Abstract
Androgens within physiological ranges protect castrated male mice from cerebral ischemic injury. Yet, underlying mechanisms are unclear. Here, we report that, after middle cerebral artery occlusion (MCAO), salt-induced kinase 1 (SIK1) was induced by a potent androgen—dihydrotestosterone (DHT) at protective doses. To investigate whether SIK1 contributes to DHT neuroprotection after cerebral ischemia, we constructed lentivirus-expressing small interference RNA (siRNA) against SIK1. The SIK1 knockdown by siRNA exacerbated oxygen–glucose deprivation (OGD)-induced cell death in primary cortical neurons, suggesting that SIK1 is an endogenous neuroprotective gene against cerebral ischemia. Furthermore, lentivirus-mediated SIK1 knockdown increased both cortical and striatal infarct sizes in castrated mice treated with a protective dose of DHT. Earlier studies show that SIK1 inhibits histone deacetylase (HDAC) activities by acting as a class IIa HDAC kinase. We observed that SIK1 knockdown decreased histone H3 acetylation in primary neurons. The SIK1 siRNA also exacerbated OGD-induced neuronal death in the presence of trichostatin A (TSA), an HDAC inhibitor, and decreased histone H3 acetylation at 4 hours reoxygenation in TSA-treated neurons. Finally, we showed that DHT at protective doses prevented ischemia-induced histone deacetylation after MCAO. Our finding suggests that SIK1 contributes to neuroprotection by androgens within physiological ranges by inhibiting histone deacetylation.
Keywords: androgen, cerebral ischemia, dihydrotestosterone, neuroprotection, salt-induced kinase 1
Introduction
The role of androgens in cerebral ischemia remains controversial. Epidemiological studies suggest that male gender is a risk factor for stroke (Foulkes et al, 1988); therefore, androgens are generally assumed to have deleterious roles in stroke. However, currently available clinical evidence suggests that physiological levels of androgens are protective after stroke, because subnormal blood levels of testosterone, the major circulating androgen, correlate with higher stroke incidences, larger infarcts, and poorer recovery after stroke (Dash et al, 1991; Jeppesen et al, 1996; Hollander et al, 2003; Yeap et al, 2009). Numerous in vitro studies also show that androgens directly protect neurons from various insults, including oxidative stress (Ahlbom et al, 2001; Pike et al, 2008). We recently also observed that physiological levels of androgens, including testosterone and a more potent androgen dihydrotestosterone (DHT), reduce infarct damage after middle cerebral artery occlusion (MCAO) in castrated mice through an androgen receptor (AR)-dependent mechanism (Uchida et al, 2009).
As it is well known that androgens act through AR-mediated transcriptional mechanisms to regulate gene expression, we previously used DNA microarray to globally examine post-MCAO gene transcription (Cheng et al, 2007). We identified salt-induced kinase 1 (SIK1) as an androgen-responsive gene that was robustly induced by DHT after MCAO. The SIK1 belongs to the sucrose-nonfermenting 1 kinase/adenosine monophosphate-activated kinase family, and SIK1 mRNA levels are high in the brain (Feldman et al, 2000). Recent studies indicate that SIK1 functions as a class IIa histone deacetylase (HDAC) kinase to inhibit the deacetylase activities associated with class IIa HDACs (Berdeaux et al, 2007; Gordon et al, 2009; Takemori et al, 2009; van der Linden et al, 2007). This function of SIK1 has been confirmed in the brain. Interestingly, HDAC inhibition is emerging as a protective mechanism in various neurodegenerative diseases, including stroke (Kazantsev and Thompson, 2008; Kim et al, 2007; Meisel et al, 2006). Thus, in this study, we test the hypothesis that SIK1 is an endogenous neuroprotective gene that is induced by DHT within physiological ranges and contributes to androgen neuroprotection by inhibiting histone deacetylation. Our findings contribute to the effect of androgens on CNS injury, and they suggest that SIK1 may serve as a novel therapeutic target for treating stroke.
Materials and methods
Middle Cerebral Artery Occlusion and Animal Groups
Young adult male Wistar rats (3 months old) and C57/BL6 mice (3 months old) were purchased from Charles River (Wilmington, MA, USA). The rats and mice were subjected to 2 hours and to 90 minutes of reversible MCAO, respectively, using the intraluminal filament technique as described earlier (Uchida et al, 2009; Cheng et al, 2007). Briefly, animals were anesthetized with isoflurane, and temporalis temperatures were monitored and maintained at 37°C±0.5°C by heat lamps and water pads. A femoral artery catheter was placed to monitor mean artery blood pressure and arterial blood pH and PaCO2. Adequacy of vessel occlusion and reperfusion was assessed by laser-Doppler flowmetry (Moor Instruments Ltd, Oxford, UK). At 24 hours reperfusion, brains were harvested for infarct volume analysis and plasma was collected for radioimmunoassay of DHT, as described earlier (Cheng et al, 2007). Brains were also harvested at 12 hours reperfusion for quantitative polymerase chain reaction (qPCR) assay and immunohistochemistry analysis of SIK1 induction or Western blot analysis of histone H3 acetylation.
To confirm DHT neuroprotection and DHT induction of SIK1 in the rat MCAO model, male rats were castrated and randomized to receive subcutaneous DHT pellets (15 mg/pellet) with or without AR antagonist flutamide (15 mg/pellet) 1 week before MCAO, as described earlier (Cheng et al, 2007). To examine the role of SIK1 in androgen neuroprotection, C57/BL6 male mice were randomized to receive intrastriatal injection of lentivirus-expressing SIK1 small interference RNA (siRNA) or nonsense siRNA 2 weeks before MCAO. One week after lentiviral injection, these mice were castrated and subcutaneously implanted with a protective dose of DHT (0.5 mg) (Uchida et al, 2009). This study was conducted in accordance with the NIH guidelines, and all protocols were approved by the Animal Care and Use Committee of Oregon Health and Science University.
Infarct Volume Analysis
Infarction analysis was performed at 24 hours reperfusion after MCAO to confirm neuroprotective effects of DHT in rats or to explore the role of SIK1 in DHT neuroprotection in mice. Briefly, animals were anesthetized with isoflurane (4.0% to 5.0%) and then decapitated for brain removal. The rat brain was cut into seven 2-mm-thick coronal sections; and the mouse brain was cut into five 2-mm-thick coronal sections. Brain slices were incubated in 2% 2,3,5-triphenyltetrazolium chloride (Sigma, St Louis, MO, USA) for 10 minutes on each side, fixed in 10% formalin overnight, and then photographed. Unstained (infarcted) and stained (uninfarcted) areas were analyzed across slices with digital image analysis software (SigmaScan Pro, Jandel, San Rafael, CA, USA). Infarction sizes are expressed as percentages of the contralateral structures.
qPCR Assay and Immunohistochemistry Analysis of Salt-Induced Kinase 1 Induction After Middle Cerebral Artery Occlusion
The qPCR was performed to determine whether SIK1 mRNA was induced by a protective dose of DHT after MCAO. Brain tissue was extracted from rat cortical and striatal penumbra at 12 hours reperfusion, as described in our earlier publication (Cheng et al, 2007). Briefly, rat brains were perfused with saline, harvested, and sectioned into seven 2-mm-thick coronal sections at 12 hours reperfusion. Two slices that encompass the MCA territory (between +2 and −2 mm relative to Bregma) were frozen on dry ice, and tissue samples were micropunched from cortical and striatal peri-infarct zones using 1-mm internal diameter Micron Punch (Zivic Laboratories, Pittsburgh, PA, USA). For each animal, penumbral tissue was extracted from the identical locations on ipsilateral as well as contralateral sides. Micropunch samples were then homogenized in 100 μL lysis buffer. Total RNA was purified, eluted from column with 20 μL RNase-free elution buffer, and further treated with Turbo DNase (Ambion, Austin, TX, USA). The cDNA was reverse transcribed from 10 μL of total RNA (∼500 ng) using a high capacity cDNA synthesis kit (Applied Biosystem, Foster City, CA, USA) as per the manufacturer's instructions. The qPCR reactions were performed on ABI Prism 7000 DNA Detection System as described (Cheng et al, 2007). Taqman primers and probes were obtained from Applied Biosystem for qPCR measurement of rat SIK1 mRNA (Cat #: Rn01429325_m1).
Immunohistochemistry was performed to confirm postischemic SIK1 induction by DHT at a protein level and to examine the cell identities responsible for SIK1 induction. At 12 hours reperfusion, rats were deeply anesthetized with isoflurane and transcardially perfused with 0.9% saline followed by 4% phosphate-buffered paraformaldehyde. Brains were harvested, cut into 2 mm coronal sections, postfixed with 4% paraformaldehyde and embedded in paraffin. Tissue blocks were serially cut into 6 μm coronal sections with a microtome and incubated at 4°C overnight with the rabbit anti-SIK1 polyclonal antibodies (1:400; Proteintech Group, Chicago, IL, USA) and neuronal nuclei monoclonal antibodies (NeuN; 1:2000). Then, sections were washed with 0.1 mol/L phosphate buffer (pH 7.4) for 30 minutes, incubated for 2 hours at room temperature in biotinylated IgG (goat anti-rabbit, 1:200; Amersham Bioscience, Pittsburg, PA, USA). After rinsing with phosphate buffer, the sections were incubated for 1 hour at room temperature in streptavidin-conjugated Cy3 and Cy2 (Jackson ImmunoResearch, Plymouth, PA, USA; 1:600), rinsed for 3 to 6 hours in phosphate buffer, and protected with a glycerol–glycine buffer (2:1; pH 8.6) containing 5% n-propyl gallate to reduce photobleaching. Slides were photographed using a Nikon (Tokyo, Japan) E800 microscope.
Construction, Production, and In Vitro Knockdown Efficiency of Lentivirus-Expressing Salt-Induced Kinase 1 Small Interference RNA
Replication-defective lentivirus-expressing SIK1 siRNA and enhanced green fluorescence protein (eGFP) was produced by three-plasmid transient transfection of 293FT cells as described (Blesch, 2004; Lois et al, 2002). Empty lentivirus-expressing eGFP only or lentivirus-expressing nonsense siRNA and eGFP, neither of which showed efficiency in knocking down SIK1 mRNA, was also produced by three-plasmid transient transfection and served as a control in this study. Lentivirus-expressing nonsense siRNA was used as a control as, unlike empty virus, lentivirus-expressing nonsense siRNA activates the cellular small RNA-responsive pathways as does lentivirus-expressing functional siRNA (Sun et al, 2009), thus eliminating the potential influence of activation of these pathways on outcomes. The plasmids used for lentivirus production were (1) FUGWlinker, containing the HIV flap sequence that increases lentiviral infection in nondividing cells and HIV 5′LTRs and 3′LTRs modified to delete the TAT signal; (2) pHCMV-G vector, containing the vesicular stomatitis virus envelope protein gene driven by human CMV promoter; and (3) CMV δ8.2 vector, containing Gag-Pol precursor and Rev genes necessary for virus production and packaging but not other virus genes. The intermediate vector pCMV-U6 del, containing U6 promoter, was used to introduce siRNA into FUGWlinker. Five pairs of short hairpin RNA sequences were designed based on the mouse SIK1 mRNA sequence (Genebank accession number: NM_010831), with incorporation of BbsI enzymatic site at 5′-terminus of the sense sequences and BglII site at 5′-terminus of the complimentary sequences. Oligos were synthesized (Invitrogen, Carlsbad, CA, USA), annealed, and inserted into BbsI-BglII sites of pCMV-U6 del. Recombinant pCMV-U6 del was digested with NheI and BstBI, resulting in a 560-bp DNA segment containing the short hairpin RNA DNA template and its upstream U6 promoter sequence. The 560-bp DNA segment was then inserted into NheI-BstBI sites of FUGWlinker, resulting in recombinant FUGWlinker.
For lentivirus production, 293FT cells plated to 70% confluency were cotransfected with recombinant FUGWlinker, pHCMV-G, and CMV δ8.2 using calcium phosphate transfection methods. Medium was collected at 48 hours after transfection, and lentivirus was concentrated from medium by ultracentrifugation at 20,000 r.p.m. for 2 hours. The resulting pellets were resuspended in phosphate-buffered saline (PBS, pH 7.2) and stored at −80°C. Viral titers were measured by transducing 293FT cells and counting eGFP-positive colonies.
Knockdown efficiency of SIK1 siRNA-expressing lentivirus was assessed on primary cortical neurons. Briefly, mouse cortical neurons cultured on 24-well plates were infected with 2 μL 107 transducing units/mL lentivirus per well at day 6 in vitro. After 4 days infection, neurons were harvested from each well. RNAqueous-Micro kit (Ambion) was used to extract total RNA as per the manufacturer's instructions, and qPCR was performed using Taqman primers and probe for mouse SIK1 purchased from Applied Biosystem (Cat #: Mm00440322_g1), as described earlier.
Neuronal Cell Culture, In Vitro Lentiviral Infection, and Oxygen–Glucose Deprivation Model
Primary cortical neuronal cultures were derived from embryos (16-day gestation) of C57/BL6 mice (Charles River). Cultures were prepared as described previously with modifications (Yang et al, 2007). Dissociated cells were plated onto Poly--lysine-coated plates (24-well plates, 2.85 × 105 cells/well). Cells were maintained in the culture medium (Neurobasal medium supplemented with 2% B27 and 2 mmol/L Glutamax) at 37°C in 100% humidity and a 95% room air/5% CO2 atmosphere. Neurons were infected with lentivirus at 6 days in vitro and subjected to oxygen–glucose deprivation (OGD) at 4 days after infection (10 days in vitro). The OGD was performed as described earlier (Yang et al, 2007). Briefly, culture medium was replaced with OGD buffer (Dulbecco PBS supplemented with 1 mmol/L CaCl2 and 0.8 mmol/L MgCl2), and neurons were then placed in an anoxia chamber filled with 90% N2, 5% H2, and 5% CO2 for 2.5 hours. For reoxygenation, cells were removed from the anoxia chamber, OGD medium was replaced with culture medium, and cells were returned to normoxia. Cell death was assessed at 24 hours reoxygenation with lactate dehydrogenase release assay. Final results were normalized to maximum lactate dehydrogenase release when all cells were killed by triton.
Intrastriatal Microinjection and In Vivo Knockdown Efficiency of Lentivirus-Expressing Salt-Induced Kinase 1 Small Interference RNA
Mice were anesthetized under isoflurane anesthesia and placed onto a stereotaxic apparatus (Cartesian Res, Sandy, OR, USA). A small hole was drilled through the skull at 0.5 mm anterior to the bregma, 2.0 mm lateral to the midline, and 2.3 mm below the pia. A measure of 2 μL of concentrated lentivirus (109 transducing units/mL) were injected at the rate of 0.5 μL/min with a 30-gauge needle on a 2-μL Hamilton syringe. The lentiviral infection was confirmed by fluorescence microscopic examination of eGFP-positive cells in brain slices at 1 and 2 weeks after injection. To confirm the in vivo knockdown efficiency of SIK1 siRNA-expressing lentivirus, tissue was extracted from infected areas in the mouse striatum using a 0.5-mm internal diameter Micron Punch (Zivic Laboratories) under a fluorescence microscope. Total RNA was extracted from micropunched tissue samples using RNAaqueous-Micro kit (Ambion), and qPCR measurement of SIK1 was performed using Taqman primers and probe purchased from Applied Biosystem (Cat #: Mm00440322_g1), as described earlier.
Western Blot Analysis of Histone H3 Acetylation in Primary Neurons and Cortical Penumbral Tissue
To investigate whether SIK1 protects ischemic neurons by acting as an HDAC kinase to inhibit histone deacetylation, we assessed histone H3 acetylation in primary cortical neurons. Neurons were cultured on 24-well plates. To measure basal histone H3 acetylation, the primary neurons were infected with lentiviruses at day 6 in vitro and cells were harvested for histone protein extraction at 4 days after infection. To examine the effects of SIK1 knockdown on post-OGD histone H3 acetylation, neurons were infected with lentiviruses at 6 days in vitro and then incubated with 300 nmol/L trichostatin A (TSA) 12 hours before OGD. At 4 days after infection (10 days in vitro), neurons were subjected to 2.5 hours OGD. Cells were harvested for histone protein extraction at 4 hours reoxygenation.
For histone protein extraction, neurons cultured on 24-well plates were washed twice with cold PBS. After washing, 60 μL triton extraction buffer (PBS containing 0.5% triton and 2 mmol/L phenylmethylsulfonyl fluoride) was added to each well at room temperature; and cells were then scraped and transferred to a 1.5-mL tube. After incubation on ice for 10 minutes with gentle stirring, lysates were centrifuged at 6500g for 10 minutes at 4°C, washed once in half-volume of triton extraction buffer and centrifuged again as before. Pellets were then resuspended in 20 μL 0.2 N HCl and stored at 4°C to extract histone protein. After overnight acid extraction, samples were centrifuged at 6500g for 10 minutes at 4°C; and supernatant was collected and frozen at −80°C. A 10-μL sample of acid-extracted protein was subjected to 12% SDS-PAGE electrophoresis. After electrophoresis, proteins were transferred onto a nitrocellulose membrane. Membranes were incubated with 1:1000 diluted rabbit antiacetylated or total histone H3 antibodies overnight. After washing with PBS containing 0.5% tween three times, membranes were incubated with anti-rabbit IgG conjugated to horseradish peroxidase (Jackson Immunoresearch) for 1 hour. Protein bands were visualized with Supersignal (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. Blots were quantitatively analyzed using Quantity One image analysis software (Bio-Rad laboratory, Hercules, CA, USA). Final results were expressed as the ratios of the optical densities of acetylated histone H3 to those of total histone H3.
To investigate whether DHT at protective doses impacts histone H3 acetylation after MCAO, rats were castrated and implanted with 15 mg DHT 1 week before MCAO. Cortical penumbral tissue was extracted from brains at 12 hours reperfusion using a micropunch technique. Then, histone proteins were extracted and Western blot analysis of histone H3 acetylation was performed as described above for cellular samples.
Statistical Analysis
Values are expressed as mean±s.e.m. and subjected to Student's t-test (between two groups), and one-way or two-way analysis of variance with post hoc Newman–Keuls test. Statistical significance was set at P<0.05. All statistical analyses were performed using SigmaStat Statistical Software, Version 2.0 (SPSS, Inc., Chicago, IL, USA).
Results
Dihydrotestosterone Within Physiological Ranges Decreased Infarct Sizes in Castrated Rats After Middle Cerebral Artery Occlusion
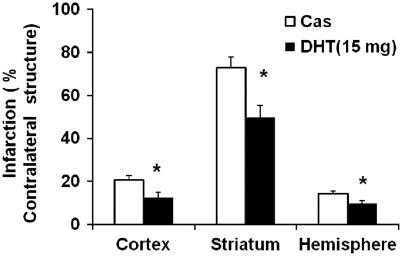
Plasma DHT levels were not detectable in unsupplemented castrated rats. Supplementation of 15-mg DHT in castrated rats elevated plasma DHT levels to 1.1±0.2 ng/mL, which was close to the reported physiological DHT levels in naive rats (0.7±0.4 ng/mL) (Gonzales et al, 2009) but significantly lower than the levels (2.6±1.3 ng/mL) at which DHT exacerbates ischemic damage in castrated rats (Cheng et al, 2007). Physiological parameters were not different between the two treatment groups during MCAO, neither were the intraischemic laser-Doppler flowmetry values (relative to the baseline: 36%±7% in DHT-supplemented castrates versus 32%±3% in nonsupplemented castrates). No rats died before 24 hours reperfusion. Castrated rats supplemented with 15-mg DHT sustained smaller cortical and striatal infarct volumes than unsupplemented castrates at 24 hours reperfusion (Figure 1).
Figure 1.
Analysis of infarct volumes in castrated (Cas) and dihydrotestosterone (DHT) (15 mg)-supplemented castrated male rats. Infarct volumes in the cortex, striatum, and hemisphere were significantly decreased in DHT-supplemented castrated males (n=9) compared with nonsupplemented castrated males (n=8). *P<0.05 versus Cas. Infarct volumes (castrates versus DHT-supplemented castrates): 63.9±7.3 versus 42.5±15.0 mm3 in the cortex, 42.4±3.7 versus 30.7±10.8 mm3 in the striatum, and 106.4±10.9 versus 74.6±26.3 mm3 in the hemisphere.
Salt-Induced Kinase 1 Expression was Induced by Dihydrotestosterone Through an Androgen Receptor-Dependent Mechanism After Middle Cerebral Artery Occlusion
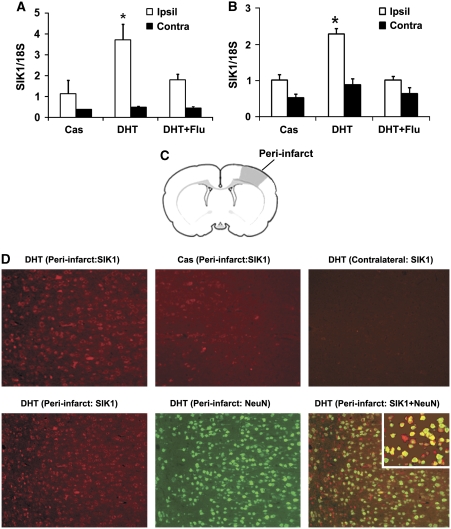
The qPCR was performed to determine whether DHT at the protective dose (15 mg) induced postischemic SIK1 mRNA expression in rats as it did in mice (Uchida et al, 2009). At 6 hours reperfusion, SIK1 mRNA levels in cortical penumbra were higher in DHT-supplemented castrated rats than in castrated rats without DHT supplementation (data not shown). We further confirmed that, at 12 hours reperfusion, SIK1 mRNA levels both in cortical and striatal penumbra were also higher in DHT-supplemented castrated rats than in castrates without DHT implantation (Figures 2A and 2B). Flutamide, an AR antagonist, attenuated DHT-induced postischemic SIK1 transcription (Figures 2A and 2B). In agreement with qPCR results, at 12 hours reperfusion, induced expression of SIK1 protein was detected with immunohistochemistry in cortical peri-infarct areas of DHT-implanted castrated rats (Figure 2D, upper left image) as compared with SIK1 protein expression in contralateral sides (Figure 2D, upper right image); and to a lesser degree, SIK1 protein was also induced in peri-infarct regions of castrated rats without DHT implantation (Figure 2D, upper center image). To identify the cell type responsible for SIK1 induction, immunofluorescence labeling was performed in DHT-treated castrated rats at 12 hours reperfusion with antibodies against SIK1 and neuronal-specific marker NeuN. In cortical peri-infarct regions, SIK1 immunofluorescence predominantly colocalized with NeuN (Figure 2D, lower panel: SIK1 (red) and NeuN (green)).
Figure 2.
Salt-induced kinase 1 (SIK1) expression was induced in rat brains at 12 hours reperfusion after middle cerebral artery occlusion (MCAO). The SIK1 mRNA was significantly increased in ipsilateral (Ipsil) cortical penumbra (A) and striatal penumbra (B) of dihydrotestosterone (DHT) (15 mg)-supplemented castrates (n=5) compared with unsupplemented castrates (Cas, n=6). In DHT plus flutamide-treated castrates (DHT+Flu, n=3), DHT induction of SIK1 mRNA was attenuated by flutamide. *P<0.05 versus Cas and DHT+Flu; Contra: contralateral side. (C) Schematic drawing of a rat coronal brain slice illustrating the region (shaded area) for immunohistochemistry analysis. (D) Immunohistochemistry analysis of SIK1 induction. At 12 hours reperfusion, SIK1 protein (red) was induced in cortical peri-infarct regions of DHT-supplemented rats compared with contralateral sides; and SIK1 protein was translated in the peri-infarct of castrates (upper panel). The SIK1 immunofluorescence predominantly colocalized with the neuronal marker NeuN (green, lower panel). The inset in the lower right image showed SIK1 (red) and NeuN (green) colocalization at a higher magnification. The color reproduction of this figure is available on the html full text version of the article.
Salt-Induced Kinase 1 Knockdown Exacerbated Cell Death in the Neuronal Oxygen–Glucose Deprivation Model
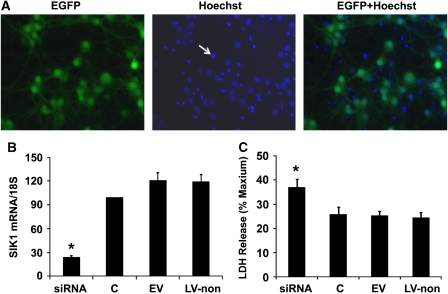
As shown in Figure 3A, 4 days after lentiviral infection, lentivirus-infected primary cortical neurons were positive for eGFP (Figure 3A, the left image: green fluorescence). We further stained lentivirus-infected neurons with a nuclear dye Hoechst 33258 (Figure 3A, the center image: blue fluorescence), and found that eGFP fluorescence, which indicated lentiviral infection, almost completely colocalized with Hoechst staining of live cells (Figure 3A, the right image), suggesting that lentivirus-transduced primary cortical neurons with almost 100% efficiency.
Figure 3.
Salt-induced kinase 1 (SIK1) knockdown exacerbated oxygen–glucose deprivation (OGD)-induced neuronal death. (A) Photomicrographs of primary neurons infected with lentivirus. At 4 days after infection, neurons were positive for enhanced green fluorescence protein (EGFP) (the left image: green fluorescence). Lentivirus-infected neurons were further stained with a nuclear dye Hoechst 33258 (the center image: blue fluorescence); and eGFP fluorescence, which indicated lentiviral infection, almost completely colocalized with Hoechst staining of live cells (the right image). In the center and right images, densely packed Hoechst staining, as pointed by an arrow in the center image, indicated the nuclei of dead cells. Cell death was not induced by lentiviral infection as control neurons without lentiviral infection at day 10 in vitro also displayed similar percentages of densely packed Hoechst staining. (B) Lentivirus-expressing SIK1 small interference RNA (siRNA) decreased SIK1 mRNA by 75% in primary neurons compared with control neurons without lentiviral infection or neurons infected with EV or LV-non (C, control neurons without lentiviral infection; EV, empty virus-expressing eGFP only; LV-non, lentivirus-expressing nonsense siRNA). (C) The SIK1 knockdown by lentivirus-delivered siRNA significantly increased cell death induced by 2.5 hours OGD in primary cortical neurons. Cell death was measured with lactate dehydrogenase (LDH) release assay at 24 hours reoxygenation. Results are expressed as ratios to maximum release when all cells are killed by triton. eGFP. *P<0.05 versus C, EV, or LV-non (n=7). The color reproduction of this figure is available on the html full text version of the article.
Consistent with the immunohistochemistry results that neurons were the primary cell type responsible for SIK1 induction after MCAO (Figure 2D), qPCR assay further revealed that SIK1 mRNA was preferentially expressed in primary cortical neurons but not in primary astrocytes (>40 folds, data not shown). We also validated with qPCR that neurons infected with the lentiviral construct containing the short hairpin RNA sequence (5′-GCCGCCATTTACTACCTCCTACTCG AGTAGGAGGTAGTAAATGGCGGCTTTTT-3′) displayed >75% reduction in SIK1 mRNA as compared with control neurons without lentiviral infection or neurons infected with empty virus or virus-expressing nonsense siRNA (n=5; Figure 3B). Next, we tested whether SIK1 knockdown exacerbates OGD-induced cell death in primary neurons. Using MTT metabolization assays, we first confirmed that infection with lentivirus-expressing SIK1 siRNA did not affect neuronal survival before OGD as compared with nonlentiviral infection or infection with empty virus or lentivirus-expressing nonsense siRNA (data not shown). However, lentivirus-expressing SIK1 siRNA sensitized primary neurons to OGD-induced cell death, as, at 24 hours reoxygenation, more lactate dehydrogenase release was detected in cultures of neurons infected with SIK1 siRNA-expressing lentivirus than in cultures of neurons without lentiviral infection or infected with empty virus or nonsense siRNA-expressing virus (Figure 3C).
Striatal Injection of Salt-Induced Kinase 1 Small Interference RNA Increased Infarction in Dihydrotestosterone-Supplemented Castrated Mice After Middle Cerebral Artery Occlusion
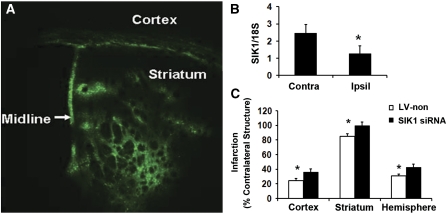
We further tested the hypothesis that SIK1 knockdown blocks DHT neuroprotection in the mouse MCAO model. Lentiviruse-expressing SIK1 siRNA or nonsense siRNA (LV-non) was stereotaxically injected into the mouse striatum. At 2 weeks after injection, lentivirus effectively infected the mouse striatum, as manifested by large quantities of eGFP-positive cells in the striatum under fluorescence microscopy (Figure 4A). Gross pathological inspection further revealed that lentivral injection did not induce pronounced inflammatory changes in the brain. Next, we determined whether SIK1 siRNA-expressing lentiviruses reduced SIK1 mRNA in the infected areas. As shown in Figure 4B, 2 weeks after injection, SIK1 siRNA-expressing lentivirus significantly reduced SIK1 mRNA levels in ipsilateral versus contralateral sides. However, lentivirus-expressing nonsense siRNA did not reduce SIK1 mRNA expression (data not shown). We have reported that 0.5-mg DHT implantation provided physiological plasma levels of DHT and reduced infarct damage in castrated mice after MCAO (Uchida et al, 2009). Thus, we determined whether lentivirus-mediated SIK1 knockdown increased striatal infarction in DHT (0.5 mg)-supplemented castrated mice. Physiological parameters were not different between the two treatment groups. At 24 hours after MCAO, 2 out of 12 mice died in the nonsense siRNA-injected group, and 5 out of 14 mice died in the SIK1 siRNA-injected groups. As expected, at 24 hours reperfusion, intrastriatal injection of SIK1 siRNA-expressing lentivirus increased striatal infarction (15%) in DHT-supplemented castrates compared with intrastriatal injection of lentivirus-expressing nonsense siRNA (Figure 4C). Unexpectedly, SIK1 siRNA-expressing lentivirus injected into the striatum also increased cortical infarct volumes by 50% at 24 hours reperfusion compared with lentivirus-expressing nonsense siRNA (Figure 4C), although we did not observe pronounced lentiviral leaking from the striatum into the cortex by microscopic examination of eGFP fluorescence (Figure 4A).
Figure 4.
Striatal injection of lentivirus-expressing salt-induced kinase 1 (SIK1) small interference RNA (siRNA) increased infarct sizes in castrated mice supplemented with a protective dose of dihydrotestosterone (DHT) (0.5 mg). (A) Intrastriatal injection of lentivirus resulted in robust enhanced green fluorescence protein (eGFP) signal in the striatum at 2 weeks after injection, and eGFP signal was absent in the noninjected, contralateral side. (B) Lentivirus-delivered SIK1 siRNA significantly decreased striatal SIK1 mRNA expression in injected, ipsilateral sides (Ipsil) versus noninjected, contralateral sides (Contra) at 2 weeks after injection (n=5). *P<0.05. (C) In castrated mice implanted with the protective dose of DHT, intrastriatal injection of lentivirus-expressing SIK1 siRNA (n=9) increased striatal, cortical, and hemispheric infarct volumes compared with injection of lentivirus-expressing nonsense siRNA (LV-non, n=10). *P<0.05. Infarct volumes (DHT-supplemented castrates injected with LV-non versus DHT-supplemented castrates injected with siRNA): 12.1±1.7 versus 19.6±2.5 mm3 in the cortex, 8.5±0.6 versus 9.9±0.5 mm3 in the striatum, and 36.8±3.0 versus 55.0±6.2 mm3 in the hemisphere.
Salt-Induced Kinase 1 Knockdown Decreased Basal and Post-Oxygen–Glucose Deprivation Histone H3 Acetylation in Primary Neurons
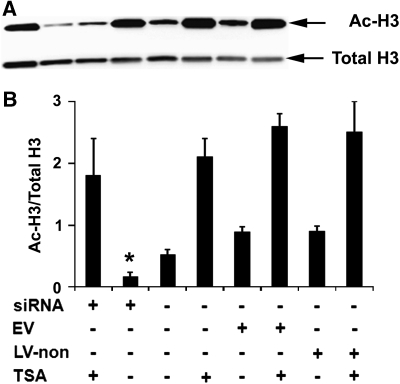
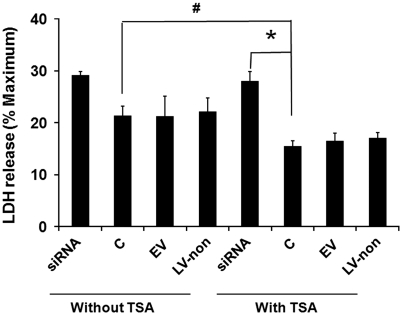
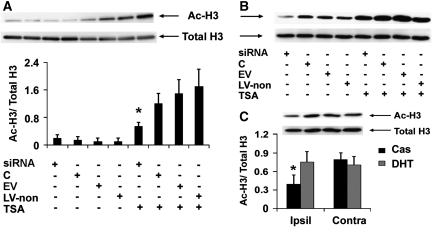
To investigate whether SIK1 knockdown exacerbates ischemia-induced neuronal death by decreasing histone acetylation, we examined the effect of SIK1 knockdown on histone H3 acetylation levels in primary neurons. At the time point when cultures were exposed to OGD, basal acetylated histone H3 levels were lower in primary neurons infected with SIK1 siRNA-expressing lentivirus than in control neurons without lentiviral infection or neurons infected with empty virus or virus-expressing nonsense siRNA (Figure 5). The TSA is an HDAC inhibitor that increases histone acetylation and thus protects neurons against injury from OGD in vitro and MCAO in vivo (Meisel et al, 2006; Yildirim et al, 2008). Consistent with these published results, we found that TSA treatment increased basal histone H3 acetylation levels (Figure 5). Although siRNA-mediated SIK1 knockdown did not reduce TSA-elevated basal histone acetylation (Figure 5), SIK1 knockdown exacerbated OGD-induced neuronal death in the presence of TSA (Figure 6) and decreased histone H3 acetylation at 4 hours reoxygenation in TSA-treated neurons (Figure 7A). We also examined histone H3 acetylation in primary neurons treated with OGD buffer containing glucose under normoxic conditions for 2.5 hours followed by incubation with normal neural basal medium for 4 hours. The histone H3 acetylation patterns in these normoxic control cultures (Figure 7B) were not different from basal histone H3 acetylation patterns (Figure 5).
Figure 5.
Salt-induced kinase 1 (SIK1) knockdown decreased basal histone H3 acetylation but did not decrease trichostatin A (TSA)-elevated basal H3 acetylation levels in primary neurons. Representative images of Western blot analysis of basal acetylated (Ac-H3) and total histone H3 (Total H3) in primary neurons at the time point when cultures were exposed to oxygen–glucose deprivation (OGD) insults (A) and bar graph of the Western blot results (B). C, control neurons without lentiviral infection; EV, neurons transduced with empty virus-expressing enhanced green fluorescence protein (eGFP) only; LV-non, neurons infected with lentivirus-expressing nonsense small interference RNA (siRNA) and eGFP. *P<0.05 versus C, EV, or LV-non in the absence of TSA (n=5).
Figure 6.
Salt-induced kinase 1 (SIK1) gene knockdown increased oxygen–glucose deprivation (OGD)-induced neuronal cell death in the presence of the histone deacetylase (HDAC) inhibitor trichostatin A (TSA). Cell death was measured by lactate dehydrogenase (LDH) release at 24 hours reoxygenation. Results are expressed as ratios to maximum release when all cells are killed by triton. #P<0.05 versus control neurons (C) without TSA; *P<0.05 versus SIK1 small interference RNA (siRNA)-expressing lentivirus (siRNA)-transduced neurons with TSA treatment (n=7).
Figure 7.
Salt-induced kinase 1 (SIK1) knockdown decreased trichostatin A (TSA)-elevated histone H3 acetylation after oxygen–glucose deprivation (OGD) and dihydrotestosterone (DHT) prevented ischemia-induced loss of histone H3 acetylation after middle cerebral artery occlusion (MCAO). (A) Representative image of Western blot analysis of acetylated (Ac-H3) and total histone H3 (Total H3) in primary neurons at 4 hours reoxygenation after 2.5 hours OGD and bar graph of the Western blot results. *P<0.05 versus C, EV, or LV-non in the presence of TSA (n=3; C, control neurons without lentiviral infection; EV, empty virus-expressing enhanced green fluorescence protein (eGFP) only; LV-non, lentivirus-expressing nonsense small interference RNA (siRNA) and eGFP). (B) Image of Western blot analysis of histone H3 acetylation patterns in normoxic control neurons, which were not different from basal histone H3 acetylation patterns (as shown in Figure 5). (C) Representative image of Western blot analysis of acetylated and total histone H3 in the rat brain at 12 hours reperfusion after MCAO and bar graph of the Western blot results. Compared with castration, DHT at the protective dose prevented ischemia-induced loss of histone H3 acetylation in ipsilateral cortical peri-infarct regions. *P<0.05 versus Cas (Ipsil), DHT (Contra), and Cas (Contra) (n=3).
Dihydrotestosterone at the Protective Dose Prevented Ischemia-Induced Loss of Acetylated Histone H3
As we found that DHT at protective doses induced SIK1 expression after MCAO (Figure 2) and that SIK1 knockdown reduced basal and TSA-elevated histone H3 acetylation after OGD in primary neurons (Figures 5 and 7A), we examined whether DHT treatment impacts histone H3 acetylation after MCAO. As shown in Figure 7C, in castrated rats, histone H3 acetylation in the ipsilateral cortical peri-infarct areas was reduced at 12 hours reperfusion compared with that in contralateral sides. However, protective doses of DHT prevented ischemia-induced loss of acetylated histone H3 in the cortical peri-infarct regions (Figure 7C).
Discussion
The major findings in this study are (1) DHT at a protective dose-enhanced postischemic SIK1 expression through an AR-dependent mechanism; (2) SIK1 knockdown exacerbated OGD-induced cell death in primary neurons and increased infarct sizes after MCAO in castrated mice treated with a protective dose of DHT; and (3) SIK1 knockdown reduced histone H3 acetylation and exacerbated OGD-induced neuronal cell death even in the presence of the HDAC inhibitor TSA, whereas DHT at a protective dose prevented ischemia-induced loss of acetylated histone H3 after MCAO. Taken together, our results suggest that postischemic induction of SIK1 contributes to androgen neuroprotection in part by preserving histone acetylation after cerebral ischemia.
We previously used DNA microarray to globally identify androgen-responsive genes after MCAO, and found that DHT at supra-physiological doses altered postischemic transcription of numerous neuroprotective and damaging gene products (Cheng et al, 2007). Accordingly, we were unable to distinguish overall tissue benefit (or lack of) imparted by DHT. Here, we carefully evaluated a low-DHT treatment, previously shown in our model to reduce infarct size, and confirmed that expression of the novel gene SIK1 was induced by protective doses of DHT in the postischemic penumbra. To a lesser degree, SIK1 expression was also induced by ischemia alone as compared with contralateral sides in unsupplemented castrates. As SIK1 was induced in salvageable penumbral areas during early reperfusion, SIK1 should be considered in the ever lengthening list of genes that serve as endogenous protectants in cerebral ischemia.
Our finding that AR antagonist flutamide attenuated DHT-enhanced postischemic SIK1 transcription suggests an AR-dependent mechanism for DHT induction of SIK1. However, there are no known AR-binding sites in the SIK1 promoter. Instead, SIK1 expression in many cell types, including neurons, is transcriptionally regulated by the activation of cAMP-response-element-binding protein (Berdeaux et al, 2007; Koo et al, 2005; Li et al, 2009). Androgens also rapidly but sustainably activate cAMP-response-element-binding protein in neurons through an AR-dependent mechanism (Pike et al, 2008), and it may be that DHT enhances SIK1 transcription by activating cAMP-response-element-binding protein. Further studies are underway to evaluate regulation of SIK1 after injury.
To investigate whether SIK1 induction contributes to DHT neuroprotection, we first showed that SIK1 knockdown by siRNA exacerbated OGD-induced cell death in primary neurons, thus confirming that SIK1 is an endogenous neuroprotective gene in an in vitro ischemia model. Then, we tested whether SIK1 knockdown blocked DHT neuroprotection in a mouse MCAO model. Lentivirus-delivered nonsense siRNA did not alter striatal infarct sizes of castrated mice supplemented with a protective dose of DHT (Uchida et al, 2009). Indeed, striatal infarct sizes of these nonsense siRNA-injected mice were comparable to those we reported for DHT-supplemented castrates in our earlier publication (Uchida et al, 2009). As expected, in DHT-supplemented castrated mice, intrastriatal injection of lentivirus-expressing SIK1 siRNA increased striatal infarct volumes by 15% compared with lentiviral injection of nonsense siRNA. Thus, these results provide first evidence, from a clinically relevant ischemia model, that SIK1 profoundly affects stroke pathogenesis and contributes to androgen neuroprotection in cerebral ischemia.
Unexpectedly, in DHT-treated castrates, intrastriatal injection of SIK1 siRNA-expressing lentivirus also increased cortical infarct size by 50% compared with injection of lentivirus-expressing nonsense siRNA. However, we did not observe eGFP-positive cells in the cortex that would suggest viral leakage. Standby effects may in part account for these unexpected results. For example, lentivirus-mediated gene knockdown exerts standby effects after global cerebral ischemia, that is, survival of noninfected neurons was affected indirectly by neurons infected with lentivirus-expressing miRNAs that knocked down the endogenous Akt inhibitor, carboxyl-terminal modulator protein (CTMP) (Miyawaki et al, 2009). Furthermore, virus-mediated knockdown has more robust effects in vivo than initially expected. For instance, when injected intraventricularly, lentivirus-expressing an miRNA and eGFP infects spleen cells effectively enough to alter brain pathology in an autoimmune disease model. Yet, lentiviral infection cannot be detected in the spleen by fluorescence microscopic identification of eGFP-positive cells (Du et al, 2009). Furthermore, SIK1 siRNA, when delivered by adenovirus through tail vein injection, blocks fasting-mediated SIK1 induction in the liver and systematically promotes fasting hyperglycemia or reprograms gene expression in the liver (Koo et al, 2005; Yoon et al, 2009). Regardless of the mechanisms, our results emphasize the importance of SIK1 as an endogenous neuroprotective gene.
Although it is currently unclear how SIK1 protects the ischemic brain, recent finding that SIK1 functions as a class IIa HDAC kinase (Berdeaux et al, 2007; Gordon et al, 2009; Takemori et al, 2009; van der Linden et al, 2007) suggests a possible mechanism for SIK1 neuroprotection. The HDACs are a class of enzymes that remove acetyl groups from histones. Although class IIa HDACs are enzymatically inactive in vivo, they are thought to exert deacetylase activity by forming complexes with highly active class I HDAC3 in the nucleus (Bottomley et al, 2008; Fischle et al, 2002; Haberland et al, 2009; Lahm et al, 2007). Thus, cerebral overexpression of class IIa HDAC4 or HDAC5 decreases histone H3 acetylation in the brain (Kumar et al, 2005; Tsankova et al, 2006). The SIK1 phosphorylates class IIa HDACs and promotes their nuclear export (Berdeaux et al, 2007; Gordon et al, 2009), thereby inhibiting deacetylase activities associated with them. This function of SIK1 has been confirmed in the brain (van der Linden et al, 2007) and links SIK1 to neuroprotection as HDAC inhibition is increasingly recognized as a neuroprotective mechanism in various neurodegenerative diseases, including stroke (Kazantsev and Thompson, 2008; Kim et al, 2007; Meisel et al, 2006). After MCAO, histone acetylation levels markedly decrease, which could be related to HDAC activation (Kim et al, 2007; Ren et al, 2004). Pan-HDAC inhibitors prevent ischemia-induced histone hypoacetylation and provide neuroprotection in experimental stroke both in vivo and in vitro (Meisel et al, 2006; Yildirim et al, 2008; Ren et al, 2004; Faraco et al, 2006; Qi et al, 2004).
Our results support the notion that SIK1 neuroprotection is linked to its function as a class IIa HDAC kinase. We found that SIK1 knockdown decreased basal histone H3 acetylation and exacerbated OGD-induced cell death in primary neurons. Furthermore, SIK1 knockdown exacerbated OGD-induced cell death in the presence of the HDAC inhibitor TSA; and the exacerbating effects were associated with decreased histone H3 acetylation in TSA-treated neurons after OGD. Finally, we linked DHT neuroprotection to histone acetylation by showing that DHT at the protective dose prevented ischemia-induced loss of acetylated histone H3 in the in vivo MCAO model. Collectively, these results suggest a possible mechanism by which androgens protect the brain via inducing SIK1 and consequently preserving histone acetylation after cerebral ischemia.
There are several limitations to this study. First, we only showed that SIK1 contributes to androgen neuroprotection at early reperfusion (24 hours). Yet, our recent findings indicate that DHT neuroprotection persists into later reperfusion (4 days, unpublished data). Thus, further studies are needed to verify whether SIK1 is continuously elevated by DHT and thereby contributes to androgen neuroprotection during later reperfusion. Second, in this study, we only focused on a neuronal mechanism. However, it should be emphasized that SIK1 is expressed and SIK1-HDAC molecular machinery is also present in other cell types, including microglia and vascular smooth muscle cells (Gordon et al, 2009), which are the key components of neurovascular units. Thus, further studies are needed to determine whether SIK1 also acts through these cell types to protect the ischemic brain especially as we have shown that SIK1 has profound effects on ischemic damage.
In summary, our results indicate that SIK1 is an integral player in mechanisms of androgen neuroprotection against cerebral ischemia and that SIK1 itself is neuroprotective. These findings have important clinical implication relevant to the understanding of androgen action in stroke and the development of stroke therapies devoid of the side effects associated with sex steroids.
Acknowledgments
The authors thank Ms Kathy Gage, Grants, and Publications Writer for the Department of Anesthesiology and Peri-Operative Medicine (APOM), OHSU, for her editorial help in the preparation of this paper. The authors also thank Xiaojin Nie for expert assistance with immunohistochemistry.
The authors declare no conflict of interest.
Footnotes
This research was funded by the American Heart Association Grant 0825526G to JC and NIH Grant NS49210 to PDH.
References
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Berdeaux R, Goebel N, Banaszynski L, Takemori H, Wandless T, Shelton GD, Montminy M. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33:164–172. doi: 10.1016/j.ymeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Bottomley MJ, Lo Surdo P, Di Giovine P, Cirillo A, Scarpelli R, Ferrigno F, Jones P, Neddermann P, De Francesco R, Steinkuhler C, Gallinari P, Carfi A. Structural and functional analysis of the human HDAC4 catalytic domain reveals a regulatory structural zinc-binding domain. J Biol Chem. 2008;283:26694–26704. doi: 10.1074/jbc.M803514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash RJ, Sethi BK, Nalini K, Singh S. Circulating testosterone in pure motor stroke. Funct Neurol. 1991;6:29–34. [PubMed] [Google Scholar]
- Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, Moroni F, Chiarugi A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol. 2006;70:1876–1884. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- Feldman JD, Vician L, Crispino M, Hoe W, Baudry M, Herschman HR. The salt-inducible kinase, SIK, is induced by depolarization in brain. J Neurochem. 2000;74:2227–2238. doi: 10.1046/j.1471-4159.2000.0742227.x. [DOI] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Duckles SP, Krause DN. Dihydrotestosterone stimulates cerebrovascular inflammation through NFkappaB, modulating contractile function. J Cereb Blood Flow Metab. 2009;29:244–253. doi: 10.1038/jcbfm.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JW, Pagiatakis C, Salma J, Du M, Andreucci JJ, Zhao J, Hou G, Perry RL, Dan Q, Courtman D, Bendeck MP, McDermott JC. PKA regulated assembly of a MEF2/HDAC4 repressor complex controls C-jun expression in vascular smooth muscle cells. J Biol Chem. 2009;284:19027–19042. doi: 10.1074/jbc.M109.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996;16:749–754. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, Koch U, De Francesco R, Steinkuhler C, Gallinari P. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci USA. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang C, Takemori H, Zhou Y, Xiong ZQ. TORC1 regulates activity-dependent CREB-target gene transcription and dendritic growth of developing cortical neurons. J Neurosci. 2009;29:2334–2343. doi: 10.1523/JNEUROSCI.2296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Meisel A, Harms C, Yildirim F, Bosel J, Kronenberg G, Harms U, Fink KB, Endres M. Inhibition of histone deacetylation protects wild-type but not gelsolin-deficient neurons from oxygen/glucose deprivation. J Neurochem. 2006;98:1019–1031. doi: 10.1111/j.1471-4159.2006.04016.x. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, Zukin RS. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53:693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66:899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, Golde TE, Orser BA, Macdonald JF, Tymianski M. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Takemori H, Katoh Hashimoto Y, Nakae J, Olson EN, Okamoto M. Inactivation of HDAC5 by SIK1 in AICAR-treated C2C12 myoblasts. Endocr J. 2009;56:121–130. doi: 10.1507/endocrj.k08e-173. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, Devries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden AM, Nolan KM, Sengupta P. KIN-29 SIK regulates chemoreceptor gene expression via an MEF2 transcription factor and a class II HDAC. EMBO J. 2007;26:358–370. doi: 10.1038/sj.emboj.7601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Bhardwaj A, Cheng J, Alkayed NJ, Hurn PD, Kirsch JR. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth Analg. 2007;104:1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94:2353–2359. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- Yildirim F, Gertz K, Kronenberg G, Harms C, Fink KB, Meisel A, Endres M. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol. 2008;210:531–542. doi: 10.1016/j.expneurol.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Seo WY, Lee MW, Kim ST, Koo SH. Salt-inducible kinase regulates hepatic lipogenesis by controlling SREBP-1c phosphorylation. J Biol Chem. 2009;284:10446–10452. doi: 10.1074/jbc.M900096200. [DOI] [PMC free article] [PubMed] [Google Scholar]