Abstract
The pathogenesis of cerebral malaria (CM) remains largely unknown. There is growing evidence that combination of both parasite and host factors could be involved in blood–brain barrier (BBB) breakdown. However, lack of adequate in vitro model of human BBB so far hampered molecular studies. In this article, we propose the use of hCMEC/D3 cells, a well-established human cerebral microvascular endothelial cell (EC) line, to study BBB breakdown induced by Plasmodium falciparum-parasitized red blood cells and environmental conditions. We show that coculture of parasitized erythrocytes with hCMEC/D3 cells induces cell adhesion and paracellular permeability increase, which correlates with disorganization of zonula occludens protein 1 expression pattern. Permeability increase and modification of tight junction proteins distribution are cytoadhesion independent. Finally, we show that permeability of hCMEC/D3 cell monolayers is mediated through parasite induced metabolic acidosis, which in turns correlates with apoptosis of parasitized erythrocytes. This new coculture model represents a very useful tool, which will improve the knowledge of BBB breakdown and the development of adjuvant therapies, together with antiparasitic drugs.
Keywords: brain endothelial cell line, cerebral malaria, human ICAM-1, metabolic acidosis, permeability, Plasmodium falciparum
Introduction
Malaria is a major public heath issue in developing countries with almost 1 million deaths per year (WHO, 2008). Plasmodium falciparum infection causes a range of clinical symptoms, from asymptomatic or mild flu-like illness to complications of severe disease, including severe anemia, respiratory complications, metabolic acidosis, multiorgan failure, and cerebral malaria (CM) (Dondorp et al, 2008). The pattern of vital organ dysfunction is different in adults and children, although CM is common at all age groups (White and Ho, 1992). The disease has a high mortality rate even when treated actively with antiparasite medication, supportive intensive care, and active management of associated complications (Mishra and Newton, 2009).
Despite the effort of the scientific community, the pathogenesis of CM remains unknown. Several studies have pointed out that sequestration of parasitized red blood cells (PRBCs) is at the origin of this pathology, as it results in microcirculatory obstruction, decreased oxygen delivery, tissue hypoxia, metabolic acidosis, hyperlactatemia, and organ damage. This phenomenon has been reported to be higher in the brain of patients dying from CM than in other organs in the same patient and also higher than in patients without CM (MacPherson et al, 1985; Pongponratn et al, 1991). Therefore, PRBCs sequestration is necessary to the pathogenesis, but there is also evidence that it might not be sufficient by itself in initiating or maintaining the processes leading to CM and death, as demonstrated by the presence of sequestered PRBCs in non-CM cases (MacPherson et al, 1985; Montgomery et al, 2006).
There is growing evidence that a combination of both parasite and host environment could be implied in the blood–brain barrier (BBB) breakdown and in the pathogenesis of CM (Medana and Turner, 2006). Immunohistochemical and ultrastructural studies of postmortem brain tissue have shown brain endothelial cell (EC) activation, with formation of villous processes, which adhere to sequestered parasites (Brown et al, 1999a; MacPherson et al, 1985). It has also been described an upregulation of constitutively expressed molecules, such as human intercellular adhesion molecule 1 (hICAM-1) (Chakravorty and Craig, 2005; Tripathi et al, 2006), and reduction of junctional proteins expression at the BBB endothelium (Brown et al, 1999a; Pongponratn et al, 2003). Furthermore, the fact that plasma proteins leakage has been observed into the central nervous system (Boonpucknavig et al, 1990; Brown et al, 1999b) confirms that there is an alteration in the BBB integrity.
Until now, the hypothesis of the implication of PRBC interaction with the EC in BBB dysfunction has been difficult to study because of the lack of an adequate in vitro model of human BBB. To overcome this limitation, recent studies have used primary EC from either animal origin, such as porcine (Treeratanapiboon et al, 2005), simian cells (Gay et al, 1995), or human origin from different organs, including skin (Yipp et al, 2003), lung (Taoufiq et al, 2008), and brain (Tripathi et al, 2007). Nevertheless, the procedure of cell isolation remains a delicate and time-consuming step. Furthermore, it is not systematically reproducible in terms of quality, as it depends on the histological state of organs, and the time between death and cell isolation.
New progresses have been recently achieved in the generation of a new human cerebral EC line named hCMEC/D3. These brain cells have been immortalized and have been shown to display a large number of human BBB characteristics, including tight junction proteins, adhesion molecules, chemokines receptors, and multidrug resistance proteins expression (Weksler et al, 2005). This cell line is widely used as a powerful tool to mimic BBB behavior in a large number of fields: studies of pathogens behavior, HIV (Afonso et al, 2008), meningoccocus (Coureuil et al, 2009), pathologies (prion protein) (Viegas et al, 2006), multiple sclerosis (Rampon et al, 2008), stroke (Cucullo et al, 2008), Alzheimer's disease (Vukic et al, 2009), inflammatory situation, and signaling pathway (tight junction regulation) (Eum et al, 2008; Sade et al, 2009).
We propose here the use of this well-established human cerebral EC line hCMEC/D3 to explore the implication of parasite factors and environmental conditions in the BBB breakdown and in the pathogenesis of CM.
Materials and methods
Cell Culture
The hCMEC/D3 cells were cultured on collagen type I (BD Biosciences, Bedford, MA, USA)-coated culture dishes at 37°C and 5% CO2 as described (Afonso et al, 2008). The EC culture medium (ECCm) contained EBM-2 (endothelial basal medium) basal medium (Lonza, Walkersville, MD, USA), supplemented with 5% heat-inactivated fetal bovine serum (Gibco), 10 mmol/L HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco, Paisley, UK), 1.4 μmol/L hydrocortisone, 5 μg/mL ascorbic acid, and 1 ng/mL basic fibroblast growth factor (Sigma-Aldrich, Steinheim, Germany). Mouse brain EC bEnd5 cell line was a generous gift from Professor Engelhardt (Theodor Kocher Institut, University of Bern) and was cultured on gelatine (Sigma-Aldrich)-coated cell culture dishes in DMEM (Dulbecco's Modified Eagle Medium) (4.5 g/L glucose) supplemented with 10% fetal bovine serum, 2 mmol/L -glutamine, 1 × MEM nonessential amino acids, 1 mmol/L sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco), and 50 μmol/L 2-β-mercaptoethanol (Sigma-Aldrich).
P. falciparum Parasite Culture and Tryspin Treatment
P. falciparum RAOL isolate was obtained from a patient who suffered from severe malaria, kindly supplied by Dr Pierre Buffet (Institute Pasteur, Paris, France). Parasites were cultured at a hematocrit ranging from 2.5% to 8% with human RBCs. Parasite culture medium (PCm) was composed of RPMI (Roswell Park Memorial Institute) 1640 medium supplemented with -glutamine, 25 mmol/L HEPES (Gibco), 10% human serum (Etablissement Français du Sang), 0.1 mmol/L hypoxanthine (Sigma-Aldrich), 0.22% sodium bicarbonate (Gibco), and 20 μg/mL gentamicin (Gibco). Parasites were cultured in a humidified chamber at 37°C under 3% CO2, 1% oxygen, and 96% azote.
For trypsin treatment, parasites were washed in Hank's balanced salt buffer, after gelatine enrichment, and incubated either in buffer or in 0.5 mg/mL trypsin/EDTA (ethylenediaminetetraacetic acid) at 10% final hematocrit for 5 minutes at room temperature. Reaction was stopped by adding RPMI 1640/5% fetal bovine serum medium.
Cytoadhesion Assay
The hCMEC/D3 cells were seeded onto collagen type I-coated eight-well Lab-Tek chamber slides (Nunc, Rochester, NY, USA), grown in ECCm to confluence and stimulated with 150 U/mL of tumor necrosis factor-α (TNFα) (Biosource, Camarillo, CA, USA) for 24 hours before the assay. Cells were fixed with 4% paraformaldehyde for 15 minutes and kept at 4°C until used. Mature forms (late trophozoites and schizonts) of P. falciparum PRBCs were obtained after gelatine enrichment. These mature forms were cocultured with TNFα-stimulated hCMEC/D3 cells at a given parasitemia and hematocrit for 1 hour at 37°C and 5% CO2. Parasite load used were calculated as parasites/cm2 of culture support. In all, 5 × 107/cm2 was the starting parasite concentration, equivalent to 60% parasitemia and 5% hematocrit. The assays were performed using the cytoadherence culture medium, in which composition was similar to PCm but without bicarbonate (pH 6.8 to 6.9, which favors PRBC binding). During coincubation, gentle agitation was performed every 15 minutes. At the end of the incubation, cultures were washed in RPMI 1640 medium to remove unfixed PRBCs, by removing Lab-Tek wells. The slides were gently washed up and down in the medium, fixed in 2% glutaraldehyde, and stained with Giemsa (CML). The washing step was controlled by using nonparasitized RBCs, which do not adhere to the cells (negative control). In each experiment, we conclude that nonattached parasitized and nonparasitized RBCs were removed, when after at least three washing steps, there was any RBC in the control well. This was verified by microscopy.
Alternatively, cells nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) after fixation. In parallel, gelatine-enriched parasites were stained with 2 μmol/L (((4-chloromethyl) benzoyl) amino) tetramethylrhodamine, orange cell tracking dye (Interchim, Montluçon, France), according to the manufacturer's instructions. Stained cells and PRBCs were incubated for 1 hour in cytoadherence culture medium at 37°C and 5% CO2, with gentle agitation every 15 minutes. Cocultures were washed in RPMI 1640 medium, air-dried, methanol fixed, and mounted for fluorescence microscopy analysis. For each well, 16 homogeneously distributed fields (magnification × 200) were imaged under the red filter (band pass, BP 610/75 nm) for PRBCs and under DAPI filter (BP 470/40 nm) for nuclei using a Leica epifluorescence microscope (Leica DMI 4000B, Leica Microsystems, Wetzlar, Germany) and a Zeiss camera (AxioCam MRc5, Oberkochen, Germany). In each wavelength, four images were assembled together using Zeiss software mounting tool and resulting TIFF images were analyzed using Image J software. The number of adherent PRBCs was counted on red filter images, and cells were counted on DAPI filter images by counting nuclei. The results were expressed as the number of adherent PRBCs per adherent cell.
Parasite Panning
Gelatine-enriched RAOL PRBCs were coincubated with TNFα-stimulated hCMEC/D3 cells in cytoadherence culture medium at 5% hematocrit for 1 hour at 37°C and 5% CO2, with gentle agitation every 15 minutes. Nonadherent parasites were removed by washing with RPMI 1640 medium. Adherent parasites were harvested by slight pipette flow, directly onto cells, with complete PCm at pH=7.18, which facilitates PRBC detachment from cells. The obtained parasites were cultured, and the medium was changed the next day.
RNA Interference
The hCMEC/D3 cells were transfected with 20 nmol/L siRNA targeting either hICAM-1 or the corresponding siRNA scramble using Lipofectamine RNAimax (Invitrogen, Carlsbad, CA, USA). Transfected cells were allowed to settle down for 4 hours and then stimulated with 150 U/mL of TNFα for 24 hours. Transfected cells were analyzed for hICAM-1 expression by flow cytometry or used for PRBCs cytoadherence assay.
Flow Cytometry Analysis
The hCMEC/D3 or bEnd5 cells were harvested from culture dishes by treatment with 0.5 mg/L heated trypsin/EDTA (Gibco) for a very short period of time (3 minutes) and stained with anti-human ICAM-1 fluorescein-conjugated monoclonal antibody, or with the isotype control antibody (R and D system, Minneapolis, MN, USA). Short-time trypsin treatment did not alter surface antigens expression. Cells were washed with phosphate-buffered saline (PBS) and fixed with 1% formaldehyde and analyzed by flow cytometry using an EPICS XL cytometer (Beckman Coulter, Villepinte, France).
Lentiviral Production and Transduction
The lentiviral construct pTRIP-CMV-hICAM-1 ΔU3 was generated by gateway recombination cloning (Invitrogen). Briefly, the hICAM-1-coding sequence was PCR amplified from a plasmid using the following primers: hICAM-1-S 5′-ATGGCTCCCAGCAGCCCCCG-3′ and hICAM-1-AS 5′-TCAGGGAGGCGTGGCTTGTG-3′. The 1.6-kb resulting PCR product was cloned into the pENTR/D/TOPO plasmid (Invitrogen) to generate entry clone. The LR clonase II (Gateway Cloning System) recombination was performed using the entry clone and the pTRIP-CMV-rfa gateway ΔU3 destination vector as described previously (Russ et al, 2008). The pTRIP-CMV-GFP ΔU3 vector was used as control. Lentiviral vector stocks were produced by transient transfection of 293T cells with the p8.91 encapsidation plasmid, the VSV glycoprotein-G-encoding pHCMV-G plasmid, and the pTrip ΔU3 lentiviral vector as previously described (Zennou et al, 2000). Supernatants were treated with DNAse I (Roche Diagnostic, Mannheim, Germany) before ultracentrifugation, and the resulting pellet was resuspended in PBS, separated into aliquots and frozen at −80°C until use.
Mouse bEnd5 cells were transduced in suspension with the lentiviral vectors premixed for 15 minutes with 10 mg/mL DEAE-Dextran at 37°C. Transduced mouse cells were then seeded onto gelatine-coated culture dish in a minimal volume of bEnd5 culture medium. One hour on transduction, fresh medium was added. Two days after transduction, cells were either analyzed by flow cytometry for hICAM-1 expression or used for cytoadherence assays.
Confocal Microscopy Analysis
The hCMEC/D3 or bEnd5 cells were trypsinized and stained using an anti-hICAM-1 antibody (BioVision, Mountain View, CA, USA), followed by a phycoerythrin-conjugated secondary antibody (Sigma-Aldrich). Cells were fixed with 1% formaldehyde, mounted, and analyzed. For tight junction proteins expression, hCMEC/D3 cells were seeded onto collagen type I-coated Lab-Tek chamber slides, grown for 12 days and fixed with 4% paraformaldehyde for 10 minutes at room temperature. Cells were then permeabilized with 0.2% Triton X100 in PBS for 10 minutes, blocked in 3% bovine serum albumin/PBS for 30 minutes, and stained with an anti-zonula occludens protein 1 (ZO1) antibody for 2 hours at room temperature followed by incubation with an Alexa Fluor 488 secondary antibody (Invitrogen, Eugene, OR, USA). Cells were then washed three times with PBS, mounted in Immuno Mount (Thermo Scientific, Pittsburgh, PA, USA), and analyzed using an Olympus fluorescence confocal microscope (Olympus Europa GmbH, Hamburg, Germany).
Permeability Assay
The hCMEC/D3 cells were seeded onto 12 mm-collagen/70% ethanol-coated Millicell cell culture inserts (0.4 μm; Millipore, Carrigtwohill, Ireland). To allow a better tightness of the junctions between cells, they were cultured in ECCm for 12 days. Cells were TNFα stimulated for 24 hours before the assay. Gelatine-enriched mature parasites, at late trophozoite and schizont stages, were added in the upper chamber (luminal compartment) at a given hematocrit and parasitemia (8 × 107/cm2 was the starting parasite concentration, equivalent to 50% parasitemia and 2.5% hematocrit). To carry out coculture experiment during 20 hours, a modified culture medium (MCm) was used. The MCm contained RPMI 1640 medium supplemented with -glutamine, 35 mmol/L HEPES (Gibco), 5% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco), 1.4 μmol/L hydrocortisone, 5 μg/mL ascorbic acid, and 1 ng/mL basic fibroblast growth factor (Sigma-Aldrich). This medium lacks bicarbonate and shows reduced buffer efficiency when compared with PCm. The PRBCs and RBCs were washed and Lucifer Yellow (Sigma-Aldrich) was added to the luminal compartment. The insert with cells was changed in four wells successively every 15 minutes for 1 hour, and the lower chamber (abluminal compartment) was sampled each 15 minutes to determine Lucifer Yellow passage through the culture insert. Same experiment was performed in parallel with coated culture insert alone. Samples obtained were analyzed using a florescence multiwell-plate reader (FlexStation 3, Molecular Devices, Sunnyvale, CA, USA) at 430 and 530 nm wavelengths for excitation and emission, respectively. Permeability was expressed as the percentage of fluorescence compared with the insert alone.
Lactate Dehydrogenase Assay
Parasite lactate dehydrogenase was measured following the manufacturer's instructions (DiaMed-France S.A., Chambly, France).
Merozoites Purification
The PRBCs infected with mature forms at 50% parasitemia were cultured at 2.5% hematocrit in MCm for 20 hours to obtain the equivalent number of merozoites obtained when PRBCs 8 × 107/cm2 density were added to the culture during 20 hours. After parasites burst, supernatants were harvested and centrifuged at 1800 g. (Jouan GR412, Saint-Herblain, France) to remove the remaining cells. Pellet was discarded, and the supernatant was centrifuged at 3000 g. for 10 minutes at room temperature. Merozoites were resuspended in MCm.
Annexin V Staining
The PRBCs apoptosis was measured by annexin staining following the manufacturer's recommendation. The PRBCs were washed with ice-cold-binding buffer and stained with annexin V for 15 minutes in the dark. After washing, PRBCs were stained with propidium iodide. Samples were maintained on ice until analyzed by flow cytometry using an EPICS XL cytometer.
Statistical Analysis
Mean and standard deviation have been determined after 3 independent experiments. For multiple comparisons, analysis of variance followed by post hoc analysis with Tukey test was used. Levels of significance are denoted as follows: *P≤0.05, **P≤0.01, and ***P≤0.001.
Results
hCMEC/D3 Cells Allow Parasitized Red Blood Cell Cytoadherence
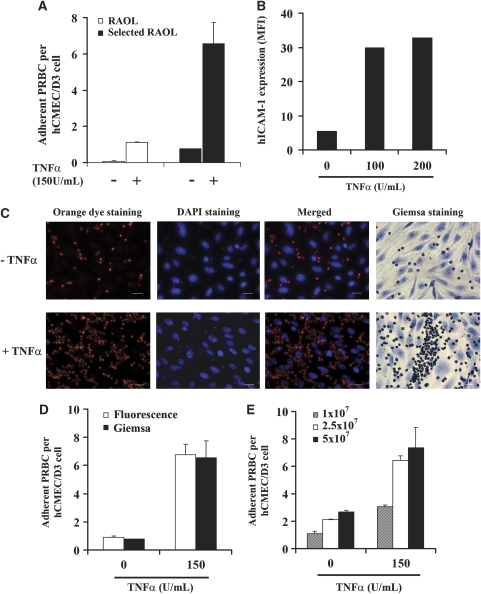
A cytoadherence assay was performed to determine whether the P. falciparum RAOL isolate could adhere to hCMEC/D3 cells. Figure 1A shows that hCMEC/D3 cells very weakly support P. falciparum PRBCs cytoadherence when unstimulated. The TNFα was used to reproduce in vivo conditions described in CM cases (Grau et al, 1989) and which modulates adhesion molecules expression. Cell stimulation with 150 U/mL of TNFα largely increases their ability to support PRBCs cytoadherence (one PRBC per cell). To better analyze cytoadherence mechanisms and subsequent effects, adherent parasites were selected by four successive rounds of cytoadherence assays using TNFα-stimulated cells and the panning method. The cytoadherence of the selected PRBCs was greatly enhanced on both unstimulated and TNFα-stimulated cells, with up to 6.6 PRBCs per cell on stimulated cells, compared with the nonselected one (Figure 1A). This selected isolate was used in the following experiments. The increase in PRBCs cytoadherence was concomitant with an increase in the expression of the adhesion molecule ICAM-1 on hCMEC/D3 cells after TNFα treatment. Flow cytometry analysis shows that stimulation of hCMEC/D3 cells with 100 U/mL of TNFα strongly increases hICAM-1 basal expression, more than fivefold (Figure 1B); stimulation of cells with 200 U/mL of TNFα does not significantly modify hICAM-1 expression compared with 100 U/mL dose. From these data, the dose of 150 U/mL was used throughout this study.
Figure 1.
Plasmodium falciparum-parasitized red blood cells (RBCs) adhere to hCMEC/D3 cells. (A) In all, 5 × 107/cm2 P. falciparum RAOL isolate parasitized RBCs (PRBCs) (60% parasitemia and 5% hematocrit) were cocultured for 1 hour with hCMEC/D3 cells, stimulated or not with 150 U/mL of tumor necrosis factor (TNFα). White bars denote cytoadherence of nonselected parasite isolate and black bars denote cytoadherence of selected RAOL isolate. (B) Flow cytometry analysis of human intercellular adhesion molecule 1 (hICAM-1) expression in hCMEC/D3 cell line, stimulated or not with TNFα for 24 hours. MFI, mean fluorescence intensity. (C) Fluorescent staining of PRBCs (orange dye) and hCMEC/D3 cells stimulated or not with TNFα for 24 hours (DAPI staining). Emission wavelengths of the orange dye and DAPI were 561 and 460 nm, respectively. Classical Giemsa staining is also shown. The scale represents 20 μm. (D) In all, 5 × 107/cm2 PRBCs and hCMEC/D3 cells were cocultured as described in section A and the cytoadherence was measured using the new fluorescence and Giemsa protocols. White bars denote cytoadherence quantification with the new fluorescence method and black bars denote cytoadherence quantification with the Giemsa staining. (E) Different doses of P. falciparum selected RAOL isolate PRBCs were cocultured with hCMEC/D3 cells, stimulated or not with TNFα for 24 hours, and PRBCs cytoadherence was assessed. Hatched bars represent cytoadherence quantification with a parasite load of 1 × 107/cm2 (60% parasitemia and 1% hematocrit), 2.5 × 107/cm2 (60% parasitemia and 2.5% hematocrit) for white bars, and 5 × 107/cm2 (60% parasitemia and 5% hematocrit) for black bars (s.d. is shown for n=3). DAPI, 4′,6-diamidino-2-phenylindole.
For a more rapid analysis of cytoadherence assay, we set up a new fluorescence-based detection protocol. The PRBCs were stained with the orange cell tracking dye and detected at 561 nm wavelength. hCMEC/D3 nuclei were stained with DAPI and detected at 460 nm wavelength (Figure 1C). The control Giemsa staining is also shown in Figure 1C. A semiautomatic quantification of the number of adherent PRBCs per hCMEC/D3 cell was obtained using Image J software. Figure 1D shows that both methods of cytoadherence quantification in nonstimulated or TNFα-stimulated cells yielded similar results. We further analyzed whether low doses of PRBCs are able to adhere to nonstimulated or stimulated hCMEC/D3 cells. As shown in Figure 1E, the lowest dose of PRBCs tested allows their adherence to nonstimulated hCMEC/D3 cells. The number of adherent PRBCs obtained using 2.5 × 107 PRBCs/cm2 increases by 2.5-fold compared with the adherent PRBCs observed using 1 × 107 PRBCs/cm2. Similar results were obtained using TNFα-stimulated hCMEC/D3 cells (Figure 1E). This result shows that hCMEC/D3 cells can support PRBCs cytoadherence even with low initial parasite load.
Human Intercellular Adhesion Molecule 1 Is Involved in Parasitized Red Blood Cells Cytoadherence to hCMEC/D3 Cells
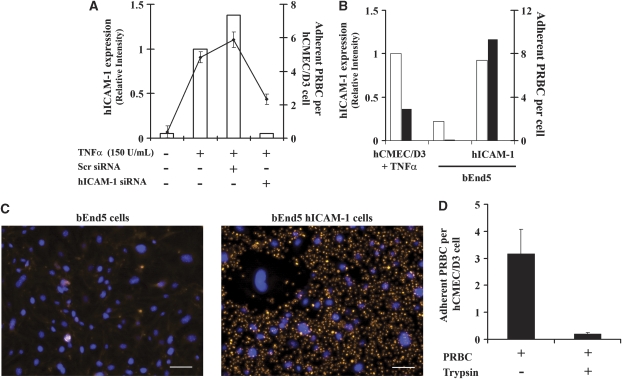
To determine the adhesion molecule(s) responsible for P. falciparum RAOL isolate cytoadherence to hCMEC/D3 cells, we used two different approaches for inhibition or induction of hICAM-1 expression: the siRNA and the lentiviral approaches. We were interested in hICAM-1, because it is one of the main adhesion molecules implicated in P. falciparum cytoadherence, particularly in the brain, and a target for new therapeutic approaches (Cojean et al, 2008; Turner et al, 1994; Wassmer et al, 2005). In the first case, hCMEC/D3 cells were transfected with a siRNA targeting hICAM-1 or an irrelevant siRNA (scramble (SCR) siRNA). The inhibition of hICAM-1 expression was evaluated by flow cytometry 48 hours on transfection of TNFα-stimulated cells (Figure 2A). The hICAM-1 siRNA-transfected cells show a 96% reduction of hICAM-1 expression compared with cells transfected with SCR siRNA. Transfected cells were then assayed for their ability to support PRBCs cytoadherence. As shown in Figure 2A, SCR siRNA does not affect PRBCs adherence to TNFα-stimulated hCMEC/D3 cells, whereas hICAM-1 siRNA-transfected hCMEC/D3 cells show a 2.5-fold reduction of PRBCs adherence.
Figure 2.
Plasmodium falciparum RAOL adhere to hCMEC/D3 cells mainly through human intercellular adhesion molecule 1 (hICAM-1). (A) The hCMEC/D3 cells were treated or not with 20 nmol/L of SCR siRNA or hICAM-1 siRNA for 48 hours and then hICAM-1 expression, as well as 2.5 × 107/cm2 (60% parasitemia and 2.5% hematocrit) parasitized red blood cells (PRBCs) adherence, was analyzed. The black line represents the adherence of PRBCs to hCMEC/D3 cells. Flow cytometry data are represented as the relative intensity with respect to tumor necrosis factor (TNFα)-stimulated hCMEC/D3 cells. (B) Mouse bEnd5 cells were transduced with lentiviral vectors bearing hICAM-1 sequence. The hICAM-1 expression in transduced cells (white bars), as well as PRBCs cytoadherence (2.5 × 107/cm2) (black bars), is shown. Flow cytometry data are represented as the relative intensity with respect to TNFα-stimulated hCMEC/D3 cells. (C) In all, 2.5 × 107/cm2 PRBCs were cocultured with control bEnd5 mouse cells or hICAM-1-transduced bEnd5 cells. Cytoadherence was detected by fluorescence, using a DMI 4000B Leica microscope, and imaged with an AxioCam MRc5 Zeiss camera. Blue staining, cell nuclei, Orange staining, PRBCs. The bar represents 50 μm. (D) In all, 2.5 × 107/cm2 PRBCs were treated or not with trypsin and then cocultured with hCMEC/D3 cells. After 20 hours of coculture, cytoadherence was estimated. SCR, scramble.
To further confirm these results, the mouse brain EC line bEnd5 was transduced using a lentiviral vector containing hICAM-1 sequence and assayed for P. falciparum PRBCs cytoadherence. Figure 2B shows the high level of hICAM-1 expression in transduced bEnd5 cells compared with control nontransduced cells, which reaches a similar magnitude than hICAM-1 in TNFα-stimulated hCMEC/D3 cells. In addition, hICAM-1-transduced cells are able to support PRBCs cytoadherence, whereas nontransduced bEnd5 cells do not (Figures 2B and 2C).
To go deeper into the mechanism of PRBCs cytoadherence, PRBCs were treated with trypsin to eliminate trypsin-sensitive P. falciparum antigens expressed on the surface of these cells. Results from Figure 2D show that a trypsin-sensitive antigen is involved in PRBCs cytoadherence as trypsin treatment strongly reduces the ability of PRBCs to adhere to EC, even on 20 hours of coculture. These results suggest that hICAM-1, expressed by hCMEC/D3 cells, and a trypsin-sensitive antigen, expressed by P. falciparum, are involved in cytoadherence.
Parasitized Red Blood Cells Induce Endothelial Cell Permeability Enhancement and Modification of Tight Junction Proteins Expression
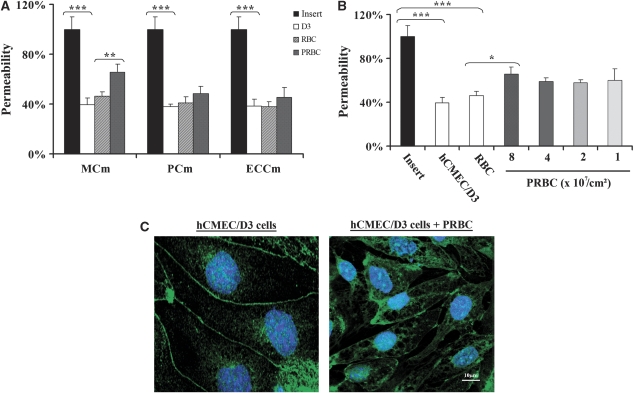
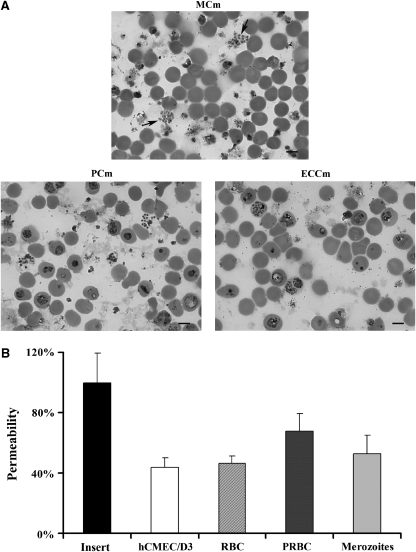
The following step of this study was to determine whether hCMEC/D3 cells could be used as a model to study the BBB breakdown triggered by PRBCs. The first parameter analyzed was the culture medium in which PRBCs and hCMEC/D3 cells could be cocultured. Cells were cultured alone or cocultured with RBCs or PRBCs in two-chamber culture inserts and the dye passing through was recovered in the lower chamber and analyzed 20 hours after coculture. We tested three different culture media: ECCm, PCm, and an MCm. Figure 3A shows that hCMEC/D3 cells form, as expected, a semipermeable barrier that reduces by 60% the amount of dye passing through the insert in all culture media analyzed. Coculture of hCMEC/D3 cells with RBCs does not modify significantly cell permeability compared with control cells. In contrast, coculture of cells with PRBCs induces a significant increase of the permeability, 43% in the MCm compared with cells cocultured with RBCs. No significant effect was observed when cells were cocultured in PCm or ECCm. As a consequence, MCm was used through the coculture experiments. We next analyzed the effect of parasite concentration on the permeability modification. Figure 3B shows that doses ranging from 1 to 4 × 107 parasites/cm2 have similar effect on the modification of the permeability. A higher dose (8 × 107 parasites/cm2) of parasite significantly increases cell permeability.
Figure 3.
Effect of Plasmodium falciparum RAOL isolate parasitized red blood cells (PRBCs) on hCMEC/D3 cell permeability. (A) The hCMEC/D3 cells were cultured or cocultured with RBCs or 8 × 107/cm2 PRBCs (50% parasitemia and 2.5% hematocrit) in 12 mm-collagen/70% ethanol-coated Millicell cell culture insert in the presence of different culture media for 20 hours, and then permeability was estimated. Permeability is represented as the percentage of Lucifer Yellow dye in the abluminal compartment compared with the insert. Black bars denote permeability quantification with the insert alone; white bars denote permeability quantification with hCMEC/D3 cells alone; hatched bars denote permeability quantification with RBCs and gray bars denote permeability quantification with PRBCs. MCm, modified culture medium; PCm, parasite culture medium; ECCm, endothelial cell culture medium. (B) The hCMEC/D3 cells were cultured or cocultured with RBCs or PRBCs at different parasitemias (1, 2, 4, and 8 × 107/cm2; hematocrit 2.5%) in MCm for 20 hours. Permeability was estimated as above. *P<0.05, **P<0.01, and ***P<0.001. (C) Immunostaining of hCMEC/D3 cells. Cells were cultured alone or cocultured with 2.5 × 107/cm2 (50% parasitemia and 2.5% hematocrit) PRBCs and samples were stained with an anti-ZO1 antibody, followed by an Alexa Fluor 488 (Eugene, OR, USA)-conjugated secondary antibody and DAPI. Cells were then analyzed by confocal microscopy. The bar represents 10 μm. Similar results were obtained in three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole.
We further analyzed whether the PRBCs-induced permeability modification could be associated with a disturbance of tight junction proteins of hCMEC/D3 cells. The expression of a tight junction protein, the submembranous tight junction-associated ZO1 protein, was determined (Figure 3C). Confocal microscopy analysis of immunostained hCMEC/D3 cells shows that ZO1 is expressed as a continuous line at the cell–cell contact. The TNFα stimulation of cells does not modify the staining of ZO1 protein (data not shown). In contrast, coculture of hCMEC/D3 and PRBCs shows a disorganization of ZO1 staining with gaps between cells and ZO1 relocalization in the intracellular compartment.
Parasitized Red Blood Cells Induced Permeability Modification Is Cytoadherence and Contact Independent
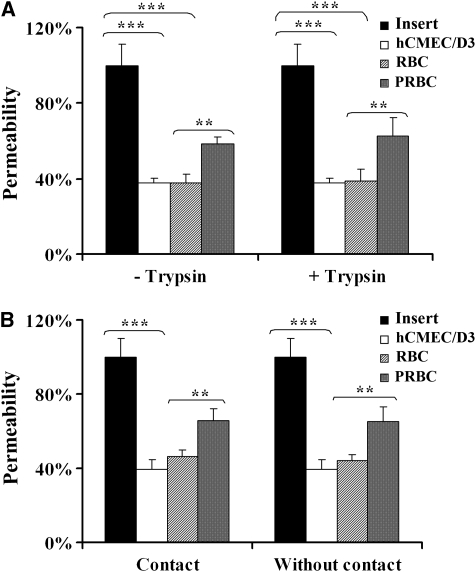
To analyze the role of cytoadherence in EC permeability, trypsin-treated and -untreated PRBCs were used in a permeability assay. Figure 4A shows that noncytoadherent trypsin-treated PRBCs are still able to induce an EC permeability increase similar to that observed in control nontrypsinized PRBCs. To exclude the hypothesis that other trypsin-resistant antigens could be involved in permeability, PRBCs were cultured in the lower chamber of the insert, preventing interaction with hCMEC/D3 cells. Figure 4B shows that independently of the contact, PRBCs could still induce permeability, compared with the control conditions.
Figure 4.
Evaluation of cytoadherence involvement in hCMEC/D3 cells permeability. (A) Parasitized red blood cells (PRBCs) (8 × 107/cm2, equivalent to 50% parasitemia and 2.5% hematocrit) were trypsinized or not and cocultured with hCMEC/D3 cells for 20 hours in modified culture medium (MCm). Percentage of cell permeability was estimated as in previous figures. (B) The hCMEC/D3 cells were cultured in contact or not with RBCs or PRBCs (8 × 107/cm2) for 20 hours and then permeability was estimated as above. Black bars denote permeability quantification with the insert alone; white bars denote permeability quantification with hCMEC/D3 cells alone; hatched bars denote permeability quantification with RBCs; and gray bars denote permeability quantification with PRBCs. *P<0.05, **P<0.01, and ***P<0.001.
Analysis of the parasite morphology after 20 hours of coculture showed that some PRBCs can mature to free merozoites (Figure 5A). Recent studies have shown that this parasite stage is implicated in the pulmonary endothelial barrier permeability (Gillrie et al, 2007). So the role of this parasite stage interacting with brain EC was also evaluated. Figure 5B shows that merozoites only induce an increase of 15% of permeability compared with cells cocultured with RBCs, whereas coculture of cells with PRBCs is able to induce an increase of 48% of the permeability compared with the control. All together, these results strongly suggest that the observed permeability is triggered through parasite cytoadherence and contact-independent mechanisms.
Figure 5.
Effect of Plasmodium falciparum merozoites in endothelial cell (EC) permeability. (A) The hCMEC/D3 cells and 8 × 107/cm2 parasitized red blood cells (PRBCs) (50% parasitemia and 2.5% hematocrit) were cocultured in the different media. After 20 hours of coculture, PRBCs smears were performed, stained with Giemsa and then analyzed by microscopy. Arrows show the presence of free merozoites in modified culture medium (MCm). Similar results were obtained in three independent experiments. The bar represents 5 μm. (B) The hCMEC/D3 cells were cultured or cocultured with RBCs, PRBCs (8 × 107/cm2) or merozoites in MCm for 20 hours. Permeability was estimated as in previous experiments (s.d. is shown for n=3).
The Role of Parasite Soluble Factors in the Endothelial Cell Permeability Enhancement
Previous studies have suggested that during parasite maturation, specific soluble factors released from PRBC mediate the decrease in human brain EC barrier resistance (Tripathi et al, 2007). Consequently, the parasite development in the different culture conditions was determined as an indirect measure of the release of parasite-specific soluble factors. Parasite development was evaluated through the measurement of the parasite lactate deshydrogenase (Figure 6A). Results show that parasite maturation, so release of specific soluble factors, was higher in the PCm and in the ECCm, both conditions, which failed to induce EC barrier permeability enhancement, than in the MCm. In parallel, PRBCs apoptosis was measured as a way to assess parasite viability in the different culture conditions. For this purpose, we performed annexin V and propidium iodide staining of PRBCs in different culture media. Figure 6B shows that PRBCs cultured in PCm and ECCm show very low level of late apoptosis, whereas PRBCs cultured in MCm show higher level of apoptosis. These results further point to a mechanism independent on a parasite-specific soluble factor.
Figure 6.
Evaluation of parasitized red blood cells (PRBCs) soluble factors release and viability on cell permeability. (A) The PRBCs (8 × 107/cm2, equivalent to 50% parasitemia and 2.5% hematocrit) were cultured for 20 hours in different culture media and parasite lactate deshydrogenase was estimated (s.d. is shown for n=3). (B) The PRBCs (8 × 107/cm2) were cultured in parasite culture medium (PCm), EC culture medium (ECCm), and modified culture medium (MCm) for 20 hours. To analyze apoptosis, PRBCs were harvested, diluted in ice-cold-binding buffer, stained with annexin V and propidium iodide, and analyzed by flow cytometry. Similar results were obtained in two independent experiments.
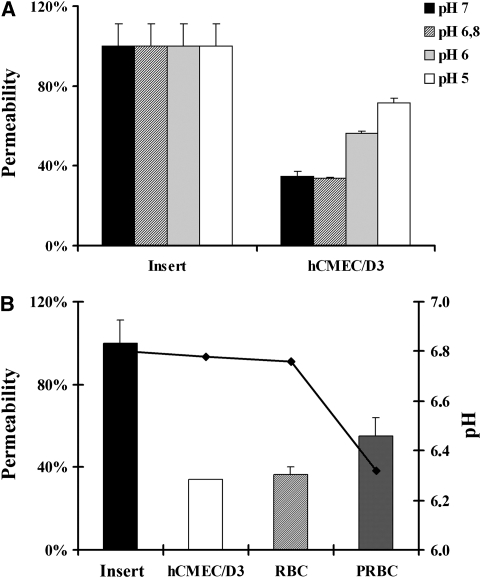
Parasitized Red Blood Cells Metabolism Induces Endothelial Cell Permeability Through the Decrease of the Environmental pH
As metabolic acidosis has been described as one of the strongest predictors of a fatal outcome in severe malaria (Dondorp et al, 2008), we decided to test whether the pH of the culture medium could be involved in the permeability enhancement observed. Results show that a decrease in the pH of the culture medium was correlated with an increase in the EC barrier permeability (Figure 7A). Consequently, we decided to study whether parasite metabolism could be responsible for an increase in the acidosis of the culture environment and associated with the permeability observed. Figure 7B shows the relationship between EC permeability and the culture medium pH measured after 20 hours of culture in the different conditions of the study. Results show a decrease in the culture medium pH because of the parasite metabolism when compared with the pH measured in the insert or in the EC conditions. Furthermore, this pH decrease was concomitant with the increase of the EC permeability. These results can be explained by the fact that MCm has a severe limitation in the buffer efficiency. This statement was determined comparing the final pH of the coculture in PCm and MCm. Difference between initial and final pH in PCm was 0.1 point, whereas in MCm it was 0.5 point. Consequently, in more alkaline media, PCm (pH=7.18) and ECCm (pH=7.31), parasite metabolism does not decrease enough the environmental pH to induce EC permeability. In contrast, in more acidic medium, MCm (pH=6.78), parasite metabolism contributes to decrease the pH and to modify EC permeability compared with that observed in the control conditions. These results strongly suggest that a slightly acidic environment together with PRBC metabolism induces EC permeability through decrease of the environmental pH.
Figure 7.
Effect of the environmental pH on endothelial cell (EC) permeability. (A) The hCMEC/D3 cells were cultured for 20 hours in modified culture medium (MCm) at different pH. Black bars denote pH medium at 7; hatched bars denote pH medium at 6.8 (MCm); gray bars denote pH medium at 6; and white bars denote pH medium at 5 (s.d. is shown for n=3). (B) The EC permeability and pH after 20 hours of hCMEC/D3 culture alone or in coculture with parasitized red blood cells (PRBCs) (8 × 107/cm2, equivalent to 50% parasitemia and 2.5% hematocrit). The bars correspond to permeability measurements and line corresponds to the pH measurements obtained in the different culture conditions.
Discussion
Cerebral malaria remains an important cause of death and therefore there is an urgent need of models to understand the pathophysiology of the disease. This study shows that hCMEC/D3 cells are a good model for studying P. falciparum-parasitized erythrocytes cytoadherence. It is also an excellent tool to study the capacity of clinical isolates to adhere on brain endothelium as well as to select cytoadherent clones present in this isolate, by panning method, for further characterization studies. This model has also permitted us to elucidate the molecular interaction between our selected P. falciparum strain and the hCMEC/D3 cell line. The remarkable capacity of these cells to withstand siRNA transfection showed that our selected strain interacts mainly with hICAM-1. This result was confirmed by the induction of P. falciparum PRBCs cytoadherence on hICAM-1-transduced mouse cells, mouse cells that are initially not permissive to PRBCs cytoadherence. These results make this model a very valuable and a powerful tool to study the molecular mechanisms underlying P. falciparum isolates cytoadherence, and opens the way to a wide range of other molecular or signaling pathways analyses. Further studies in the molecular mechanisms suggest that this interaction occurs probably via PfEMP1. We do not rule out the possibility that other trypsin-susceptible antigens would be involved in cytoadherence.
One interesting contribution of this study is that using hCMEC/D3 cell line, it has been possible to show that the selected strain, RAOL, isolated from a patient who suffered from severe malaria, induces an important permeability enhancement on the interendothelial permeability. This finding was consistent within different cell assays, suggesting that it is an intrinsic property of the parasite presence. Our results are in agreement with those previously reported, which showed the capacity of P. falciparum 3D7 PRBCs to alter BBB endothelium integrity, using primary endothelial brain cells (Tripathi et al, 2007). Furthermore, in our model, modification of permeability was concomitant with a disorganization in tight junction protein ZO1 expression pattern, as it was observed in brain tissues from fatal cases of CM (Brown et al, 1999a, 2001). All together, these results confirm the reliability of the hCMEC/D3 model to study the implication of different actors in the alteration of the BBB.
To decipher the pathways implicated in the PRBCs-induced permeability enhancement, the role of the parasite cytoadherence phenomenon was explored. Previous studies, performed using cultures of human umbilical vein EC, human dermal microvascular EC, human pulmonary EC, or animal cerebral EC lines, have suggested that PRBCs cytoadherence to EC could induce a BBB alteration via activation of intracellular signaling pathways, which leads to changes in the expression of surface adhesion molecule, release of immunoregulatory molecules, and junctions opening (Gillrie et al, 2007; Jenkins et al, 2007; Taoufiq et al, 2008; Yipp et al, 2003). In contrast, in the hCMEC/D3 model, our results showed that alteration of the paracellular permeability can be induced with noncytoadherent PRBCs, as observed in primary brain EC (Tripathi et al, 2007) or even when the PRBCs are not in contact with the cells. We then analyzed whether free merozoites that appeared after 20 hours of PRBC maturation could interact with the EC and induce the permeability enhancement as described for pulmonary EC barrier. However, this parasite stage failed to induce permeability enhancement in the hCMEC/D3 model, which suggests that the molecular mechanisms implicated in the triggering of the dermal or pulmonary permeability increase are not related with those implicated in the onset of cerebral permeability enhancement. We cannot rule out that the difference observed between our results and those obtained in the mentioned studies could rely on the different specialization of EC from different origins (Aird, 2007) or on the different parasite isolates used in each study, which can induce the alteration of the monolayer integrity by different pathways. Altogether, our results point out to a parasite cytoadherence-independent pathway.
Previous studies have proposed that parasite soluble factors such as proteins from parasite machinery released during parasite maturation and reinvasion could be at the origin of the EC barrier integrity breakdown (Gillrie et al, 2007; Tripathi et al, 2007). This hypothesis has been shown using artificial PRBCs lysate, which could introduce a bias in results obtained, as some parasite enzymes that are not naturally exposed to the EC will be released. In this study, we show that culture conditions, which allow a correct parasite maturation and reinvasion of new RBCs failed to induce a permeability enhancement, which suggests that parasite soluble factors related to the normal parasite maturation and reinvasion are not related with this phenomenon, even when working at high parasitemias such as 50% (8 × 107 PRBCs/cm2). In contrast, culture conditions, which reduce the parasite development and induce a PRBC apoptosis-like cell death trigger a permeability enhancement. Similar PRBC crisis forms have been observed in human CM (MacPherson et al, 1985), so we cannot discard the hypothesis that soluble factors produced during this apoptotic or death process can participate in the permeability enhancement observed in the hCMEC/D3 cell model. Moreover, these apoptotic structures can be more harmful as the reduced circulation in these obstructed microvascular vessels decreases the clearance of these parasitic wastes usually cleared by macrophages.
Finally, our results show a clear and expected relationship between pH of the culture medium and the integrity of the EC barrier. However, the more important and unexpected contribution of this study is to show up that, in a static condition, development of a high burden of parasites, in a slightly acidic environment with a limitation to control physiological pH, is enough to further decrease local environmental pH and alter the integrity of the EC barrier. One could conclude that culture conditions were not ideal for maintaining the integrity of the EC barrier; however, these results could have an important relevance in severe malaria. Indeed, recent published articles have shown a clear association between acidosis and the prognostic of the disease (Dondorp et al, 2008), mortality being greatest in children where acidosis coexists with impaired consciousness (Maitland and Newton, 2005). These clinical results, compared with the results obtained in our study, suggest that acidosis could be a main factor in BBB breakdown. This acidosis, because of systemic complications and reinforced by impaired blood perfusion because of parasites sequestration, leads to a deficiency to control the physiological pH (Sasi et al, 2007), which lets to think to the severe limitation in the buffer efficiency of MCm in our study. Finally, the high burden of sequestered parasites observed in the brain makes us thinking to the high parasite concentration needed in our experiments to decrease the environmental pH and to obtain the EC permeability enhancement.
The parallelism observed between our results and clinical data suggests that the culture conditions used in this study, even if they are not ideal for long-term parasite or EC culture, could be representative of the pathological situation observed in patients suffering from severe malaria. Thus, it is possible that in patients suffering from severe malaria, impaired perfusion of the brain, because of sequestered parasites, and massive parasite development are important factors in the etiology of acidosis increase in certain brain vessels and consequently in the BBB breakdown. However, the lack of metabolic disturbances or impaired perfusion of the brain could explain that infected human beings can support a high burden of sequestered parasites without undergoing CM.
In conclusion, our results establish that cytoadherence of mature parasites is not directly involved in permeability enhancement via activation of intracellular signaling pathways. In contrast, PRBCs cytoadherence together with metabolic disturbances contribute to induce EC permeability by producing a local toxic environment, which together with parasite metabolic activity, favors a decrease of the environmental pH, the apoptosis of PRBCs, and BBB breakdown. The results obtained in this study could have a strong relevance in the understanding of the step needed to the onset of CM and offer a new model to explore new therapies aimed at reducing brain EC integrity impairment.
Acknowledgments
The authors thank Catherine Blanc (flow cytometry core facility of Paris 6-Pitié-Salpêtrière) for flow cytometry assistance. Image acquisition was performed in the imaging facilities ‘Plate-forme d'Imagerie Cellulaire Pitié-Salpêtrière' (Shared Resource Facilities of CRICM, IFR14, IFR113, INSERM, UPMC). The authors thank Claude-Marie Bachelet and Aurélien Dauphin for confocal microscopy assistance. The authors also thank Dr G Snounou for his scientific advices. SZ has a fellowship from La Fondation des Treilles.
The authors declare no conflict of interest.
References
- Afonso PV, Ozden S, Cumont MC, Seilhean D, Cartier L, Rezaie P, Mason S, Lambert S, Huerre M, Gessain A, Couraud PO, Pique C, Ceccaldi PE, Romero IA. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 2008;4:e1000205. doi: 10.1371/journal.ppat.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Boonpucknavig V, Boonpucknavig S, Udomsangpetch R, Nitiyanant P. An immunofluorescence study of cerebral malaria. A correlation with histopathology. Arch Pathol Lab Med. 1990;114:1028–1034. [PubMed] [Google Scholar]
- Brown H, Hien TT, Day N, Mai NT, Chuong LV, Chau TT, Loc PP, Phu NH, Bethell D, Farrar J, Gatter K, White N, Turner G. Evidence of blood-brain barrier dysfunction in human cerebral malaria. Neuropathol Appl Neurobiol. 1999a;25:331–340. doi: 10.1046/j.1365-2990.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- Brown H, Rogerson S, Taylor T, Tembo M, Mwenechanya J, Molyneux M, Turner G. Blood-brain barrier function in cerebral malaria in Malawian children. Am J Trop Med Hyg. 2001;64:207–213. doi: 10.4269/ajtmh.2001.64.207. [DOI] [PubMed] [Google Scholar]
- Brown H, Turner G, Rogerson S, Tembo M, Mwenechanya J, Molyneux M, Taylor T. Cytokine expression in the brain in human cerebral malaria. J Infect Dis. 1999b;180:1742–1746. doi: 10.1086/315078. [DOI] [PubMed] [Google Scholar]
- Chakravorty SJ, Craig A. The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur J Cell Biol. 2005;84:15–27. doi: 10.1016/j.ejcb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Cojean S, Jafari-Guemouri S, Le Bras J, Durand R. Cytoadherence characteristics to endothelial receptors ICAM-1 and CD36 of Plasmodium falciparum populations from severe and uncomplicated malaria cases. Parasite. 2008;15:163–169. doi: 10.1051/parasite/2008152163. [DOI] [PubMed] [Google Scholar]
- Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, Dumenil G, Mege RM, Weksler BB, Romero IA, Couraud PO, Nassif X. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, Than M, Htut Y, Mohanty S, Yunus EB, Rahman R, Nosten F, Anstey NM, Day NP, White NJ. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47:151–157. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- Eum SY, Andras IE, Couraud PO, Hennig B, Toborek M. Pcbs and tight junction expression. Environ Toxicol Pharmacol. 2008;25:234–240. doi: 10.1016/j.etap.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay F, Robert C, Pouvelle B, Peyrol S, Scherf A, Gysin J. Isolation and characterization of brain microvascular endothelial cells from Saimiri monkeys. An in vitro model for sequestration of Plasmodium falciparum-infected erythrocytes. J Immunol Methods. 1995;184:15–28. doi: 10.1016/0022-1759(95)00070-q. [DOI] [PubMed] [Google Scholar]
- Gillrie MR, Krishnegowda G, Lee K, Buret AG, Robbins SM, Looareesuwan S, Gowda DC, Ho M. Src-family kinase dependent disruption of endothelial barrier function by Plasmodium falciparum merozoite proteins. Blood. 2007;110:3426–3435. doi: 10.1182/blood-2007-04-084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, Lambert PH. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Jenkins N, Wu Y, Chakravorty S, Kai O, Marsh K, Craig A. Plasmodium falciparum intercellular adhesion molecule-1-based cytoadherence-related signaling in human endothelial cells. J Infect Dis. 2007;196:321–327. doi: 10.1086/518795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- Maitland K, Newton CR. Acidosis of severe falciparum malaria: heading for a shock. Trends Parasitol. 2005;21:11–16. doi: 10.1016/j.pt.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol. 2006;36:555–568. doi: 10.1016/j.ijpara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Newton CR. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009;5:189–198. doi: 10.1038/nrneurol.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Milner DA, Jr, Tse MT, Njobvu A, Kayira K, Dzamalala CP, Taylor TE, Rogerson SJ, Craig AG, Molyneux ME. Genetic analysis of circulating and sequestered populations of Plasmodium falciparum in fatal pediatric malaria. J Infect Dis. 2006;194:115–122. doi: 10.1086/504689. [DOI] [PubMed] [Google Scholar]
- Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- Pongponratn E, Turner GD, Day NP, Phu NH, Simpson JA, Stepniewska K, Mai NT, Viriyavejakul P, Looareesuwan S, Hien TT, Ferguson DJ, White NJ. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–359. [PubMed] [Google Scholar]
- Rampon C, Weiss N, Deboux C, Chaverot N, Miller F, Buchet D, Tricoire-Leignel H, Cazaubon S, Baron-Van Evercooren A, Couraud PO. Molecular mechanism of systemic delivery of neural precursor cells to the brain: assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 2008;26:1673–1682. doi: 10.1634/stemcells.2008-0122. [DOI] [PubMed] [Google Scholar]
- Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- Sade H, Holloway K, Romero IA, Male D. Transcriptional control of occludin expression in vascular endothelia: regulation by Sp3 and YY1. Biochim Biophys Acta. 2009;1789:175–184. doi: 10.1016/j.bbagrm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Sasi P, Burns SP, Waruiru C, English M, Hobson CL, King CG, Mosobo M, Beech JS, Iles RA, Boucher BJ, Cohen RD. Metabolic acidosis and other determinants of hemoglobin-oxygen dissociation in severe childhood Plasmodium falciparum malaria. Am J Trop Med Hyg. 2007;77:256–260. [PubMed] [Google Scholar]
- Taoufiq Z, Gay F, Balvanyos J, Ciceron L, Tefit M, Lechat P, Mazier D. Rho kinase inhibition in severe malaria: thwarting parasite-induced collateral damage to endothelia. J Infect Dis. 2008;197:1062–1073. doi: 10.1086/528988. [DOI] [PubMed] [Google Scholar]
- Treeratanapiboon L, Psathaki K, Wegener J, Looareesuwan S, Galla HJ, Udomsangpetch R. In vitro study of malaria parasite induced disruption of blood-brain barrier. Biochem Biophys Res Commun. 2005;335:810–818. doi: 10.1016/j.bbrc.2005.07.151. [DOI] [PubMed] [Google Scholar]
- Tripathi AK, Sullivan DJ, Stins MF. Plasmodium falciparum-infected erythrocytes increase intercellular adhesion molecule 1 expression on brain endothelium through NF-kappaB. Infect Immun. 2006;74:3262–3270. doi: 10.1128/IAI.01625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi AK, Sullivan DJ, Stins MF. Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood-brain barrier endothelial cell monolayers. J Infect Dis. 2007;195:942–950. doi: 10.1086/512083. [DOI] [PubMed] [Google Scholar]
- Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukritayakamee S, Nagachinta B, White NJ, Berendt AR. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- Viegas P, Chaverot N, Enslen H, Perriere N, Couraud PO, Cazaubon S. Junctional expression of the prion protein PrPC by brain endothelial cells: a role in trans-endothelial migration of human monocytes. J Cell Sci. 2006;119:4634–4643. doi: 10.1242/jcs.03222. [DOI] [PubMed] [Google Scholar]
- Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, Romero IA, Weksler B, Stanimirovic DB, Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmer SC, Cianciolo GJ, Combes V, Grau GE. Inhibition of endothelial activation: a new way to treat cerebral malaria. PLoS Med. 2005;2:e245. doi: 10.1371/journal.pmed.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- White NJ, Ho M. The pathophysiology of malaria. Adv Parasitol. 1992;31:83–173. doi: 10.1016/s0065-308x(08)60021-4. [DOI] [PubMed] [Google Scholar]
- WHO 2008. World Malaria Report 2008, pp1–215.
- Yipp BG, Robbins SM, Resek ME, Baruch DI, Looareesuwan S, Ho M. Src-family kinase signaling modulates the adhesion of Plasmodium falciparum on human microvascular endothelium under flow. Blood. 2003;101:2850–2857. doi: 10.1182/blood-2002-09-2841. [DOI] [PubMed] [Google Scholar]
- Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]