Abstract
γ-Aminobutyric acid (GABA) synthesis from glutamate is catalyzed by glutamate decarboxylase (GAD) of which two isoforms, GAD65 and GAD67, have been identified. The GAD65 has repeatedly been shown to be important during intensified synaptic activity. To specifically elucidate the significance of GAD65 for maintenance of the highly compartmentalized intracellular and intercellular GABA homeostasis, GAD65 knockout and corresponding wild-type mice were injected with [1-13C]glucose and the astrocyte-specific substrate [1,2-13C]acetate. Synthesis of GABA from glutamine in the GABAergic synapses was further investigated in GAD65 knockout and wild-type mice using [1,2-13C]acetate and in some cases γ-vinylGABA (GVG, Vigabatrin), an inhibitor of GABA degradation. A detailed metabolic mapping was obtained by nuclear magnetic resonance (NMR) spectroscopic analysis of tissue extracts of cerebral cortex and hippocampus. The GABA content in both brain regions was reduced by ∼20%. Moreover, it was revealed that GAD65 is crucial for maintenance of biosynthesis of synaptic GABA particularly by direct synthesis from astrocytic glutamine via glutamate. The GAD67 was found to be important for synthesis of GABA from glutamine both via direct synthesis and via a pathway involving mitochondrial metabolism. Furthermore, a severe neuronal hypometabolism, involving glycolysis and tricarboxylic acid (TCA) cycle activity, was observed in cerebral cortex of GAD65 knockout mice.
Keywords: 13C isotopes; γ-vinylGABA (GVG, vigabatrin); glutamate decarboxylase; hypometabolism; neuronal-glial trafficking; nuclear magnetic resonance
Introduction
The most abundant neurotransmitters in the brain are glutamate and γ-aminobutyric acid (GABA) for excitatory and inhibitory transmission, respectively. A tight regulation of the synthesis and degradation of these two compounds is therefore crucial. Disturbances in this regulation are likely involved in GABA–glutamate imbalances characteristic for a number of neurodegenerative and psychiatric disorders (Sonnewald and Kondziella, 2003). The GABA synthesis from glutamate is catalyzed by the enzyme glutamate decarboxylase (GAD, EC 4.1.1.15) of which two isoforms, GAD65 and GAD67, have been identified. These isoforms are encoded for by separate genes and differ with regard to regulation and intracellular localization (Erlander et al, 1991; Kaufman et al, 1991; Esclapez et al, 1994). The GAD65 appears to exist predominantly as a dormant apoenzyme, being rapidly activated on binding of pyridoxal phosphate. In contrast, GAD67 is present as the active holoenzyme having the coenzyme bound (Martin et al, 1991). The GAD67 is localized throughout the cytosol of GABAergic neurons, whereas GAD65 is enriched in the nerve endings and has been suggested to be associated with synaptic vesicles (Kaufman et al, 1991). The fraction of total GAD accounted for by GAD65 in cerebral cortex and hippocampus has been estimated to be 50% (Feldblum et al, 1993; Sheikh et al, 1999).
The GABA in the brain is continuously renewed by the concomitant actions of GAD and GABA transaminase, i.e. the GABA shunt. In addition to the GABA shunt, turnover of GABA involves metabolism via the tricarboxylic acid (TCA) cycle, which is dependent on supply of acetyl-CoA from glucose. Although GABA synthesis is confined to the neuronal compartment, astrocyte-derived glutamine has been identified as a quantitatively important precursor (Bradford et al, 1978; Battaglioli and Martin, 1991; Sonnewald et al, 1993; Waagepetersen et al, 2001). This is likely due to the partial astrocytic clearance of GABA from the synaptic cleft combined with the lack of a significant neuronal anaplerosis (Bak et al, 2006). In neurons, glutamine is converted to glutamate catalyzed by phosphate-activated glutaminase, which is located in the inner mitochondrial membrane (Laake et al, 1999). Glutamate may instantly be decarboxylated to GABA by GAD in the cytosol, referred to as direct synthesis of GABA. Alternatively, glutamate may be converted to α-ketoglutarate and metabolized via the TCA cycle within the mitochondria, and GABA may subsequently be formed from glutamate. This pathway involving mitochondrial metabolism was shown to account for up to 60% of GABA synthesis from glutamine (Waagepetersen et al, 2001). To specifically and simultaneously unravel the importance of GAD65 in the intercellular scenario of GABA biosynthesis, GAD65 knockout mice were injected with a combination of [1-13C]glucose and the astrocyte-specific substrate [1,2-13C]acetate (Waniewski and Martin, 1998). A detailed metabolic mapping was revealed from nuclear magnetic resonance (NMR) spectroscopy of tissue extracts of cerebral cortex and hippocampus from the GAD65 knockout and the corresponding wild-type mice. To further elucidate the involvement of GAD65 in the two pathways of GABA synthesis from glutamine, that is direct GABA synthesis and GABA synthesis via the TCA cycle, mice were injected with [1,2-13C]acetate alone. Moreover, the significance of GABA degradation via the action of GABA transaminase was probed using γ-vinylGABA (GVG, Vigabatrin) to specifically and irreversibly inhibit this enzyme (Gram et al, 1988).
Materials and methods
Materials
[1-13C]Glucose, [1,2-13C]acetate, and D2O (99.9%) were purchased from Cambridge Isotopes Laboratories (Woburn, MA, USA) and ethylene glycol was from Merck (Darmstadt, Germany). The GVG (Sabrilex single dosage powders 500 mg/dose) was purchased from Sanofi-Aventis (Hørsholm, Denmark). Antibodies were bought from Abcam (Cambridge, UK) and secondary antibody (P 0260) was obtained from Dako (Glostrup, Denmark). N,N-dimethylformamide Sigma-Aldrich (St Louis, MO, USA) and N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide as well as 1% tertbutyldimethylchlorosilane were purchased from Regis Technologies Inc. (Morton Grove, IL, USA). All other chemicals were of the purest quality available from regular commercial sources.
Animals
The GAD65 knockout mice were generated as described by Kash et al (1997) and backcrossed onto the C57BL/6 background for 10 generations. The mice used in this study were obtained from heterozygous × heterozygous breeding and GAD65 knockout and corresponding wild-type mice were identified by genotyping. Breeding and genotyping were managed at Taconic (Ry, Denmark), and the mice were delivered to the animal facilities at the Norwegian University of Science and Technology, Trondheim. Animals used in the metabolic study were treated in compliance with the European Convention (ETS 123 of 1986), and all protocols were approved by the Norwegian National Animal Research Authority. Animals were maintained under standard conditions at a 12-hour light–dark cycle (lights on at 0600 hours). The animals were acclimatized to these conditions with free access to food and water for at least 1 week before the experiments were performed.
Sodium Dodecyl Sulfate/Polyacrylamide Gel Electrophoresis and Immunoblotting
Cerebral cortices were excised from 17-week-old wild-type or GAD65 knockout mice and ice-cold phosphate-buffered saline was added to a final concentration of 10% w/v. The tissue was kept on ice and ultrasound was applied using a sonicator model VCX 400 (Sonics and Materials, Newtown, CT, USA) providing a homogeneous suspension, which was subsequently centrifuged at 20,000 g for 20 minutes at 4°C. The supernatant (fraction 1) contains cytosolic GAD. Ice-cold phosphate-buffered saline containing 1% Triton X-100 was added to the pellet to a final concentration of 10% w/v to solubilize GAD, and the suspension was centrifuged at 20,000 g and 4°C for 20 minutes. The supernatant (fraction 2) contains membrane-associated GAD. For Western blot analysis, comparable aliquots of the soluble fractions (fraction 1) and the solubilized membrane fractions (fraction 2) from wild-type and knockout mice were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis on 7% or 12% gels and transferred to polyvinylidene difluoride membranes according to the standard procedures. After the transfer, the membrane was incubated for 10 minutes with tris buffered saline (TBS) block buffer (50 mmol/L Tris, 0.15 mmol/L NaCl, pH 8.0, containing 2% (v/v) Tween 20). The membrane was probed with a mouse monoclonal antibody (GAD6) to GAD65/HRP-conjugated rabbit anti-mouse immunoglobulins. The primary antibodies were diluted 1:10,000 and the secondary antibody (P 0260) was diluted 1:2500. Dilutions of antibodies were performed in 1% nonfat skim milk in the wash buffer (50 mmol/L Tris, 0.15 mmol/L NaCl, pH 8.0, containing 0.5% (v/v) Tween 20) used extensively between change of application. Detection was performed using the chemiluminescence enhancer, SuperSignal West Femto Maximum Sensitivity Substrate, as recommended by the manufacturer (Pierce Biotechnology, Rockford, IL, USA), and the results were monitored on a Las Chemiluminator (LAS-1000, Fujifilm Holdings Corp., Vedbæk, Denmark).
Metabolic Studies
Homozygous GAD65 knockout and wild-type mice at age 15–23 weeks were used for experiments. To study neuronal and astrocytic metabolism simultaneously, both genotypes were injected intraperitoneally, with a combination of [1-13C]glucose (543 mg/kg) and [1,2-13C]acetate (504 mg/kg) and after 15 minutes the animals were killed by microwave fixation, 4 kW, 1.70 seconds, instantaneously inactivating brain metabolic reactions (Model GA5013, Gerling Applied Engineering, Modesto, CA, USA). The mice were decapitated, trunk blood was collected and the cerebral cortices and hippocampi were excised. The blood sample was centrifuged at 1000 g for 5 minutes and the serum was later analyzed for total amounts of glucose and 13C enrichment by NMR spectroscopy. The tissue samples were stored at −75°C until extraction using 0.7% perchloric acid. Ultrasound was applied to the tissue using a Vibra Cell sonicator (Model VCX 750, Sonics and Materials), and the homogenized samples were centrifuged at 3000 g and 4°C for 5 minutes. The precipitates were washed with distilled water and centrifugation was repeated. The supernatants were pooled and adjusted to pH 6.5–7.5 before lyophilization.
To further elucidate the role of GAD65 in GABA synthesis from astrocytic glutamine via direct synthesis and by way of TCA cycle metabolism, respectively, other GAD65 knockout and wild-type mice were injected only with [1,2-13C]acetate. This is required due to the fact that the additional injection of [1-13C]glucose gives rise to various GABA isotopomers after successive turns of TCA cycle metabolism. Some mice were pretreated with GVG to evaluate the influence of attenuation of GABA degradation on GABA synthesis. Briefly, GAD65 knockout and wild-type mice were injected (intraperitoneally) with GVG (1 g/kg) dissolved in 0.9% saline or 0.9% saline. After 24 hours, mice were given another injection (intraperitoneally) of [1,2-13C]acetate (504 mg/kg) and after another 15 minutes, the mice were killed by microwave fixation. Cortex was excised and treated as described above.
1H and 13C-NMR
Lyophilized tissue extracts were dissolved in 99% D2O (deuterated water) containing 0.05% ethylene glycol as an internal standard, and pH was readjusted to 6.5–7.5. The samples were transferred into 5 mm Shigemi NMR microtubes (Shigemi Inc., Allison Park, PA, USA). Proton decoupled 13C-NMR spectra of cortex were obtained using a BRUKER DRX-600 spectrometer (BRUKER Analytik GmbH, Rheinstetten, Germany). The spectra were recorded at room temperature and the following parameters were used: a 30° pulse angle and 30 kHz spectral width with 64 K data points. The number of scans was typically 30,000. The acquisition time was 1.08 seconds and the relaxation delay was 0.5 seconds.
Proton NMR spectra were obtained using the same spectrometer using a pulse angle of 90° and a spectral width with 32 K data points. The number of scans was 128 for cortical extracts and 1024 for hippocampal extracts. Acquisition time was 1.36 seconds and relaxation delay was 10 seconds. Water suppression was achieved by applying a low-power presaturation pulse at the water frequency.
Relevant peaks in the two spectra were identified and integrated using XWINNMR software (BRUKER BioSpin GmbH, Rheinstetten, Germany). The amounts of 13C labeling and the total amounts of metabolites were quantified from the integrals of the peak areas using ethylene glycol as internal standard. Factors for the nuclear Overhauser and relaxation effects were applied to all relevant resonances in the 13C spectra. Results for monolabeled metabolites were corrected for the 1.1% natural abundance of 13C.
GC-MS
Owing to the low sensitivity of NMR spectroscopy and the sparse amount of hippocampal tissue available, the percentual distribution of 13C-labeled mass isotopomers in hippocampus was determined using gas chromatography and mass spectrometry (GC-MS). After 1H-NMR spectroscopy, an aliquot of the sample was adjusted to pH=2 using HCl and the sample was lyophilized. Organic and amino acids were extracted into an organic phase of ethanol and benzene, dried under air and reconstituted in N,N-dimethylformamide before derivatization with N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide in the presence of 1% tertbutyldimethylchlorosilane (Mawhinney et al, 1986). After derivatization, compounds were analyzed in an Agilent 6890GC gas chromatograph linked to an Agilent 5975 B inert MSD with an electron ionization source (all from Agilent Technologies Inc., Santa Clara, CA, USA). Results for monolabeled metabolites were corrected for natural abundance of 13C.
Labeling Patterns Obtained from Metabolism of [1,2-13C]Acetate
Acetate is taken up exclusively into astrocytes, whereas glucose is metabolized in both the neuronal and astrocytic compartment. [1,2-13C]Acetate is metabolized in the TCA cycle and gives rise to [4,5-13C]α-ketoglutarate, which is a precursor for [4,5-13C]glutamate and [4,5-13C]glutamine. Moreover, [4,5-13C]glutamate and [1,2-13C]GABA are formed subsequent to transfer of [4,5-13C]glutamine to the neuronal compartments, which are the glutamatergic and GABAergic neurons (Hassel et al, 1997; Shen et al, 1999; Qu et al, 2000; Chowdhury et al, 2007). It should be noted that the amount of glutamate located in the glutamatergic neurons accounts for >80% of the total glutamate pool (Ottersen and Storm-Mathisen, 1985; Ottersen et al, 1992). Hence, the detected [4,5-13C]glutamate originates predominantly from neuronal conversion of [4,5-13C]glutamine. When GC-MS analysis was used to detect 13C labeling in hippocampal extracts [4,5-13C]glutamine, [4,5-13C]glutamate, and [1,2-13C]GABA are observed as double-labeled glutamine, glutamate, and GABA, respectively.
After conversion of [4,5-13C]glutamine to [4,5-13C]glutamate in the inner mitochondrial membrane, [1,2-13C]GABA may be synthesized directly by decarboxylation of [4,5-13C]glutamate in the cytosol. Alternatively, [4,5-13C]glutamate may be metabolized in the TCA cycle and subsequently [3-13C]- and [4-13C]GABA may be formed from [2-13C]- and [3-13C]glutamate. To elucidate the involvement of GAD65 in the two pathways of GABA synthesis from glutamine, namely direct GABA synthesis and GABA synthesis via the TCA cycle, information obtained from mice injected with [1,2-13C]acetate alone was used. The percent direct synthesis of [1,2-13C]GABA from [4,5-13C]glutamine compared with the total amount of GABA ([1,2-13C]-, [3-13C]-, and [4-13C]GABA) labeled from glutamine was calculated using Equation 1. In order for this equation to be valid, isotopic steady state for this process is assumed, and this is realistic as TCA cycle activity is extensive.
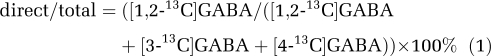 |
However, [3-13C]- and [4-13C]GABA may also be formed from [2-13C]- and [3-13C]glutamine generated after a subsequent turn of astrocytic TCA cycle metabolism and relocation to the neuronal compartment. To evaluate the influence of such preceding astrocytic TCA cycle metabolism for the labeling of glutamine and glutamate, the percent synthesis of either of these metabolites (glx) from the first turn of TCA cycle metabolism of [1,2-13C]acetate compared with the total amount of 13C-labeled synthesized was calculated using Equation 2.
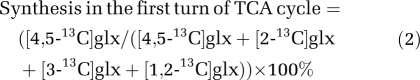 |
Labeling Patterns Obtained from Metabolism of [1-13C]Glucose
[1-13C]Glucose is metabolized to [3-13C]pyruvate and after formation and subsequent entrance of [2-13C]acetyl-CoA into the TCA cycle, [4-13C]glutamate is formed due to rapid equilibration with [4-13C]α-ketoglutarate (Mason et al, 1992). [4-13C]Glutamine can be formed directly in the astrocytic compartment from [1-13C]glucose via [2-13C]acetyl-CoA and TCA cycle metabolism or from neuronal [4-13C]glutamate subsequent to transmitter release and astrocytic uptake. Using the gliotoxin fluoroacetate, it has been shown that 40% of glutamine is derived from [4-13C]glutamate initially labeled in the neuronal compartment (Hassel et al, 1997). Other studies based on mathematical modeling suggest that 70% to 80% of glutamine synthesis relies on conversion of neurotransmitter glutamate and GABA and accordingly depends on glutamate/GABA–glutamine cycle activity (Sibson et al, 2001; Patel et al, 2005). Thus, a substantial fraction of the measured [4-13C]glutamine is likely to reflect neuronal metabolism of [1-13C]glucose. A nominal TCA cycle activity was calculated from 13C-NMR data on the basis of glutamate labeling from [1-13C]glucose in the second turn relative to the first turn using Equation 3.
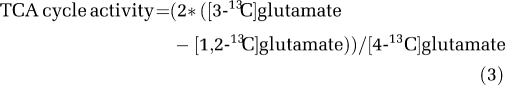 |
In addition to oxidation of pyruvate via pyruvate dehydrogenase, pyruvate contributes to anaplerosis of TCA cycle intermediates via carboxylation to oxaloacetate in astrocytes. However, elucidation of the pyruvate carboxylation pathway is hampered by the complexity of the glutamate isotopomers arising after successive turns in the TCA cycle after simultaneous injection of [1-13C]glucose and [1,2-13C]acetate. Therefore, no attempts were made to elucidate the relative importance of these metabolic pathways. Glutamate, glutamine, and GABA labeled from [1-13C]glucose was monitored as monolabeled substrates in the mass spectrum.
Data Analysis
Data are presented as averages±standard error of the mean (s.e.m.). Statistical differences between GAD65 knockout and wild-type mice as well as the effects of GVG treatment in each genotype were determined using an unpaired two-tailed Student's t-test where P<0.05 was taken to indicate statistically significant differences.
Results
Western Blot
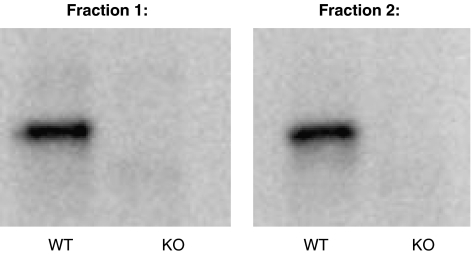
The expression of GAD65 in the wild-type and GAD65 knockout animals was examined using Western blotting. The results verified the absence of GAD65 in the knockout mice and its presence in wild type (Figure 1). In addition, it was observed that GAD65 in wild-type mice appeared to exist in a cytosolic as well as a membrane-associated form as it was detected in both fractions 1 and 2.
Figure 1.
Western blot analysis of expression of GAD65 in extracts of cerebral cortex from wild-type (WT) and GAD65 knockout (KO) mice. Cortex was excised from decapitated mice (WT and KO) and homogenized in phosphate-buffered saline (PBS) by sonication (see Materials and methods). Cytoplasmic GAD65 was saved as the supernatant after centrifugation of 10% homogenates (fraction 1). Membrane-bound GAD65 was extracted from the precipitate (fraction 2) by resolubilization in PBS containing 1% Triton X-100 followed by centrifugation. The extracts were diluted 10-fold in PBS containing 0.1% Triton X-100 and analyzed for content of GAD65 by Western blotting (see Materials and methods). GAD, glutamate decarboxylase.
Content of Metabolites in Cerebral Cortex and Hippocampus
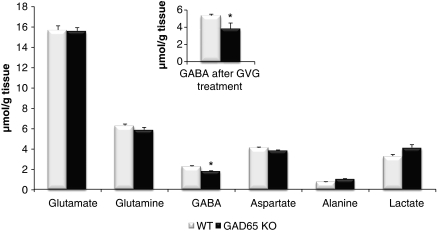
The cortical and hippocampal contents of metabolites were determined by 1H-NMR analysis. In mice injected with both [1,2-13C]acetate and [1-13C]glucose, the GABA content was significantly lower in the GAD65 knockout by ∼20% in cortex (Figure 2) and 30% in hippocampus (P=0.036; results not shown). In contrast, the content of aspartate, glutamate, glutamine, and alanine was unaffected by the lack of GAD65 in both brain regions. These findings are compatible with those obtained in cerebral cortex of mice injected with [1,2-13C]acetate alone (results not shown). Treatment with GVG, inhibiting GABA degradation, significantly increased the GABA content in both GAD65 knockout and wild type by ∼200% (P<0.001; Figure 2, inset). The relative reduction in cortical GABA content in GAD65 knockout mice compared with wild type was maintained after GVG treatment (Figure 2, inset). Treatment with GVG exhibited no effect on the cortical content of aspartate, glutamate, and glutamine neither in the wild-type nor in GAD65 knockout mice (results not shown).
Figure 2.
The amount of metabolites (μmol/g tissue) in extracts from cerebral cortex of wild-type (WT; open bars) and GAD65 knockout (GAD65 KO; filled bars) mice was determined by 1H-nuclear magnetic resonance spectroscopy. The inset shows the amount of γ-aminobutyric acid (GABA; μmol/g tissue) after treatment with γ-vinylGABA (GVG). Results are averages±s.e.m. (n=5), and statistically significant differences (P<0.05) between GAD65 knockout and wild-type mice are indicated with an asterisk. GAD, glutamate decarboxylase.
Metabolism of [1,2-13C]Acetate in GAD65 Knockout and Wild-Type Mice
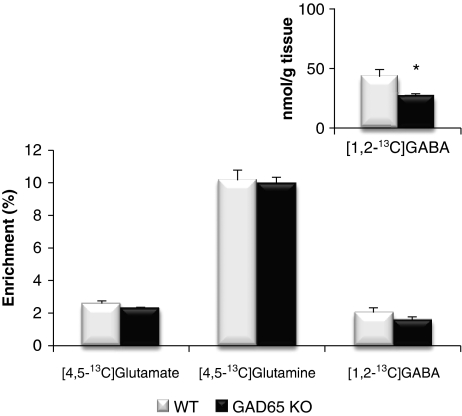
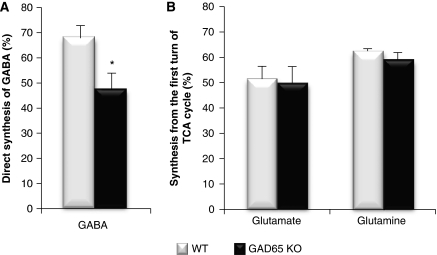
When injecting [1-13C]glucose and [1,2-13C]acetate simultaneously, the relative 13C enrichment of [4,5-13C]glutamate, [4,5-13C]glutamine, and [1,2-13C]GABA obtained from metabolism of [1,2-13C]acetate is compatible with previous studies (van den Berg et al, 1969; Chowdhury et al, 2007; Bogen et al, 2008). The percent enrichment in [4,5-13C]glutamine formed from [1,2-13C]acetate was similar in the GAD65 knockout and wild-type mice, indicating that the expression of GAD65 does not influence astrocytic TCA cycle metabolism and glutamine synthesis (Figure 3). In agreement with the lower total GABA content reported above, the amount of [1,2-13C]GABA (nmol/g tissue) labeled from [1,2-13C]acetate was significantly reduced in the GAD65 knockout mice (Figure 3, inset). As the relative decrements were similar in the total amount of GABA and that synthesized from acetate via glutamine, the percent enrichment was not different in the knockouts compared with wild type. Regarding the precursors of GABA, that is glutamine and glutamate, no differences were observed neither in the total amount (Figure 2) nor in the percent 13C enrichment (Figure 3). Similar findings were obtained in the hippocampus of these mice and in cortex of mice injected only with [1,2-13C]acetate (results not shown). This study, using only [1,2-13C]acetate, was explicitly designed to provide information regarding the involvement of GAD65 in GABA synthesis from glutamine both via direct synthesis and by way of TCA cycle metabolism. The amount of [1,2-13C]GABA generated by direct synthesis from [4,5-13C]glutamine was significantly reduced in GAD65 knockout mice, whereas [3-13C]- and [4-13C]GABA formed from [4,5-13C]glutamine via the TCA cycle were unaltered (Table 1). On the basis of the amounts of GABA isotopomers, the percent GABA synthesized via direct synthesis from glutamine was calculated using Eq. 1, and this was reduced from ∼67% in wild-type to ∼47% in GAD65 knockout mice (Figure 4A). It may be argued that alterations in the labeling of GABA from glutamine are based on the labeling of the precursors glutamate and glutamine, which is influenced by successive turns of astrocytic TCA cycle metabolism. However, the amounts of glutamate and glutamine isotopomers labeled from [1,2-13C]acetate after the first or second turn of TCA cycle metabolism were similar in GAD65 knockout and wild-type mice (Table 1). In analogy to the ratio of the direct versus the TCA cycle pathways for GABA synthesis, we calculated the percent of glutamate and glutamine synthesized in the first turn of the TCA cycle from [1,2-13C]acetate using Eq. 2, and no differences were observed between GAD65 knockout mice and wild type (Figure 4B).
Figure 3.
Percent enrichment of [4,5-13C]glutamate, [4,5-13C]glutamine, and [1,2-13C]GABA labeled from [1,2-13C]acetate in wild-type (WT; open bars) and GAD65 knockout (GAD65 KO; filled bars) mice. The animals were injected with [1-13C]glucose and [1,2-13C]acetate and killed 15 minutes later as detailed in Materials and methods. The enrichment was determined from 13C-nuclear magnetic resonance spectroscopic analysis of brain extracts as detailed in Materials and methods. The inset shows the amount of [1,2-13C]GABA (nmol/g tissue). Results are averages±s.e.m. (n=5), and the asterisk indicates a statistically significant difference between GAD65 knockout and wild-type mice (P<0.05). GABA, γ-aminobutyric acid; GAD, glutamate decarboxylase.
Table 1. Content of isotopomers of glutamate, glutamine, and GABA labeled from [1,2-13C]acetate.
|
Content of isotopomer (nmol/g tissue) |
||
|---|---|---|
| Isotopomer | Wild type | GAD65 knockout |
| [4,5-13C]Glutamate | 293.5±30.0 | 273.1±20.9 |
| [2-13C]Glutamate | 67.8±7.0 | 78.3±11.4 |
| [3-13C]Glutamate | 112.1±8.7 | 107.1±9.6 |
| [1,2-13C]Glutamate | 94.0±9.2 | 88.8±2.7 |
| [4,5-13C]Glutamine | 387.1±52.4 | 417.8±46.4 |
| [2-13C]Glutamine | 78.2±13.6 | 68.7±9.3 |
| [3-13C]Glutamine | 111.7±13.6 | 91.5±6.2 |
| [1,2-13C]Glutamine | 119.4±7.2 | 122.6±18.2 |
| [1,2-13C]GABA | 36.4±3.0 | 23.1±3.0* |
| [3-13C]GABA | 9.8±2.3 | 10.2±1.6 |
| [4-13C]GABA | 13.8±3.3 | 9.7±1.0 |
GABA, γ-aminobutyric acid; GAD, glutamate decarboxylase.
The content of isotopomers of glutamate, glutamine, and GABA labeled from [1,2-13C]acetate. The animals were injected with [1,2-13C]acetate and killed 15 minutes later as detailed in Materials and methods. The amount of isotopomers was determined from 13C-nuclear magnetic resonance spectroscopic analysis of cortical extracts as detailed in Materials and methods. Results are averages±s.e.m. (n=6–9).
*Statistically significant difference between GAD65 knockout and wild-type mice (P<0.01).
Figure 4.
Percent γ-aminobutyric acid (GABA) formed via direct synthesis from [4,5-13C]glutamine (A) and percent of glutamate and glutamine synthesized in the first turn of the tricarboxylic acid (TCA) cycle from [1,2-13C]acetate (B) in wild-type (WT; open bars) and GAD65 knockout (GAD65 KO; filled bars) mice. The animals were injected with [1,2-13C]acetate and killed 15 minutes later as detailed in Materials and methods. The amount of 13C incorporation was determined from 13C-nuclear magnetic resonance spectroscopic analysis of brain extracts as detailed in Materials and methods. The percent direct synthesis of GABA from glutamine was calculated by dividing the amount of [1,2-13C]GABA by the sum of [1,2-13C]GABA, [3-13C]GABA, and [4-13C]GABA. The percent synthesis of glutamate was calculated by dividing the amount of [4,5-13C]glutamate by the sum of [4,5-13C]glutamate, [2-13C]glutamate, [3-13C]glutamate, and [1,2-13C]glutamate. The corresponding calculation was performed for glutamine. Results are averages±s.e.m. (n=5), and the asterisk indicates a statistically significant difference between GAD65 knockout and wild-type mice (P=0.03). GAD, glutamate decarboxylase.
Although inhibition of GABA degradation by GVG treatment led to a dramatic increase in the cortical GABA content, it had no impact on the amount of GABA, glutamate, or glutamine labeled directly from [1,2-13C]acetate, neither in GAD65 knockout nor in wild-type mice (results not shown).
Metabolism of [1-13C]Glucose in GAD65 Knockout and Wild-Type Mice
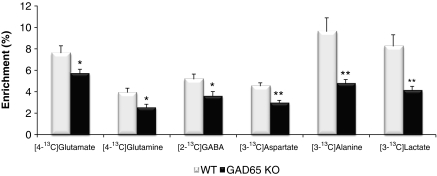
The relative 13C enrichment from [1-13C]glucose in metabolites extracted from the cerebral cortex of wild-type mice is compatible with previous findings (Chowdhury et al, 2007; Bogen et al, 2008). The total amount and the percent enrichment of glucose in the brain were not different in GAD65 knockout compared with wild-type mice (results not shown). Moreover, the percent 13C enrichment in plasma was unaltered in GAD65 knockout mice compared with wild type (GAD65 knockout: 32.1%±1.8% and wild type 37.4%±4.5% P=0.21). Nevertheless, the percent enrichment of [3-13C]lactate and [3-13C]alanine labeled via glycolysis was dramatically decreased in cortex of GAD65 knockout mice to half of that observed in wild-type mice (Figure 5). Furthermore, the percent enrichment of [4-13C]glutamate, [3-13C]aspartate, and [2-13C]GABA was reduced by ∼30%. The percent enrichment of [4-13C]glutamine was decreased by ∼35%, which may seem somewhat surprising as astrocytic TCA cycle metabolism and glutamine synthesis were shown not to be affected in the GAD65 knockout mice as observed from the labeling obtained using [1,2-13C]acetate as the substrate. The decrease in percent enrichment seen in [4-13C]glutamine, which was comparable to the decrease in neuronal [4-13C]glutamate, is most likely reflecting glutamine synthesis subsequent to glutamatergic neurotransmission and not astrocytic TCA cycle metabolism. Importantly, the TCA cycling activity calculated on the basis of glutamate labeling from [1-13C]glucose revealed a reduced (∼35%) activity in the GAD65 knockout (0.32±0.02) compared with wild-type mice (0.48±0.04; P=0.044). In contrast to this general neuronal hypometabolism observed in cortex, only the amount of monolabeled GABA, reflecting [2-13C]GABA, was significantly lower in hippocampus of GAD65 knockout mice compared with wild type.
Figure 5.
Percent enrichment of [4-13C]glutamate, [4-13C]glutamine, [2-13C]GABA, [3-13C]aspartate, [3-13C]alanine, and [3-13C]lactate labeled from [1-13C]glucose in wild-type (WT; open bars) and GAD65 knockout (GAD65 KO; filled bars) mice. The animals were injected with [1-13C]glucose and [1,2-13C]acetate and killed 15 minutes later as detailed in Materials and methods. The enrichment was determined from 13C-nuclear magnetic resonance spectroscopic analysis of brain extracts as detailed in Materials and methods. Results are averages±s.e.m. (n=5) and statistically significant differences between GAD65 knockout and wild-type mice are indicated with asterisks (*P<0.05; **P<0.01). GABA, γ-aminobutyric acid; GAD, glutamate decarboxylase.
Discussion
Two isoforms of GAD exist in the brain, and these differ according to intracellular localization and regulation (Erlander et al, 1991; Kaufman et al, 1991; Esclapez et al, 1994). Mice lacking GAD67 are not viable (Asada et al, 1997), whereas GAD65 knockout mice exhibit a limited lifespan with a survival rate of ∼70% after 7 months (Kash et al, 1997; Stork et al, 2000). The GAD65 knockout mice are more susceptible to seizures than the wild-type mice and during EEG recordings high-voltage seizure bursts have been observed (Asada et al, 1996; Kash et al, 1997; Stork et al, 2000). Cerebral cortex and hippocampus are strongly related brain regions exhibiting a close interaction in the evolvement and progression of seizures.
GAD65 Is Essential for Maintenance of the γ-Aminobutyric Acid Content in Brain
We observed a reduction in the GABA level in GAD65 knockout mice, which is an obvious repercussion of the absence of GAD65 in these mice. The reduction of ∼20% indicates a significant contribution and impact of GAD65 activity on the maintenance of the total GABA content in brain. This finding is in agreement with that previously found in mice lacking GAD65 in which GABA was reduced by ∼35% compared with that in wild-type mice (Stork et al, 2000). It should be noted, however, that the cerebral GABA content in GAD65 knockout mice is normal at birth (Asada et al, 1996; Stork et al, 2000). Although the GABA content in wild type increases significantly after birth, this remains unchanged in GAD65 knockout mice leading to a significantly lower GABA level in GAD65 knockout mice compared with wild type at 2 months after birth (Stork et al, 2000). The functional and metabolic consequences of a deficit in the average brain GABA level are difficult to predict because of the highly compartmentalized intracellular and intercellular distribution of GABA, which may be coupled to the multifaceted role of GABA (Waagepetersen et al, 1999a). Distinct pools of GABA may be differentially affected by inhibition of GABA degradation by GVG. However, even though GABA levels were elevated by GVG, the reduction in the cortical GABA level of ∼30% was preserved in GAD65 knockout mice compared with controls when GABA transaminase was inhibited clearly demonstrating that the inhibition exerted by GVG was ubiquitous and likely affected all pools of GABA. This was underlined by the fact that the rate of GABA synthesis monitored by the amount of 13C incorporated into GABA from [1,2-13C]acetate was not affected by GVG treatment neither in GAD65 knockout nor in wild-type mice. Consistently, a pronounced increase in both the cytosolic and the vesicular GABA pools of cultured GABAergic neurons after exposure to GVG has been reported (Gram et al, 1988).
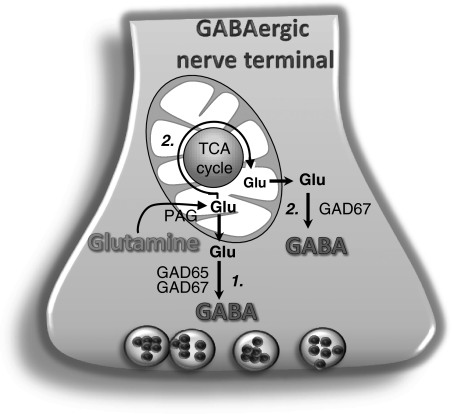
Subsequent to vesicular release of GABA and interaction with the GABA receptors, GABA is taken up either by the presynaptic neuron or by the adjacent astrocytic processes (Schousboe et al, 2004). To the extent that GABA is taken up by the astrocytes, maintenance of GABA homeostasis requires a transfer of glutamine from astrocytes to neurons, a process which is likely quantitatively coupled to neuronal activity and restricted to the synaptic area (Behar and Rothman, 2001). Thus, the 13C labeling in GABA from [1,2-13C]acetate is assumed to reflect only neurotransmitter GABA synthesized from astrocyte-derived glutamine and the observed decrease in the amount of [1,2-13C]GABA in the GAD65-deficient mouse therefore is in line with the localization of GAD65 in nerve terminals. This observation emphasizes the importance of glutamine as precursor for de novo synthesis of vesicular GABA in vivo as reported previously (Patel et al, 2005). However, the relatively significant amount of [1,2-13C]GABA synthesized in both cerebral cortex and hippocampus of GAD65 knockout mice demonstrates that not only GAD65, but also GAD67, catalyzes the synthesis of GABA from glial precursors. That GAD67 contributes to vesicular GABA synthesis would be compatible with the finding that inhibitory postsynaptic currents were sustained in GAD65 knockout mice (Tian et al, 1999; Kubo et al, 2009). In addition to the direct synthesis of [1,2-13C]GABA from [4,5-13C]glutamine, GABA may be formed from astrocytic glutamine via TCA cycle metabolism (Waagepetersen et al, 1999b). Conversion of glutamine into GABA via this pathway appears to be governed predominantly by GAD67 as observed by the similar amounts of [3-13C]GABA and [4-13C]GABA in GAD65 knockout and wild-type mice. In contrast, the fraction of glutamine converted to GABA via direct synthesis was reduced from 67% in wild-type mice to ∼47% in mice lacking GAD65. These observations underline a functional separation of the GABA synthesizing machinery, GAD65 being important mainly for direct synthesis of GABA from glutamine (pathway 1 in Figure 6). However, GAD67 is important for the synthesis of GABA from glutamine both via the direct way (pathway 1 in Figure 6) and via the TCA cycle (pathway 2 in Figure 6). It should be noted that although the expression of GAD67 is unchanged in GAD65 knockout mice, this isozyme might undergo adaptive compensatory changes, which could complicate the interpretation of the obtained results.
Figure 6.
Schematic illustration of synthesis of γ-aminobutyric acid (GABA) from glutamine directly and via tricarboxylic acid (TCA) cycle metabolism. Glutamine is converted to glutamate by phosphate-activated glutaminase (PAG) and pathway 1 illustrates direct synthesis of GABA from glutamate in the cytosol. GABA synthesis via this pathway is as indicated governed by both GAD65 and GAD67. Pathway 2 shows the deamination of glutamate and metabolism of α-ketoglutarate in the TCA cycle before GABA synthesis catalyzed by GAD67. GAD, glutamate decarboxylase.
Although the synthesis of GABA from glutamine is likely confined to the synaptic area (Bradford et al, 1978), the 13C labeling from glucose represents GABA synthesis taking place ubiquitously in the GABAergic neuron. Moreover, the labeling from glucose maps glycolytic activity and TCA cycle metabolism. In hippocampus, only the enrichment in monolabeled GABA was affected by lack of GAD65. The lack of hypometabolism in the hippocampal region is possibly related to a higher neuronal activity associated with the pro-convulsive state of the knockout animals. In contrast, labeling from glucose was dramatically decreased in lactate and alanine in cerebral cortex reflecting attenuated glycolysis. In accordance with this, oxidative metabolism via the TCA cycle was also decreased in cortex of GAD65 knockout mice. This scenario implies a severe neuronal hypometabolism in cortex of GAD65 knockout mice that can explain the decreased 13C enrichment seen in all metabolites including GABA. Interestingly, hypometabolism has previously been associated with various neurological disorders including schizophrenia, epilepsy, and depression (Tamminga and Holcomb, 2005; Kondziella et al, 2007). In fact, GAD65 knockout mice were shown to exhibit pronounced deficits in prepulse inhibition (Heldt et al, 2004), a phenomenon well characterized in schizophrenia (Geyer, 2006). This implies a close relationship between metabolic and synaptic events associated with neurological disorders.
Altogether, we have provided evidence for a significant role of GAD65 in GABA homeostasis. It was revealed that GAD65 is crucial for maintenance of biosynthesis of synaptic GABA from astrocytic glutamine. In this regard, GAD65 is important specifically for synthesis of GABA from glutamine via direct synthesis, whereas GAD67 is important for GABA synthesis from glutamine both via the direct way and via a pathway involving TCA cycle metabolism.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from manufacturer Vilhelm Pedersen and wife Memorial Legacy, a support granted on recommendation from the Novo Nordisk Foundation, the Novo Nordisk, the Hørslev, and the Lundbeck Foundations.
References
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Battaglioli G, Martin DL. GABA synthesis in brain slices is dependent on glutamine produced in astrocytes. Neurochem Res. 1991;16:151–156. doi: 10.1007/BF00965703. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL. In vivo nuclear magnetic resonance studies of glutamate-gamma-aminobutyric acid-glutamine cycling in rodent and human cortex: the central role of glutamine. J Nutr. 2001;131:2498S–2504S. doi: 10.1093/jn/131.9.2498S. [DOI] [PubMed] [Google Scholar]
- Bogen IL, Risa O, Haug KH, Sonnewald U, Fonnum F, Walaas SI. Distinct changes in neuronal and astrocytic amino acid neurotransmitter metabolism in mice with reduced numbers of synaptic vesicles. J Neurochem. 2008;105:2524–2534. doi: 10.1111/j.1471-4159.2008.05344.x. [DOI] [PubMed] [Google Scholar]
- Bradford HF, Ward HK, Thomas AJ. Glutamine—a major substrate for nerve endings. J Neurochem. 1978;30:1453–1459. doi: 10.1111/j.1471-4159.1978.tb10477.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury GM, Gupta M, Gibson KM, Patel AB, Behar KL. Altered cerebral glucose and acetate metabolism in succinic semialdehyde dehydrogenase-deficient mice: evidence for glial dysfunction and reduced glutamate/glutamine cycling. J Neurochem. 2007;103:2077–2091. doi: 10.1111/j.1471-4159.2007.04887.x. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest thatthe two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- Gram L, Larsson OM, Johnsen AH, Schousboe A. Effects of valproate, vigabatrin and aminooxyacetic acid on release of endogenous and exogenous GABA from cultured neurons. Epilepsy Res. 1988;2:87–95. doi: 10.1016/0920-1211(88)90024-1. [DOI] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps. Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab. 1997;17:1230–1238. doi: 10.1097/00004647-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziella D, Alvestad S, Vaaler A, Sonnewald U. Which clinical and experimental data link temporal lobe epilepsy with depression. J Neurochem. 2007;103:2136–2152. doi: 10.1111/j.1471-4159.2007.04926.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Nishikawa K, Ishizeki J, Hardy-Yamada M, Yanagawa Y, Saito S. Thermal hyperalgesia via supraspinal mechanisms in mice lacking glutamate decarboxylase 65. J Pharmacol Exp Ther. 2009;331:162–169. doi: 10.1124/jpet.109.156034. [DOI] [PubMed] [Google Scholar]
- Laake JH, Takumi Y, Eidet J, Torgner IA, Roberg B, Kvamme E, Ottersen OP. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 1999;88:1137–1151. doi: 10.1016/s0306-4522(98)00298-x. [DOI] [PubMed] [Google Scholar]
- Martin DL, Martin SB, Wu SJ, Espina N. Regulatory properties of brain glutamate decarboxylase (GAD): the apoenzyme of GAD is present principally as the smaller of two molecular forms of GAD in brain. J Neurosci. 1991;11:2725–2731. doi: 10.1523/JNEUROSCI.11-09-02725.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Rothman DL, Behar KL, Shulman RG. NMR determination of the TCA cycle rate and α-ketoglutarate/glutamate exchange rate in rat brain. J Cereb Blood Flow Metab. 1992;12:434–447. doi: 10.1038/jcbfm.1992.61. [DOI] [PubMed] [Google Scholar]
- Mawhinney TP, Robinett RS, Atalay A, Madson MA. Analysis of amino acids as their tert.-butyldimethylsilyl derivatives by gas-liquid chromatography and mass spectrometry. J Chromatogr. 1986;358:231–242. doi: 10.1016/s0021-9673(01)90333-4. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Different neuronal localization of aspartate-like and glutamate-like immunoreactivities in the hippocampus of rat, guinea-pig and Senegalese baboon (Papio papio), with a note on the distribution of gamma-aminobutyrate. Neuroscience. 1985;16:589–606. doi: 10.1016/0306-4522(85)90194-0. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46:519–534. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci USA. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Haberg A, Haraldseth O, Unsgard G, Sonnewald U. (13)C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci. 2000;22:429–436. doi: 10.1159/000017472. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sheikh SN, Martin SB, Martin DL. Regional distribution and relative amounts of glutamate decarboxylase isoforms in rat and mouse brain. Neurochem Int. 1999;35:73–80. doi: 10.1016/s0197-0186(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci USA. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG. In vivo (13)C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during [2-13C]glucose infusion. J Neurochem. 2001;76:975–989. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Kondziella D. Neuronal glial interaction in different neurological diseases studied by ex vivo 13C NMR spectroscopy. NMR Biomed. 2003;16:424–429. doi: 10.1002/nbm.837. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A, Svendsen JS, Unsgard G, Petersen SB. Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem Int. 1993;22:19–29. doi: 10.1016/0197-0186(93)90064-c. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10:27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci USA. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg CJ, Krzalic L, Mela P, Waelsch H. Compartmentation of glutamate metabolism in brain. Evidence for the existence of two different tricarboxylic acid cycles in brain. Biochem J. 1969;113:281–290. doi: 10.1042/bj1130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Schousboe A. The GABA paradox: multiple roles as metabolite, neurotransmitter, and neurodifferentiative agent. J Neurochem. 1999a;73:1335–1342. doi: 10.1046/j.1471-4159.1999.0731335.x. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. Synthesis of vesicular GABA from glutamine involves TCA cycle metabolism in neocortical neurons. J Neurosci Res. 1999b;57:342–349. [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Gegelashvili G, Larsson OM, Schousboe A. Metabolic distinction between vesicular and cytosolic GABA in cultured GABAergic neurons using 13C magnetic resonance spectroscopy. J Neurosci Res. 2001;63:347–355. doi: 10.1002/1097-4547(20010215)63:4<347::AID-JNR1029>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]