 48 | 3.55 |
CBCL, Child Behavior Check List; MNI, Montreal Neurological Institute; rCBF, regional cerebral blood flow.
The negative coordinates correspond to the left hemisphere.
x, y, z are coordinates in MNI space at height threshold.
Simple Regression of Intelligence Quotient Against Relative Regional Cerebral Blood Flow
The WISC-III scores in patients with PWS were 63 for the verbal quotient, 67.5 for the performance quotient, and 60.5 for the total intelligence quotient. There was no relationship between mean WISC-III results and relative rCBF.
Discussion
In this functional neuroimaging study, the globally normalized relative rCBF of patients with PWS was different from that of control subjects in the anterior cingulum and the superior temporal regions. For the first time, imaging data obtained from unsedated patients with PWS displayed perfusion abnormalities in brain regions that may explain some of the major behavioral and psychiatric disturbances described in these patients. This analysis was conducted on the whole brain, without any attempt to focus on the brain regions known to be involved in PWS. Indeed hypoperfusion is not necessarily dysfunctional or causal, but in this discussion we will consider that the relative low rCBF is likely a manifestation of impaired activity of the brain region compared with the control.
Anterior Cingulum and Superior Temporal Region Hypoperfusion
The two main hypoperfused regions, the anterior cingulum and the superior temporal lobe, may plausibly be implicated in the corresponding behavioral and social deficits of PWS.
Functional imaging studies have pointed out the role of the anterior cingulum (BA32) and cingulate gyrus (BA24) in the mechanisms underlying TOM and empathy. The anterior cingulum also has a regulatory role between cognitive and emotional processes through its links to hypothalamic-pituitary and brainstem structures (Mayberg, 1997). It is involved in pain sensation as well, particularly in the sensory component of pain and the empathic experience of pain (Singer et al, 2004). The anterior cingulum network also has a role in executive functions (Miller and Cohen, 2001), which require the selection of action by the dorsolateral prefrontal cortex (cognitive control) and motivation to engage in the action before performing it. Thus, the cingulate network is involved in the initiation and maintenance of motivation to complete an action (Koechlin and Summerfield, 2007).
Hypoperfusion of the anterior cingulum and the cingulate gyrus in patients with PWS may be related to their lack of empathy and TOM, which causes them difficulties in interacting with peers and understanding their social environment (Koenig et al, 2004), and may also be related to the high emotional lability and episodes of uncontrolled emotion that they characteristically display. Indeed, damage or dysfunction in the neural circuitry of emotional self-regulation and empathy has been linked to the failure to regulate emotions, impulsive aggression, and violence (Guroglu et al, 2008). In addition, patients with PWS have a high threshold for pain (Holm et al, 1993) and their empathic experience of pain is frequently abnormal. Finally, we note that the relative hypoperfusion in the anterior cingulum may be related to deficits in their motivation to engage in an action and their lack of initiative and enthusiasm for proposing or carrying out activities.
The second markedly hypoperfused region in PWS patients was the superior temporal lobe, which is implicated in the initial processing of auditory information. In addition, this region is involved in the highest levels of auditory processing and spoken language comprehension, and it therefore provides access to understanding. In our study, hypoperfusion of the temporal region in PWS patients was located in the left hemisphere, which may explain part of the understanding deficit of these patients.
Contradictory results have been reported in patients with PWS (Kim et al, 2006) with an increased metabolism in bilateral anterior cingulated gyri. These discrepancies may be attributed to the procedures and particularly to the use of sedation. Indeed, the PET procedure chosen in this study was performed under significant sedation (choral hydrate 50 mg/kg). Therefore, it seems difficult to compare the results. We acknowledge that four of our nine patients were receiving psychotropic medications, which did not seem to strongly influence the brain perfusion.
Interestingly, abnormalities in these two regions, the anterior cingulum and the superior temporal lobe, have been also described in autism (Boddaert et al, 2004; Haznedar et al, 2000; Kennedy and Courchesne, 2008; Zilbovicius et al, 2000).
Other Hypoperfused Regions
Other brain regions were hypoperfused in patients with PWS in our study, mainly the areas BA11 and BA2.
Hypoperfusion in the right orbitofrontal gyrus (BA11), like in the cingulate region discussed above, may be linked to disinhibition, irritability, and mood lability which are the main characteristics of these patients (Knutson et al, 2008). These dysfunctions may thus be due not only to reduced understanding of social cues in association with a lack of empathy, but also owing to a dysfunction in the orbitofrontal region.
The BA2 region, which corresponds to the postcentral gyrus of the parietal lobe, drives the control of motor activity and is involved in the execution of gestural or graphic sequences in motor program elaboration, and therefore it is involved in visually guided movements (Leibenluft et al, 2007). Abnormalities in this region result in perceptive agnosia, particularly in incorrect perceptions of the consistency, size, weight, and shape of objects. Interestingly, patients with PWS showed a significant delay in the ‘block design scale' of the Wechsler test, which targets the ability to combine visual and motor processes. Hypoperfusion in the BA2 region may explain their difficulties in object perception and overall spatial organization.
Simple Regression of Intelligence Quotient, Child Behavior Check List Against Relative Regional Cerebral Blood Flow
The WISC-III data obtained for patients with PWS in our study are consistent with the literature (Diene et al, 2007; Dykens and Kasari, 1997; van Lieshout et al, 1998). We found no relationship between the WISC-III score and relative rCBF in our population of PWS patients.
The CBCL showed classic abnormalities, particularly pathologic social skills in terms of activity participation, interaction with others, thought problems, and behavioral disorder. The links found between CBCL scores and relative rCBF suggest a pathologic significance of hypoperfusion in these areas. Indeed, this pattern of hypoperfusion may be associated with attention disorder, poor engagement in activity, and depressive states, all of which are regularly described in PWS patients.
Conclusions
The neuroimaging results obtained in this study document the perfusion abnormalities in specific brain regions of PWS patients. These abnormalities may cause functional disorders, which in turn could explain the behavioral and social disorders observed in these patients. This study confirms that PWS is a neurodevelopmental brain disorder and offers promising perspectives in terms of pathophysiology and therapeutic developments.
The identification of perfusion abnormalities in specific brain regions is a preliminary step toward understanding the pathophysiology of this very complex disorder, particularly regarding the links between hypothalamic dysfunction, hypoactivity, and psychiatric and social disorders. Moreover, these hypoperfused brain regions have also been implicated in the social deficits observed in autism. Comparative brain imaging studies may provide important clues about these two neurodevelopmental disorders, which are characterized by similar disturbances in behavior and social interactions.
Acknowledgments
Mathé Tauber takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Isabelle Kieffer for helping in the writing of this paper.
The authors declare no conflict of interest.
Footnotes
This work was partly supported by Fondation Orange and Conseil Régional de Midi-Pyrénées (71/2007).
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families; 2001. [Google Scholar]
- Bachere N, Diene G, Delagnes V, Molinas C, Moulin P, Tauber M. Early diagnosis and multidisciplinary care reduce the hospitalization time and duration of tube feeding and prevent early obesity in PWS infants. Horm Res. 2008;69:45–52. doi: 10.1159/000111795. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Tager-Flusberg H, Cohen D.(eds) (2000Understanding Other Minds: Perpectives from Developmental Neuroscience2nd ed.Oxford: Oxford University Press [Google Scholar]
- Bendriem B, Casey M, Dahlbom M, Trebossen R, Blohm K, Nutt R, Syrota A. Evaluation of the ECAT EXACT HR+: a new positron camera with 2D/3D acquisition capabilities and nearly isotropic spatial resolution. J Nucl Med. 1996;37:170. [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Chai JH, Locke DP, Greally JM, Knoll JH, Ohta T, Dunai J, Yavor A, Eichler EE, Nicholls RD. Identification of four highly conserved genes between breakpoint hotspots BP1 and BP2 of the Prader-Willi/Angelman syndromes deletion region that have undergone evolutionary transposition mediated by flanking duplicons. Am J Hum Genet. 2003;73:898–925. doi: 10.1086/378816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DJ, Boer H, Chung MC, Sturmey P, Webb T. Maladaptive behaviour in Prader-Willi syndrome in adult life. J Intellect Disabil Res. 1996;40 (Part 2:159–165. doi: 10.1046/j.1365-2788.1996.743743.x. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Boer H, Whittington J, Holland A, Butler J, Webb T. Prader-Willi syndrome, compulsive and ritualistic behaviours: the first population-based survey. Br J Psychiatry. 2002;180:358–362. doi: 10.1192/bjp.180.4.358. [DOI] [PubMed] [Google Scholar]
- Diene G, Postel-Vinay A, Pinto G, Polak M, Tauber M. [The Prader-Willi syndrome] Ann Endocrinol (Paris) 2007;68:129–137. doi: 10.1016/j.ando.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Blackford J, Walden T, Thompson T. Compulsive behavior in Prader-Willi syndrome: examining severity in early childhood. Res Dev Disabil. 2006;27:190–202. doi: 10.1016/j.ridd.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Kasari C. Maladaptive behavior in children with Prader-Willi syndrome, Down syndrome, and nonspecific mental retardation. Am J Ment Retard. 1997;102:228–237. doi: 10.1352/0895-8017(1997)102<0228:MBICWP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes F. Handbook of Child Psychology: Social, Emotional, and Personality Development. New York: Wiley; 2006. Prosocial development; pp. 646–718. [Google Scholar]
- Friston KJ.1994Satistical parametric mapping Functional Neuroimaging(Thatcher RW, Hallett M, Zeffiro T, John ER, Heurta M, eds),San Diego: Academic Press; 79–93. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93:4183–4197. doi: 10.1210/jc.2008-0649. [DOI] [PubMed] [Google Scholar]
- Guroglu B, Haselager GJ, van Lieshout CF, Takashima A, Rijpkema M, Fernandez G. Why are friends special? Implementing a social interaction simulation task to probe the neural correlates of friendship. Neuroimage. 2008;39:903–910. doi: 10.1016/j.neuroimage.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91:398–402. [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Chambers R, Butler MG, Bittel DC, Brooks WM, Thompson TI, Savage CR. Genetic subtype differences in neural circuitry of food motivation in Prader-Willi syndrome. Int J Obes (Lond) 2009;33:273–283. doi: 10.1038/ijo.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregi J, Arias C, Vegas O, Alen F, Martinez S, Copet P, Thuilleaux D. A neuropsychological assessment of frontal cognitive functions in Prader-Willi syndrome. J Intellect Disabil Res. 2007;51:350–365. doi: 10.1111/j.1365-2788.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Jin DK, Cho SS, Kim JH, Hong SD, Paik KH, Oh YJ, Kim AH, Kwon EK, Choe YH. Regional cerebral glucose metabolic abnormality in Prader-Willi syndrome: a 18F-FDG PET study under sedation. J Nucl Med. 2006;47:1088–1092. [PubMed] [Google Scholar]
- Knutson KM, Zamboni G, Tierney MC, Grafman J. Neural correlates of caregiver burden in cortical basal syndrome and frontotemporal dementia. Dement Geriatr Cogn Disord. 2008;26:467–474. doi: 10.1159/000167268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Koenig K, Klin A, Schultz R. Deficits in social attribution ability in Prader-Willi syndrome. J Autism Dev Disord. 2004;34:573–582. doi: 10.1007/s10803-004-2551-z. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller JL, James GA, Goldstone AP, Couch JA, He G, Driscoll DJ, Liu Y. Enhanced activation of reward mediating prefrontal regions in response to food stimuli in Prader-Willi syndrome. J Neurol Neurosurg Psychiatry. 2007;78:615–619. doi: 10.1136/jnnp.2006.099044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Shinohara M, Ohno K, Mori E. Frontal behavioral syndromes in Prader-Willi syndrome. Brain Dev. 2008;30:469–476. doi: 10.1016/j.braindev.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev. 2008;32:123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Shapira NA, Lessig MC, He AG, James GA, Driscoll DJ, Liu Y. Satiety dysfunction in Prader-Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry. 2005;76:260–262. doi: 10.1136/jnnp.2004.039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain: 3-D Proportional System. An Approach to Cerebral Imaging. Stuttgart: Georg Thieme Verlag/Thieme Medical Publishers; 1988. [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lieshout CF, de Meyer RE, Curfs LM, Koot HM, Fryns JP. Problem behaviors and personality of children and adolescents with Prader-Willi syndrome. J Pediatr Psychol. 1998;23:111–120. doi: 10.1093/jpepsy/23.2.111. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. London: Psychological Corporation, Harcourt Brace & Co; 1993. [Google Scholar]
- Whittington J, Holland A, Webb T, Butler J, Clarke D, Boer H. Cognitive abilities and genotype in a population-based sample of people with Prader-Willi syndrome. J Intellect Disabil Res. 2004;48:172–187. doi: 10.1111/j.1365-2788.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Boddaert N, Belin P, Poline JB, Remy P, Mangin JF, Thivard L, Barthelemy C, Samson Y. Temporal lobe dysfunction in childhood autism: a PET study. Positron emission tomography. Am J Psychiatry. 2000;157:1988–1993. doi: 10.1176/appi.ajp.157.12.1988. [DOI] [PubMed] [Google Scholar]