Abstract
High-mobility group protein box-1 (HMGB1) has recently been recognized as a novel candidate in a specific upstream pathway promoting inflammation after brain ischemia. However, its downstream pathway and underlying mechanism have yet to be elucidated. The HMGB1 level in the acute cerebral infarct (ACI) group was significantly increased compared with that of control group, and correlated with the severity of neurologic impairment of ACI patients. Further, recombinant human HMGB1 (rhHMGB1) had no effect on microglia derived from mice lacking the Toll-like receptor 4 (TLR4−/−). Intracerebroventricular injection of rhHMGB1 in TLR4+/+ mice cause significantly more injury after cerebral ischemia–reperfusion than control group. But, TLR4−/− mice administered with rhHMGB1 showed moderate impairment after ischemia–reperfusion than TLR4+/+ mice. To determine the potential downstream signaling of HMGB1/TLR4 in cerebral ischemic injury, we used the ischemic–reperfusion model with Toll/interleukin-1 receptor domain-containing adaptor-inducing interferon-β knockout mice (TRIF−/−) and evaluated the activity and expression of TRIF pathway-related kinases. The results suggest that the TRIF pathway is not likely to be involved in TLR4-mediated ischemia brain injury. Finally, we found that TLR4 expressed by immigrant macrophages was involved in the development of ischemic brain damage. These results suggest that HMBG1 mediates ischemia–reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. The TLR4 expressed by immigrant macrophages may be involved in the development of ischemic brain damage.
Keywords: acute cerebral infarct, HMGB1, ischemia–reperfusion injury, TLR4
Introduction
Accumulating evidence shows that ischemic injury and inflammation account for the pathogenic progression of stroke (Kriz, 2006; Tuttolomondo et al, 2008; Wang et al, 2007; Yilmaz and Granger, 2008; Stoll et al, 1998; Xia et al, 2010; Brea et al, 2009). The distal cascade of inflammation response that results in organ damage after ischemia/reperfusion (I/R) injury has been studied extensively. However, the extent to which the initial cellular injury contributes to propagation of the inflammatory response and further tissue damage is poorly understood.
The high-mobility group protein box-1 (HMGB1) protein is a 215-amino acid nuclear protein and has been long considered a key regulator of nucleosome formation and gene transcription (Allain et al, 1999; Ellwood et al, 2000; Verrijdt et al, 2002; Park et al, 2006; Stros et al, 2002). Recently, it has also been characterized as a cytokine (Lotze and Tracey, 2005; Yang and Tracey, 2005) initiating inflammatory response after tissue injury. The HMGB1 participates in extracellular signaling by interacting with different receptors, such as the receptor for advanced glycation end products (RAGE), or the TLR2 and TLR4, on the plasma membrane of various cell types (Yang et al, 2010). It has been reported that HMGB1 is released from neurons early after ischemic injury and functions as a proinflammatory cytokine that connects excitotoxicity-induced acute damage and inflammatory processes in the postischemic brain (Kim et al, 2006; Qiu et al, 2008). Serum HMGB1 levels have been shown to be significantly elevated in cerebral ischemia (Goldstein et al, 2006). Moreover, it has been shown that in a mouse model of cerebral ischemia, HMGB1 released from ischemic brain tissue mediates ischemic brain damage through the RAGE receptor (Muhammad et al, 2008). In vitro studies have also provided evidence that recombinant human HMGB1 (rhHMGB1) prompts induction of proinflammatory mediators, such as inducible nitric oxide (NO) synthase, cyclooxygenase-2 (COX-2), interleukin (IL)-1β, and tumor necrosis factor (TNF)-α, and increases excitotoxicity and ischemic neuronal death (Kim et al, 2006). These findings indicate the important role of HMGB1 as a potential candidate in a specific upstream pathway promoting inflammation after brain ischemia.
The TLR4 have been demonstrated to be involved in cellular activation by HMGB1. In our previous studies, we have shown that increased expression of TLR4 correlates with severity of acute cerebral infarction (Yang et al, 2008). Moreover, we have observed that TLR4-deficient mice exhibited reduced cerebral ischemia–reperfusion injury as well as downregulation of inflammatory cytokines (Cao et al, 2007), suggesting that TLR4 has a pivotal role in ischemia–reperfusion injury. It has also been reported that activation of TLR4 signaling contributes to hippocampal neuronal death after global cerebral I/R (Hua et al, 2007). However, whether the interaction of HMGB1 and TLR4 is involved in the initial inflammatory response after ischemic brain injury remains unknown. In this study, we investigated the possible role of HMGB1 and TLR4 signaling in ischemic brain injury and its probable downstream pathway.
Materials and methods
Clinical Investigation
Clinical subjects
Study subjects were selected according to the inclusion and exclusion criteria as previously described in detail (Yang et al, 2008). A total of 21 acute cerebral infarct (ACI) patients who were admitted to the Stroke Unit of the department within 24 hours after the onset of the symptoms were selected (Table 1). All the patients were diagnosed and classified according to the criteria adopted by the Trial of Org 10172 in Acute Stroke Treatment (Kolominsky-Rabas et al, 2001). The exclusion criteria included intracerebral hemorrhage (confirmed by computed tomography or magnetic resonance imaging); significant stenosis of extracerebral and intracerebral carotid arteries (confirmed by transcranial doppler (TCD) and ultrasonography); arrhythmias and intracardiac thrombi (confirmed by electrocardiography and echocardiography); symptoms lasting >24 hours; obvious inflammatory conditions (e.g., infectious disease, systemic lupus erythematosus, rheumatism, and rheumatoid disease) within the half-year before enrollment; hospital-acquired infection (confirmed by the patient's symptoms and laboratory findings); acute myocardial infarction (diagnosed according to the patient's symptoms and electrocardiographic findings); acute ischemia of liver (confirmed by liver function test); autoimmune disease (diagnosed according to the patient's symptoms and positive autoimmune antibodies); patients who took glucocorticosteroids or immunodepressants; patients who were incompliant to the study protocol or could not undergo all the tests required by the study. A total of 19 healthy people who underwent regular physical examinations at our hospital were included in the control group. The controls were matched with the patients in terms of age and gender, and they did not have acute ischemia of the heart, peripheral tissues, or brain within 12 months before enrollment. Signed informed consent was obtained from all the study subjects and the study protocol was approved by the Medical Ethics Committee of the hospital.
Table 1. Clinical characteristics of control subjects and ACI patients.
| Control (n=19) | ACI (n=21) | P-value | |
|---|---|---|---|
| Age (years) | 65.6±10.4 | 66.5±9.8 | NS |
| Sex (F/M, %) | 58.2/41.8 | 52.6/47.4 | NS |
| Smoking (%) | 40.2 | 38.8 | NS |
| Hypertension (%) | 46.3 | 79.5 | <0.001 |
| Diabetes mellitus (%) | 32.2 | 28.8 | NS |
| Hypercholesterolemia (%) | 28.8 | 60.3 | <0.001 |
| Peripheral artery disease (%) | 20.1 | 18.6 | NS |
| Prior medication with | |||
| Lipid-lowering drugs | 17.6 | 36.8 | <0.01 |
| Platelet inhibitors | 22 | 46 | <0.001 |
| Atrial fibrillation | 3.5 | 18.2 | <0.001 |
| Platelet count ( × 109/L) | 245±32 | 238±28 | NS |
| Leukocytes ( × 109/L) | 5.6±2.2 | 7.9±2.8 | NS |
| C-reactive protein (mg/mL) | 0.3±0.2 | 15.4±6.6 | <0.001 |
Abbreviation: ACI, actue cerebral infarct.
The National Institutes of Health Stroke Scale was used to rate the severity of stroke (Lyden et al, 1994), and modified Rankin scores were used to assess the functional status of patients at 3 months after the onset of stroke.
Sample Collection and Preparation
A total of 10 mL of blood sample was drawn from the median cubital vein of one patient after he had stayed in the hospital for 18 hours and in the other patients immediately after admission. The 2-mL blood not treated with heparin was used to isolate serum at room temperature. Serum samples were stored at −80°C before measurement.
Animals
The TLR4−/− mice (8 weeks old, weighing 18 to 22 g) were purchased from American Jackson Laboratories (Bar Harbor, ME, USA) and bred with C57BL/c mice in our laboratory. The TRIF−/− mice (10 weeks old, weighing 20 to 25 g) were purchased from Jackson laboratory (005037, C57/6J-Ticam1lps2/J). Unlike wild-type macrophages, macrophages derived from these animals (TLR4−/− mice; 8 weeks old, weighing 18 to 22 g) fail to respond to synthetic lipid A, lipopolysaccharide, and dsRNA with production of TNF. Macrophages are less susceptible to lipopolysaccharide-induced cytotoxicity. Nitrous oxide and type I interferon production in activated macrophages is impaired. Homozygotes positive exhibit increased susceptibility to mouse cytomegalovirus.
Enzyme-Linked Immunosorbent Assay
An enzyme-linked immunosorbent assay (ELISA) kit for human HMGB1 assay (R&D Systems Inc., Minneapolis, MN, USA) was used to determine the concentration of serum HMGB1. The detection threshold of this assay is <1 ng/mL. The between-assay coefficient of variations is <10%. Serum levels of TNF-α, IL-1β were determined by ELISA according to the instructions provided with the kits (Beijing Jingmei co, Beijing, China). Interferon regulatory factor-3 (IRF-3) activity of mice was determined by ELISA according to the instructions provided with the kits (Beijing Jingmei co).
Real-Time Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from cultured microglia using TRIzol reagent kit (Invitrogen, Gaithersburg, MD, USA). To determine the levels of expression of COX-2, TNF-α, IL-1β, and β-actin, 1 μg total RNA was used to perform quantitative real-time reverse transcription-polymerase chain reaction with reverse transcription-polymerase chain reaction kit (Roche, Mannheim, Germany). The primer sequences were as follows: COX-2 forward, 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′, COX-2 reverse 5′-AGATCATCTCTGCCTGAGATATCTT-3′ IL-1β forward, 5′-AAACAGATGA-AGTGCTCCTTCCAGG-3′, IL-1β reverse, 5′-TGGAGAACACCACTTGTTGCTC-CA-3′ TNF-α forward, 5′-CCATTCCTG-AGTTCTGCAAAG-3', TNF-α reverse 5′-GCAAATATAAATAGAGGGGGGC-3′ β-actin forward 5′-CTCCTTAATGTCACGCACGATTTC-3′, β-actin reverse 5′-GTGGGGCGCCCCAGGCACCA-3′ (TaKaRa, Dalian, China). Standard curves were established with SYBR Green I kit (Roche).
Western Blot
Proteins were prepared from serum and brain tissue. Briefly, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto Hybond electrogenerated chemiluminescence (ECL) membranes (Amersham Pharmacia, Piscataway, NJ, USA). The ECL membranes were incubated with the primary antibodies including: mouse anti-human HMGB1 (1:1000, Santa Cruz, CA, USA), anti-TBK1 (1:1000), anti-IKKα (1:500), and anti-pIKKɛ (1:2000) (Cell Signaling Technology, Danvers, MA, USA), followed by incubation with peroxidase-conjugated secondary antibodies (1:2000, Jingmei). The signals were detected with ECL system (Amersham Pharmacia). The same membranes were probed with antibody for glyceradehyde-3-phosphate dehydrogenase after being washed with stripping buffer. The signals were quantified by scanning densitometry and computer-assisted image analysis.
Mouse Model of 6 hours of Ischemia/24 hours of Reperfusion
All mice were kept and bred in-house under pathogen-free condition. The Animal Care and Use Committee of the Third Military Medical University approved the experimental protocol. We constructed the mouse models of middle cerebral artery reperfusion as described previously (Cao et al, 2007). The mice were anesthetized by intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg), immobilized in a dorsal position, and incised at the cervical median line, and the left common carotid artery (CCA), external carotid artery, internal carotid artery, and the deep branch pterygopalatine artery were isolated. After that, the CCA was ligated at the proximal part and the external carotid artery at the distal part, leaving the ligature suture at the proximal part of the CCA crotch. After occlusion of the pterygopalatine artery and the distal internal carotid artery with mini-bulldog clamps, we made a small incision at the site (1.0 mm away from the CCA crotch) with microscissors, and inserted a prepared nylon suture into the internal carotid artery until it reached the cerebral prerolandic artery (about 8.29±0.54 mm depth) and blocked the blood supply of the middle cerebral artery. After that, we tightened the ligature line at CCA, and performed regular saturation on cervical incision. After 6 hours ischemia, the ligature line was pulled into the CCA to achieve reperfusion until 24 hours. We maintained the room temperature at about 25°C during and after surgery, and exposed the animals to incandescent lamp to keep their rectal temperature at (37°C±1°C) until its palinesthesia. The signs of a successful model are (1) left Horner's symptom and (2) right-side hemiparalysis mainly for the forward limb. Table 2 lists the measurements of I/R models. The success rate of model reproduction was 93.8%, and the failure rate was 6.2% (including the death rate of 5.4%).
Table 2. Physiological parameters of control and TLR4−/− mice 30 minutes before and 30 minutes after MCAO.
| Parameter |
TLR4+/+ |
TLR4−/− |
||
|---|---|---|---|---|
| Before MACO (n=6) | After MACO (n=6) | Before MACO (n=6) | After MACO (n=6) | |
| MABP (mm Hg) | 72.4±2.6 | 70.6±3.4 | 72.0±2.8 | 69.6±3.6 |
| Heart rate (per minute) | 255.6±29.8 | 260.5±32.4 | 266.3±27.4 | 267.5±30.2 |
| Glucose (mg/dL) | 210.5±139.5 | 202.6±132.2 | 212.4±100.8 | 218.0±152.2 |
| Arterial pH | 7.26±0.05 | 7.16±0.06 | 7.31±0.08 | 7.20±0.09 |
| Arterial pO2 (mm Hg) | 117.5±16.2 | 98.5±8.6 | 106.7±12.4 | 92.3±10.6 |
| Arterial pCO2 (mm Hg) | 44.5±9.5 | 47.2±10.2 | 40.1±12.6 | 45.8±8.8 |
| Base excess | −9.2±2.3 | −11.6±4.5 | −8.3±6.0 | 10.1±2.9 |
| Hb (g/L) | 15.2±1.3 | 14.8±1.6 | 13.5±2.4 | 16.1±0.3 |
| Laser Doppler (% before MCAO) | 14.2±3.8 | 12.8±6.4 | ||
| Body weight (g) | 26.7±4.0 | 23.8±5.2 | ||
Abbreviations: MABP, mean arterial blood pressure; MCAO, middle cerebral artery occlusion; TLR4, Toll-like receptor 4.
There was no significance between the genotypes (t-test).
2,3,5-Trphenyl Tetrazolium Chloride Staining of Mice Brain
The brains were dissected coronally into 2 mm brain slices using a brain matrix. Slices were immediately stained by immersion in 2% 2,3,5-trphenyl tetrazolium chloride (Sigma Co, Shanghai, China) at 37°C for 30 minutes and then in 4% paraformaldehyde for preservation.
Cerebral Infarction Size Assay of Mice
Mice were killed by excess narcosis with 2% pentobarbital sodium. This was followed by immediate decapitation, and the brain tissue was removed and placed in −70°C for 10 minutes for quick freezing. Then, the procerebrum was obtained and cut into five coronal brain slices of the same thickness (2 mm) from the frontal to the occipital pole. The slices were immediately placed in 2% tetrazolium chloride (Sigma Co) phosphate-buffered saline and protected from light, incubated at 37°C for 30 minutes, and fixed in 4% paraformaldehyde for 2 hours. Normal brain tissue stained bright red, whereas infarction focus was pale white. We arranged the fixed brain slices in order, and measured the whole area of prosencephalon and cerebral infarction with Image Pro Plus 5.0 image processing software (Media Cybernetics Inc., Bethesda, MD, USA). We also calculated the area according to the formula V=t × A2+… An), where V is infarction volume or prosencephalon volume, t is the thickness of slice, and A is the infarction size. We calculated the cerebral infarction volume and prosencephalon volume, as well as the volume ratio of cerebral infarction (cerebral infarction volume/prosencephalon volume).
Measurement of Cerebral Water Content of Mice
We randomly took six mice for each group, euthanized them after 24 hours of reperfusion by decapitation, and levered the skull within 1 minute to take out brain tissues. We then blotted up the water on the surface of the left hemisphere with filter paper and took the humid weights (GW) on an electronic balance. Then we heated them for 48 hours at 110°C in an electrothermostatic blast oven and took their dry weights (DW). The cerebral water content was calculated by the formula cerebral water content %=(GW−DW)/GW × 100%.
Cell Culture
For primary microglial culture, cerebral hemispheres of 1- to 2-day-old postnatal mice were ground and digested with 0.1% trypsin, and then the digestion products were seeded into six-well plate at a density of 1 × 106/mL with Dulbecco's Modified Eagle Media (DMEM) (Sigma, St Louis, MO, USA) containing 10% FBS (Hyclone, Logan, UT, USA). Culture media were changed twice per week for 2 weeks and then microglia were detached by mild shaking and filtered through a nylon mesh to remove astrocytes. After centrifugation at 1000g for 10 minutes, the cells were resuspended in a fresh DMEM supplemented with 10% FBS and plated at a final density of 4 × 104/mL cells on a 24-well culture plate. The following day, cells were subjected to the experiments. The cell purity was determined by immunohistochemical staining using microglia-specific antibody CD11b. The microglial cultures used were >95% pure.
For peritoneal macrophage preparation, mice were intraperitoneally injected with 15 mL Roswell Park Memorial Institute medium (RPMI) (Sigma) containing 10% FBS, streptomycin (100 IU/mL), penicillin (100 U/mL), and glutamine (2 mmol/L). After gentle massage of the anterior and lateral walls of the abdomen, peritoneum washes were collected and centrifuged at 1000g for 10 minutes. Cells were resuspended in RPMI medium as above, plated in six-well plate at a density of 1 × 106/mL, and incubated at 37°C and 5% CO2 for 6 hours until 90% of cells attached. Cell viability was determined by trypsin (Hyclone) exclusion assay to be 95%.
Treatment of Recombinant High-Mobility Group Protein Box-1 to Mice
Primary culture microglial were treated with 500 ng/mL rhHMGB1 (Sigma) for 1 hour after which the culture medium was replaced. Twenty-four hours later, the supernatant was collected for the assessment of NO, and the cells were collected for the assessment of nuclear factor (NF)-κB activity and the expression of COX-2 mRNA, IL-1β mRNA, and TNF-α mRNA. The endotoxin content of rhHMGB1 was determined with a standard endotoxin-specific Limulus amebocyte lysate reagent (Endosate, Wilmington, MA, USA). Endotoxic content was always <0.05 EU/mL.
Intracerebroventricular Injection of Recombinant Human High-Mobility Group Protein Box-1 in Mice
Mice were anesthetized with chloral hydrate (40 mg/kg body weight, intraperitoneally), then immobilized in a stereotaxic apparatus (Stoelting, Kiel, WI, USA). The rhHMGB1 was administered into the lateral ventricle of mice using the following coordinates: 0.6 mm posterior to the bregma, 1.0 mm lateral to the midline, and 2.5 mm under the dura. A small hole was made on the skull with a high-speed drill and 10 μg rhHMGB1 was injected with microinjection syringe. The total volume of injection was 1 mL. A measure of 1 mL of normal saline was injected in the control group. After surgery, the skin was sutured and the mouse recovered on heating pad. For cerebral ischemia mice, rhHMGB1 was administered 1 hour after ischemia, which was followed by 24 hours reperfusion.
Determination of Nuclear Factor-κB Activity by Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assay (Yang et al, 2008) was performed as follows: nucleoprotein was extracted from culture microglia, 10 μg nucleoprotein of each sample was incubated with the reaction buffer at room temperature for 15 minutes, then 32P-labeled oligonucleotide (50-GGGGACTTTCC-30; Life Technologies, Gaithersburg, MD, USA), which binds to NF-κB, was added in the reaction buffer and incubated for 15 minutes. After incubation at 25°C for 20 minutes, the reaction mixture was subjected to 6% nondegeneration polyacrylamide gel electrophoresis. Autoradiography was performed at 70°C. Finally, the images were analyzed using Bio-Rad Image Analyzer (CA, USA) and the results were expressed as optical density.
Neurologic Impairment Scores of Mice
Two hours after the surgery, the mice awoke from narcosis. We then screened the model according to the standard of Longa 5 (Longa et al, 1989) grading method, as described previously (Yang et al, 2008; Cao et al, 2007).
Establishment of Chimeric Mice
Chimeric mice were produced by transfer of donor bone marrow cells into irradiated recipient animals using combinations of TLR4+/+ and TLR4−/− mice in the following donor/recipient groups: TLR4+/+/TLR4+/+, TLR4+/+/TLR4−/−, TLR4−/−/TLR4−/−, and TLR4−/−/TLR4+/+. Each recipient mouse was exposed to a lethal 60Coc of 1000 cGy at 6 hours before receiving 0.5 to 1 × 106 bone marrow cells. The bone marrow cells were prepared from the tibia and femur bones of the donor mice under sterile conditions. Eight weeks after bone marrow cells transfer, the peripheral blood samples were taken for reverse transcription-polymerase chain reaction detection of the successful engraftment. After 6 to 8 weeks, the chimeric mice underwent 6 hours of cerebral ischemia and 24 hours of reperfusion.
Statistical Analysis
Measurement data are shown as mean±s.d. or percentages. One-way analysis of variance and Student–Newman–Keuls test in post hoc tests were used to analyze differences between the three groups. Pearson's correlation analysis was used to reveal the correlation among HMGB1, National Institutes of Health Stroke Scale, and modified Rankin scores, and the correlation among HMGB1, TNF-α, and IL-1β. SPSS11.5 software (Chicago, IL, USA) was used in all statistical analyses. A P-value <0.05 was deemed statistically significant.
Results
Increase of High-Mobility Group Protein Box-1 in Patients with Acute Cerebral Infarct
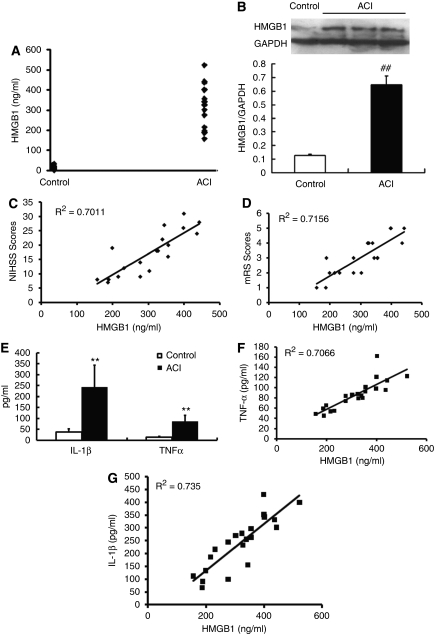
The average serum HMGB1 levels in control subjects were <14.5 ng/mL, which is consistent with previous reports (Goldstein et al, 2006). The serum HMGB1 level in the ACI group was significantly elevated as compared with the control group (14.5±6.8 ng/mL versus 303.4±36.5 ng/mL, P<0.0001) (Figure 1A). Western blot also showed significantly increased HMGB1 in ACI group as compared with that of control groups (Figure 1B). Correlation analysis showed a positive correlation of the serum level of HMGB1 with both National Institutes of Health Stroke Scale scores (R2=0.7011, P=0.0003; Figure 1C) and modified Rankin scores (R2=0.7156, P=0.002; Figure 1D), suggesting that serum HMGB1 level correlates with the severity of neurologic impairment of ACI patients.
Figure 1.
Increase of high-mobility group protein box-1 (HMGB1) correlates with severity of acute cerebral infarction in the acute cerebral infarct (ACI) group of clinical patients. (A) The serum HMGB1 level in the ACI group was significantly elevated compared with the control group. (B) Western blot showed a significant increase of HMGB1 in ACI subjects (##P<0.0001 versus control). (C, D) Correlation analysis showed a positive correlation of the serum level of HMGB1 with both National Institutes of Health Stroke Scale (NIHSS) scores (R2=0.7011, P=0.0003, C) and modified Rankin scores (mRS) (R2=0.7156, P=0.002, D). (E–G) In the ACI group, increased tumor necrosis factor (TNF)-α and interleukin (IL)-1β displayed a positive correlation with the elevation of serum HMGB1 (**P<0.0001 versus control). GAPDH, glyceradehyde-3-phosphate dehydrogenase.
In our previous study, we have shown that TLR4 is upregulated in peripheral blood monocytes from patients with ACI, and this change parallels an elevation in serum TNF-α and IL-6 in ACI group (Yang et al, 2008). Therefore, we measured the serum levels of inflammatory factors TNF-α and IL-1β to determine whether they correlate with increased HMGB1 level. The data showed that serum levels of TNF-α and IL-1β exhibit a dramatic increase in ACI group compared with that of the control group (TNF-α: 13.3±5.2 ng/mL versus 86.3±27.7 ng/mL, P<0.0001; IL-1β: 38.3±13.6 ng/mL versus 241.2±100.9 ng/mL, P<0.0001) (Figure 1E). Interestingly, both the increase of TNF-α and IL-1β displayed a positive correlation with the augmentation of serum HMGB1 measured in ACI group (TNF-α: R2=0.7066, P=0.006; Figure 1F and IL-1β: R2=0.735, P=0.0004; Figure 1G), indicating that ACI-induced elevation of HMGB1 may lead to inflammation-related brain injury.
High-Mobility Group Protein Box-1-Induced Activation of Microglia via Toll-Like Receptor 4
Microglial activation, the hallmark of brain inflammation, has an important role in the postischemic brain (Kim et al, 2006). Reactive microglial cells are the main resources of inflammatory factors, such as TNF-α and IL-1β, in the injured brain (Kim et al, 2006). In this study, we have shown that the increase of HMGB1 is related to the elevation of TNF-α and IL-1β. Therefore, it is intriguing to know whether HMGB1 can trigger microglial activation in the ischemic brain, and what might be the possible underlying mechanism. The TLR4 has been reported as one of the receptors for HMGB1 (Kim et al, 2006; Kaczorowski et al, 2009; van Zoelen et al, 2009). In our previous study, we found a significant increase of TLR4 in patients with ACI that was correlated with severity of acute cerebral infarction. So, next we determined whether TLR4 is involved in HMGB1-mediated microglial activation.
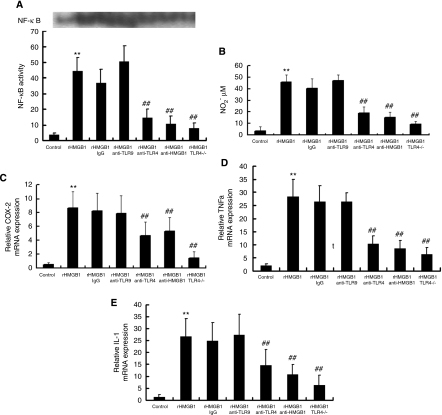
To test this hypothesis, we used TLR4−/− mice (Krüger et al, 2009; Kilic et al, 2008). Primary-cultured microglia derived from TLR4+/+ and TLR4−/− mice were treated with 500 ng/mL hHMGB1 and NF-κB activity was measured with electrophoretic mobility shift assay (EMSA) (Figure 2A). Administration of rhHMGB1 to microglial cultures significantly increases the NF-κB activity. Coadministration of rhHMGB1 with either IgG or anti-TLR9 antibody showed a similar increase in NF-κB activity. However, coadministration of rhHMGB1 with either anti-TLR4 antibody or anti-HMGB1 dramatically suppressed the effect of rhHMGB1-induced NF-κB activity. Interestingly, when microglia derived from TLR4−/− mouse were treated with rhHMGB1, no obvious increase in NF-κB activity was observed, indicating that TLR4 is involved in rhHMGB1-induced NF-κB activity.
Figure 2.
High-mobility group protein box-1 (HMGB1)-induced activation of microglia via Toll-like receptor 4 (TLR4) on murine-cultured microglia. (A) Nuclear factor (NF)-κB activity was measured with the EMSA assay on cultured microglia. (B) Production of nitric oxide (NO) in reactive microglia was measured and displayed as the concentration of nitrogen dioxide (NO2) (μmol/L). (C–E) The transcription level of cyclooxygenase-2 (COX-2) (C), tumor necrosis factor (TNF)-α (D), and interleukin (IL)-1β (E) in reactive microglia cells were quantified using real-time polymerase chain reaction (PCR). **P<0.01 versus control; ##P<0.01 versus HMGB1, n=6. EMSA, electrophoretic mobility shift assay.
Production of NO has been considered as a hallmark of microglia activation. Therefore, we further evaluated NO alteration after rhHMGB1 treatment using a NO kit (Shungong, Shanghai, China), and displayed the result as the concentration of nitrogen dioxide (μmol/L) (Figure 2B). Coapplication of rhHMGB1 with either anti-TLR4 antibody or anti-HMGB1 antibody abolished rhHMGB1-induced NO increase. Moreover, no obvious NO increase was observed in TLR4−/− microglia when they were treated with rhHMGB1.
Next, we quantified the transcription level of COX-2, TNF-α, and IL-1β using real-time polymerase chain reaction (Figures 2C–2E). Our data showed that the mRNA level of COX-2, TNF-α, and IL-1β in microglia treated with rhHMGB-1 exhibited a significant increase compared with that of untreated cells. In control experiments, coapplication of rhHMGB1 with either IgG or anti-TLR9 antibodies had no effect on the rhHMGB1-induced increase in mRNA level. However, when we neutralized rhHMGB1 with its antibody or blocked its receptor, TLR4, with anti-TLR4 antibody, the upregulations of COX-2, TNF-α, and IL-1β mRNA were significantly suppressed. The TLR4−/− microglia treated with rhHMGB1 also showed mild mRNA upregulation, which is consistent with the effect of anti-TLR4 antibody. These data suggest that rhHMGB1 can activate microglial cells through TLR4 in vitro.
Toll-Like Receptor 4 Contribute to High-Mobility Group Protein Box-1-Mediated Ischemic Brain Injury
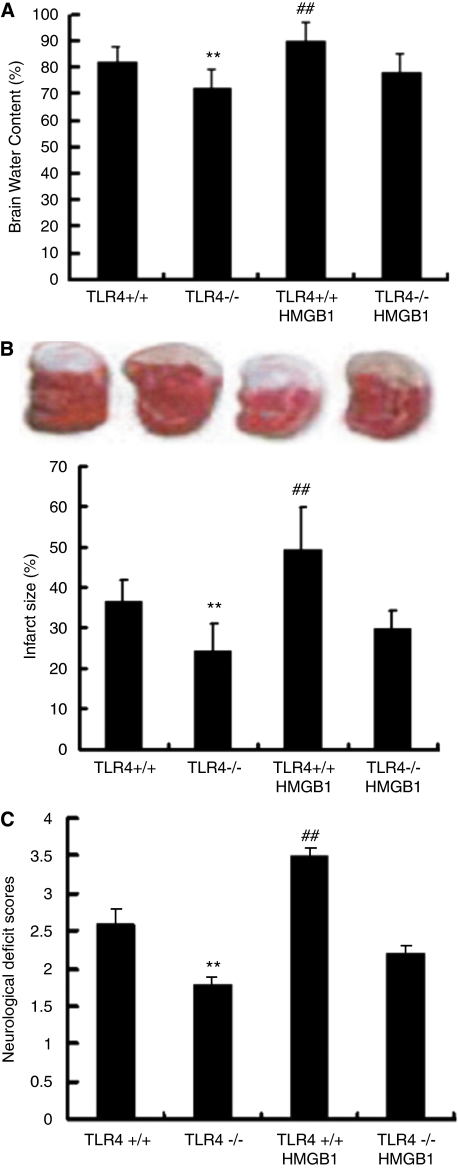
It has been reported that HMGB1 has a role in brain ischemia-induced inflammation. In this study, we have also shown that increased serum HMGB1 is associated with severity of ACI. The HMGB1 acts as a proinflammatory cytokine after interacting with its receptor, RAGE, TLR2, and TLR4. In our previous study, we have shown that increased expression of TLR4 correlates with severity of acute cerebral infarction (Yang et al, 2008). Consistent with this, it has also been reported that activation of TLR4 signaling contributes to hippocampal neuronal death after global cerebral I/R. The in vitro experiments shown here indicate that TLR4 mediates HMGB1-triggered microglial activation. But it remains unclear whether the HMGB1–TLR4 interaction also contributes to ischemia-induced brain damage. To address this question, we examined TLR4−/− mice using the cerebral ischemia–reperfusion model. In this experiment, the severity of ischemia-induced brain damage was assessed using three measurements: cerebral water content (Figure 3A), cerebral infarct size (Figure 3B), and neurologic impairment scores (Figure 3C). The physiological parameters of TLR4+/+ and TLR4−/− mice 30 minutes before and 30 minutes after middle cerebral artery occlusion are detailed in Table 2. In the first set of experiments, we compared ischemia-induced brain damage in TLR4+/+ mice and TLR4−/− mice after 6 hours of ischemia and 24 hours of reperfusion. The TLR4−/− mice displayed a significant reduction in the extent of edema (82.3±6.2 versus 72.6±6.9, P=0.002) and the cerebral infarct size (36.6±5.3 versus 24.2.6±6.8, P=0.004), and significantly lower neurologic impairment scores (2.6±0.2 versus 1.8±0.1, P=0.008), suggesting that blocking TLR4 activation dramatically reduces ischemia-induced brain damage. Further, we administered the rhHMGB1 in TLR4+/+ and TLR4−/− mice through intracerebroventricular injection. The TLR4+/+ mice treated with rhHMGB1 showed significantly worse ischemia-induced brain damage, showing as increased extent of edema (90.3±5.4, P<0.01), infarct area (49.4±10.2, P<0.01), and higher neurologic scores (3.5±0.8, P<0.01), which is consistent with our observation that the level of serum HMGB1 is associated with the severity of ACI. In contrast, TLR4−/− mice treated rhHMGB1 showed no obvious impairment compared with the wild-type animals. These results indicate that blocking TLR4 function can protect the brain from HMGB1-mediated ischemic damage.
Figure 3.
Toll-like receptor 4 (TLR4) contributes to high-mobility group protein box-1 (HMGB1)-mediated murine ischemic brain injury. The TLR 4 is involved in HMGB1-mediated ischemic brain damage. (A–C) The severity of ischemic injury was assessed using measurements of cerebral water content (A), cerebral infarct size (B), and neurologic impairment scores (C). **P<0.01 versus TLR4+/+; ##P<0.01 versus TLR4−/−, n=6.
Toll-Like Receptor 4 Contributes to Ischemic Brain Injury via TRIF-Independent Pathway
It has been established that TLR signaling pathway arises from intracytoplasmic TIR domains, which are conserved among all TLRs. Accumulating evidence has demonstrated that TIR domain-containing adaptors, such as MyD88, TIRAP, and TRIF, modulate TLR signaling pathways (Akira, 2006). MyD88 is essential for the induction of inflammatory cytokines triggered by all TLRs, whereas, TRIF is implicated in the TLR3- and TLR4-mediated MyD88-independent pathway (Zhai et al, 2004; Yamamoto et al, 2003). We previously showed that MyD88 mRNA expression did not significantly differ among the ACI and control groups, and we also observed that MyD88 knockout mice showed no significant difference in cerebral edema, cerebral infarction area, and neurologic impairment scores, suggesting that TLR4 may contribute to cerebral I/R injury through an MyD88-independent signal pathway (Yang et al, 2008).
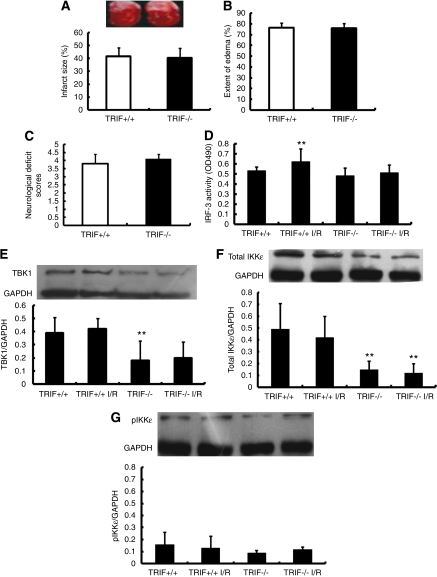
Therefore, to elucidate the intermediate or downstream signaling pathway by which TLR4 contributes to cerebral infarction, we observed cerebral I/R injury in TRIF−/− mice. Results showed no significant difference between the TRIF+/+ and TRIF−/− mice in terms of the infarct size (Figure 4A), extend of edema (Figure 4B), and neurologic deficit score (Figure 4C) after 6 hours of ischemia/24 hours of reperfusion.
Figure 4.
Toll-like receptor 4 (TLR4) contributes to ischemic brain injury via TRIF-independent pathway. (A–C) The severity of ischemic injury in TRIF−/− and TRIF+/+ mouse was compared using measurements of infarct size (A), extent of edema (B), and neurologic deficit scores, showing no obvious difference between TRIF−/− and TRIF+/+ mouse. (D) IRF-3 activity was examined in wt and TRIF−/− mice after ischemia/reperfusion (I/R) injury (**P<0.01 versus TRIF+/+, n=6). (E–G) Protein levels of TANK-binding kinase 1 (TBK1) (E), total IKKɛ (F), and pIKKɛ (G) were quantified with Western blots (**P<0.01 versus TRIF+/+, n=6). GAPDH, glyceradehyde-3-phosphate dehydrogenase; IRF-3, interferon regulatory factor-3.
We further asked whether the expression of TRIF downstream signaling alters in response to I/R. It has been well established that IRF-3 has a key role in MyD88-independent signaling pathway and the induction of inflammatory cytokines (Yamamoto et al, 2003; Sato et al, 2003). In vitro kinase assays have also found that TANK-binding kinase 1 (TBK1) and IkB kinases IKKɛ/IKKi induce IRF-3 phosphorylation. Therefore, we examined the IRF-3 activity and protein level of TBK1, total IKKɛ, and phosphorylated-IKKɛ in TRIF+/+ and TRIF−/− mice after I/R injury. The TRIF−/− mice showed no significant change in IRF-3 activity after I/R (Figure 4D). Western blots showed that compared with TRIF+/+ mice, TRIF−/− mice exhibited significantly reduced protein levels of TBK1 (Figure 4E) and total IKKɛ (Figure 4F), but not in phosphorylated-IKKɛ (Figure 4G). However, TRIF−/− mice showed no change in TBK1, total IKKɛ, and phosphorylated-IKKɛ expression in response to I/R (Figures 4E–4G). All these data suggest that the TRIF pathway is not likely to be involved in HMGB1/TLR4-mediated I/R injury.
Macrophage Toll-Like Receptor 4 Worsens Brain Injury in Ischemia Brain
The TLR4 is expressed in both microglia and macrophages (Yang et al, 2008; Lehnardt et al, 2003). In I/R injury, peripheral macrophages were recruited to the injured area from bone marrow-derived cells of myeloid origin (Lalancette-Hébert et al, 2007; Bechmann et al, 2001). Immigrant macrophages have been reported to have a detrimental role in HMGB1-induced cell death (Muhammad et al, 2008). Therefore, we further investigated whether macrophage TLR4 has a potential role in the ischemic brain.
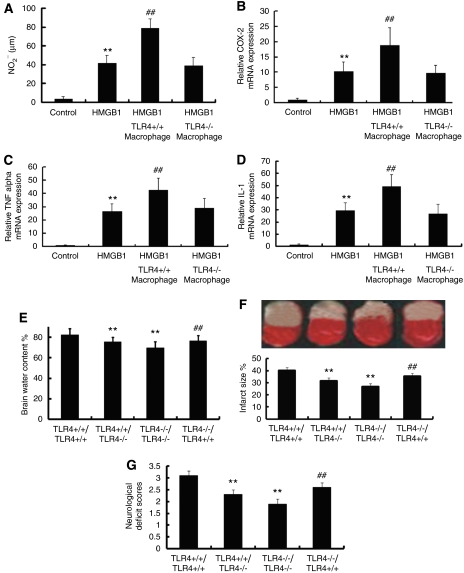
First, we evaluated the potential role of macrophages in an in vitro system by adding macrophages derived from TLR4+/+ or TLR4−/− mice to our microglial culture system as described in result 5. Result showed that macrophages derived from TLR4+/+ mice but not from TLR4−/− mice significantly potentiated microglial activation in the presence of HMGB1, demonstrated by a dramatic increased NO release and upregulation of COX-2, TNF-α, and IL-1β mRNA (Figures 5A–5D).
Figure 5.
Macrophage Toll-like receptor 4 (TLR4) worsens brain injury in ischemia brain. (A–C) Macrophages derived from TLR4+/+ mice but not from TLR4−/− mice significantly potentiated microglial activation in the presence of high-mobility group protein box-1 (HMGB1), which is demonstrated by a dramatic increase in nitric oxide (NO) release (A), elevated transcription of cyclooxygenase-2 (COX-2) (B), tumor necrosis factor (TNF)-α (C), and interleukin (IL)-1β (D). (**P<0.01 versus control; ##P<0.01 versus HMGB1, n=6). (E–G) TLR4+/+ mice that received TLR4−/− bone marrow transplants had significantly decreased extent of edema (E), smaller infarct area (F), and lower neurologic deficit scores (G) than animals that received TLR4+/+ bone marrow mice. (**P<0.01 versus TLR4+/+/TLR4+/+; ##P<0.01 versus TLR4−/−/TLR4−/−, n=6).
Further, to evaluate the role of immigrant macrophages in vivo, we generated chimeric mice by bone marrow transplantation as described in the Materials and methods. Our data showed that TLR4+/+ mice that received TLR4−/− bone marrow transplants had significantly decreased extent of edema, smaller infarct area, and lower neurologic deficit scores than animals that received TLR4+/+ bone marrow (Figures 5E–5G). Together with in vitro data, this result strongly suggests that TLR4 expressed by immigrant macrophages is involved in the development ischemic brain damage, probably by interaction with HMGB1 released from necrotic cells within the ischemic core.
Discussion
In this study, we observed that the serum HMGB1 level in ACI patients exhibited a significant increase that was correlated with the severity of neurologic impairment of ACI patients as assessed with National Institutes of Health Stroke Scale and modified Rankin scores. This increase of serum HMGB1 also displayed a positive correlation with elevation of TNF-α and IL-1β, indicating that elevation of HMGB1 in ACI may lead to inflammatory consequences in response to ischemic injury. This observation is consistent with a previous report of elevated HMGB1 levels in patients with cerebral and myocardial ischemia (Goldstein et al, 2006). More importantly, we showed the association of serum HMGB1 level with severity of neurofunctional impairment and alteration of inflammatory cytokines in response to ischemic injury, suggesting that HMGB1 level could be a potential indicator of severity of brain damage.
Further, we showed that rHMGB1 stimulated activation of microglia as demonstrated with increased NF-κB activity, production of NO, and upregulated transcription of COX-2, TNF-α, and IL-1β. However, administration of rhHMGB1 had no effect on TLR4−/− microglia. This observation provides evidence that HMGB1 may trigger microglial activation through the TLR4 receptor. Consistent with this in vitro experiment, microinjection of HMGB1 in TLR4−/− mice showed no obvious impairment after ischemia–reperfusion, suggesting that abolishing TLR4 function can protect the brain from HMGB1-mediated ischemic damage. These results indicate that HMGB1/TLR4 signaling may have an important role in inflammatory response, probably through activation of microglial cells.
The HMGB1 protein was first described as a chromosomal protein involved in the maintenance of nucleosome structure and regulation of gene transcription. Recently, it was found that HMGB1 activated an inflammatory response on release into the extracellular milieu from necrotic cells and activated macrophages with accompanying organ failure (Wang et al, 1999, 2001; Scaffidi et al, 2002). Since then, many studies have described the roles of HMGB1 in pathological states, including sepsis, atherosclerosis, and arthritis. It has been reported that after release, HMGB1 binds to the cell-surface receptors RAGE, resulting in the activation of transcription factor NF-κB and mitogen-activated protein kinase. It has also been reported that in addition to RAGE, TLR2, and TLR4 may also function as HMGB1 receptors and may mediate various cellular responses, including chemotractive cell movement and release of proinflammatory cytokines (TNF-α, IL-1β, IL-1α, IL-6, and macrophage inflammatory protein). This suggests that different receptors and downstream signaling pathways may contribute to the HMGB1-activated inflammatory response. A previous study reported that the serum level of HMGB1 was elevated in stoke patients (Goldstein et al, 2006) and that RAGE functioned as a sensor of necrotic cell death. In our study, we also found a similar increase of HMGB1 in the serum of ACI subjects. Moreover, we provide evidence that abolishing TLR4 function attenuates HMGB1-mediated ischemic/reperfusion injury, suggesting that HMGB1–TLR4 signaling is also involved in ischemic/reperfusion injury. This indicates that different pathways may contribute to HMGB1-mediated inflammation in ischemic/reperfusion injury.
We then further investigated the downstream signaling by which TLR4 functions as a sensor of elevated HMGB1 in ischemic brain damage. Toll-like receptors (TLRs) have been established to have an essential role in the activation of innate immunity. The TLR signaling pathway arises from intracytoplasmic TIR domains, which contain adaptors, such as MyD88, TIRAP, and TRIF, modulate TLR signaling pathways. MyD88 has been shown to be essential for the induction of inflammatory cytokines triggered by all TLRs, whereas, TRIF is mainly implicated in the TLR3- and TLR4-mediated MyD88-independent pathway. In our previous study, we showed that MyD88 mRNA expression did not differ significantly among the ACI and control groups, and we also observed that MyD88 knockout mice showed no significant change in cerebral edema, cerebral infarction area, and neurologic impairment scores (Yang et al, 2008), suggesting that TLR4 may contribute to cerebral I/R injury through an MyD88-independent signal pathway. Therefore, we proposed that the TRIF pathway might be the downstream signaling by which HMGB1/TLR4 is involved in inflammation in response to ischemic injury. We tested this hypothesis by introducing the cerebral I/R model to TRIF−/− mice. Interestingly, compared with wt mice, TRIF−/− mice showed no significant difference in the infarct size, extend of edema, and neurologic deficit score in response to ischemic injury. In addition, we assessed the activity and expression level of TRIF downstream kinases. Results showed that TRIF−/− mice displayed no obvious change in IRF-3 activity and expression level of TBK1, total IKKɛ, and phosphorylated-IKKɛ in response to I/R. Therefore, these data suggest that the TRIF pathway is unlikely involved in HMGB1/TLR4-mediated I/R injury.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by a grant from the National Natural Science Foundation of China (No. C30870859), the Chongqing Natural Science Foundation (CSTC, 2008BB5279).
References
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- Allain FH, Yen YM, Masse JE, Schultze P, Dieckmann T, Johnson RC, Feigon J. Solution structure of the HMG protein NHP6A and its interaction with DNA reveals the structural determinants for non-sequence-specific binding. EMBO J. 1999;18:2563–2579. doi: 10.1093/emboj/18.9.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Priller J, Kovac A, Böntert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- Brea D, Sobrino T, Ramos-Cabrer P, Castillo J. Inflammatory and neuroimmunomodulatory changes in acute cerebral ischemia. Cerebrovasc Dis. 2009;27 (Suppl 1:48–64. doi: 10.1159/000200441. [DOI] [PubMed] [Google Scholar]
- Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Ellwood KB, Yen YM, Johnson RC, Carey M. Mechanism for specificity by HMG-1 in enhanceosome assembly. Mol Cell Biol. 2000;20:4359–4370. doi: 10.1128/mcb.20.12.4359-4370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, Czura CJ, Lee DC, Ward MF, Bruchfeld AN, Wang H, Lesser ML, Church AL, Litroff AH, Sama AE, Tracey KJ. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25:571–574. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski DJ, Tsung A, Billiar TR. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed) 2009;1:91–98. doi: 10.2741/E10. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- Kriz J. Inflammation in ischemic brain injury: timing is important. Cri Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- Krüger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Krämer BK, Colvin RB, Heeger PS, Murphy BT, Schröppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci USA. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EC, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Lyden P, Brott T, Tilley B, Welch KMA, Mascha EJ, Levine S, Haley EC, Grotta J. The NINDS TPA Stroke Group. Improved reliability of the NIH Stroke Scale using video training. Stroke. 1994;25:2220–2226. doi: 10.1161/01.str.25.11.2220. [DOI] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Stros M, Ozaki T, Bacikova A, Kageyama H, Nakagawara A. HMGB1 and HMGB2 cell-specifically down-regulate the p53- and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J Biol Chem. 2002;277:7157–7164. doi: 10.1074/jbc.M110233200. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14:3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijdt G, Haelens A, Schoenmakers E, Rombauts W, Claessens F. Comparative analysis of the influence of the high-mobility group box 1 protein on DNA binding and transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocorticoid receptors. Biochem J. 2002;361 (Pt 1:97–103. doi: 10.1042/0264-6021:3610097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Han J, Huang G, Ying W. Inflammation in ischaemic brain injury: current advances and future perspectives. Clin Exp Pharmacol Physiol. 2010;37:253–258. doi: 10.1111/j.1440-1681.2009.05279.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- Yang H, Tracey KJ. High mobility group box 1 (HMGB1) Crit Care Med. 2005;33:S472–S474. doi: 10.1097/01.ccm.0000187005.81616.a9. [DOI] [PubMed] [Google Scholar]
- Yang QW, Li JC, Lu FL, Wen AQ, Xiang J, Zhang LL, Huang ZY, Wang JZ. Upregulated expression of toll-like receptor 4 in monocytes correlates with severity of acute cerebral infarction. J Cereb Blood Flow Metab. 2008;28:1588–1596. doi: 10.1038/jcbfm.2008.50. [DOI] [PubMed] [Google Scholar]
- Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q, Lu FL, Xiang J. High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:243–254. doi: 10.1038/jcbfm.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]