Abstract
Alpha-melanocyte-stimulating hormone (MSH) is a neuropeptide with profound immunomodulatory properties; we evaluated the effects of α-MSH on stroke outcome and its ability to modulate the postischemic immune response. In Lewis rats subjected to 3 hours of middle cerebral artery occlusion (MCAO), plasma concentrations of α-MSH rapidly decreased and returned to baseline over the course of days. Exogenous administration of α-MSH (100 or 500 μg/kg) improved 24 hour outcome in animals subjected to 2 hours MCAO; α-MSH 500 μg/kg also decreased infarct volume at this time point. Both doses of α-MSH were ineffective in improving outcome or decreasing infarct volume in animals subjected to 3 hours MCAO. The splenocyte response to phytohemagglutin in animals treated with α-MSH was attenuated at 24 hours after MCAO. At 1 month after MCAO, treatment with α-MSH 500 μg/kg at the time of stoke was associated with a decrease in TH1 response to myelin basic protein (MBP) in animals subjected to 2 hours MCAO, although treatment was not associated with improved outcome at this time point. Given the early benefits of α-MSH treatment and its effect on immunologic outcome, further studies to evaluate the utility of α-MSH for the treatment of cerebral ischemia are warranted.
Keywords: animal models, immunology, inflammation, neuroprotection, T cells
Introduction
The nature of postischemic immune response affects the outcome from ischemic stroke. TH1-type immune responses to central nervous system antigens are associated with worse outcome from experimental stroke, whereas Treg responses to the same are associated with improved outcome (Becker et al, 1997, 2003, 2005; Chen et al, 2003; Frenkel et al, 2003, 2005; Gee et al, 2008; Zierath et al, 2010). Strategies to prevent a TH1-type response or induce a Treg response would thus seem to be a logical therapeutic intervention for stroke. A Treg response can be induced through mucosal administration of antigen, but there are data that question the long-term safety of this strategy (Bai et al, 1998; Blanas et al, 1996; Gee et al, 2009; Genain et al, 1996; Xiao and Link, 1997). For these experiments, we sought an alternative strategy to induce the generation of central nervous system-specific Treg cells. The neuropeptide α-melanocyte-stimulating hormone (MSH) has been shown to prevent the induction of TH1 responses and to induce Treg responses to selected antigens (Namba et al, 2002; Ng et al, 2007; Taylor and Namba, 2001; Taylor et al, 1994). On the basis of these properties, it is not surprising that α-MSH has been shown to improve outcome in animal models of experimental allergic encephalomyelitis (Han et al, 2007; Taylor and Kitaichi, 2008). Moreover, α-MSH seems to have direct neuroprotective properties and has been shown to improve outcome in experimental models of stroke (Chen et al, 2008; Forslin Aronsson et al, 2006; Giuliani et al, 2006a, 2007; Huh et al, 1997). In this study, we explored the possibility of using α-MSH as an immunomodulatory peptide to improve long-term outcome after stroke.
Materials and methods
Animals
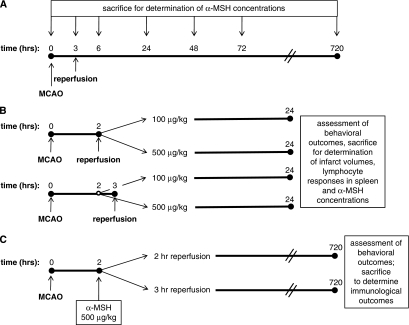
Experiments were approved by the Institution's Animal Care and Use Committee. Male Lewis rats (weighing 250 to 300 g) were used for all studies. Rats were handled before tests/surgical procedures and housed three per cage to eliminate differences in socialization. The experimental paradigms are illustrated in Figure 1.
Figure 1.
Experimental design. Three different sets of experiments were performed—assays for changes in endogenous α-MSH levels after MCAO (A), determination of neuroprotective and immunomodulatory effects at 24 hours (B), and assessment of long-term outcomes (C).
Middle Cerebral Artery Occlusion
Anesthesia was induced with 5% and maintained with 1.5% isoflurane. After midline neck incision, the right common carotid, external carotid, and pterygopalatine arteries were ligated. A monofilament suture (4.0) was inserted into the common carotid artery and advanced into the internal carotid artery as described previously (Becker et al, 2005). Animals were maintained at normothermia during surgery. Reperfusion was performed either 2 or 3 hours after middle cerebral artery occlusion (MCAO). In sham-operated animals, the suture was inserted into the carotid but not advanced.
Plasma α-Melanocyte-Stimulating Hormone Concentrations
Blood was collected by cardiac puncture at the time of killing and the plasma was stored at −80° until use. For each sample, peptides were eluted from 0.5 mL of acidified plasma using SEP columns containing 200 mg C18 (Phoenix Pharmaceuticals, Belmont, CA, USA); the samples were evaporated by centrifugal vacuum concentration and reconstituted in 125 μL buffered saline. Concentrations of α-MSH were determined using a commercially available enzyme immunoassay kit (Phoenix Pharmaceuticals). The time course of α-MSH changes after MCAO were determined at the times indicated in Figure 1A; changes in α-MSH associated with experimental manipulation were assessed as indicated in Figure 1B.
α-Melanocyte-Stimulating Hormone Administration
Two hours after the onset of MCAO (irrespective of the duration of MCAO), animals received either saline or α-MSH (100 or 500 μg/kg; Sigma-Aldrich, St Louis, MO, USA) in phosphate-buffered saline by intraperitoneal injection (Figures 1B and 1C). In a separate group of animals undergoing 2 hours MCAO, α-MSH was administered intranasally; α-MSH (100 μg/kg) was dissolved in 100 μL phosphate-buffered saline and 50 μL instilled into each nares at the time of reperfusion.
Neurologic Outcome
Neurologic outcome was assessed at set time points. Tests included a modification of the Bederson scale (Bederson et al, 1986), the ‘sticky tape test' (Hernandez and Schallert, 1988), rotarod performance (Hunter et al, 2000), and the foot-fault test (Ding et al, 2002). The ‘sticky tape test' assesses sensorimotor function; the time the animal takes to attend to a piece of adhesive tape placed on the affected forelimb is recorded. The rotarod assesses motor coordination and fatigue; animals were trained before surgery until they could remain on a rotating rod at 5 r.p.m. for 100 seconds; after surgery, the longest time animals could remain on the rotarod before falling (100 seconds maximum) was recorded (using the best of 3 trials). The foot-fault test was conducted to test forelimb motor coordination; rats were placed on a wire grid for 3 minutes and the number of times the affected front paw slipped through the grid per total number of steps taken was recorded and expressed as the percentage of the total steps taken.
Infarct Volume
At the time of killing (Figure 1B), the brains were removed and placed in phosphate-buffered saline for 10 minutes. The brains were then sectioned at 2-mm intervals and incubated in 2% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich) at 38°C for 15 minutes. Brain sections (bregma +4.20, +2.20, +0.20, −1.80, −3.80, −5.80) were scanned and digitized. Using ImageJ (version 1.37; NIH, Bethesda, MD, USA), infarct volume was determined and corrected for the presence of edema.
Enzyme-Linked Immunosorbent Spot Assays
Mononuclear cells were isolated from the spleen at 24 hours after MCAO (Figure 1B) or from the infarcted hemisphere of the brain and the spleen at 720 hours after MCAO (Figure 1C) using previously described methods (Becker et al, 2003, 2005). Mononuclear cells were cultured (1 × 105 cells per well) for 48 hours in 96-well plates (MultiScreen-IP; Millipore, Billerica, MA, USA) in lymphocyte proliferation media alone or in media supplemented with phytohemagglutinin (PHA) (Sigma-Aldrich; 2.5 μg/mL); for animals killed at 720 hours after MCAO, responses to MBP (Sigma-Aldrich; 50 μg/mL) and proteolipid protein (PLP) (Sigma-Aldrich; 10 μg/mL) were also assessed. Experiments were performed in triplicate; assays were optimized to detect interferon-γ and transforming growth factor-β1 secretion (antibodies were purchased from R&D Systems, Minneapolis, MN, USA). Spots were counted using a semi-automated system (MetaMorph, Molecular Devices, Silicon Valley, CA, USA). For response to PHA, data are presented as the relative increase in the number of cells secreting interferon-γ to PHA over that seen in media alone. For MBP and PLP, data are presented as the ratio of the relative increase in the number of cells secreting interferon-γ to the relative increase in the number of cells secreting transforming growth factor-β1 to that antigen (i.e., the T1 response).
Statistics
Categorical data were evaluated using the χ2-test statistic. Nonparametric data are displayed as the median and interquartile range and compared using the Mann–Whitney U-test or Kruskall–Wallis H-test. Significance was set at P<0.05.
Results
Endogenous Changes in α-Melanocyte-Stimulating Hormone after Middle Cerebral Artery Occlusion
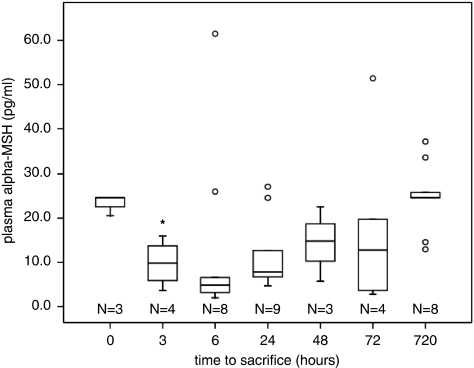
The plasma concentrations of α-MSH varied significantly over the course of time after MCAO (3 hours occlusion; P=0.026; Figure 2). The concentration reached its nadir at 6 hours after MCAO, at which point it was 4.8 (2.7 to 16.3) pg/mL, compared with 24.6 (20.6 to 24.6) pg/mL in naive animals (P=0.022); P=0.165. The variation in α-MSH levels may in part reflect the variability of the stroke model as there was a tendency for animals with the highest neurologic scores to have the lowest α-MSH concentrations; this correlation was most robust at 72 hours after MCAO (r2=−0.754, P=0.084).
Figure 2.
The plasma concentration of α-MSH decreases after MCAO and is significantly less in naive animals at 3 hours after MCAO. Box plots show median, IQR, outliers (error bars), and extreme outliers (open circles); *P<0.05 by Kruskal–Wallis H-test. α-MSH, α-melanocyte-stimulating hormone; IQR, interquartile range; MCAO, middle cerebral artery occlusion.
Effect of α-Melanocyte-Stimulating Hormone Treatment on 24-hour Outcomes
There was no difference in mortality among animals treated with α-MSH and those treated with saline (‘controls'). For animals undergoing 2 hours MCAO, mortality was 0/12 in control animals, 2/12 (17%) in animals treated with 100 μg/kg α-MSH, and 0/12 in animals treated with 500 μg/kg α-MSH; for those undergoing 3 hours MCAO, mortality was 3/15 (20%) in control animals, 2/15 (13%) in animals treated with 100 μg/kg α-MSH, and 2/11 (18%) in animals treated with 500 μg/kg α-MSH.
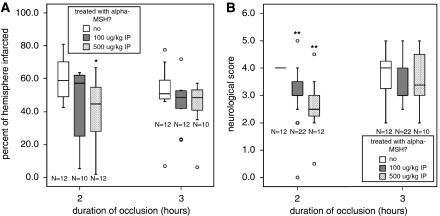
Despite the well-described antipyretic effects of α-MSH, the postischemic fever typically observed in this model of MCAO was not attenuated by α-MSH administration (data not shown). At 24 hours after MCAO, the percentage of the hemisphere in control animals subjected to 2 hours ischemia was 58.7% (48.3% to 72.2%); the percentage of the hemisphere infarcted in animals treated with α-MSH 100 μg was 57.4% (22.8% to 62.0% P=0.147) and in animals treated with α-MSH 500 μg was 44.9% (26.9% to 55.3% P=0.010) (Figure 3A). Neurologic scores were also lower (better) at this time point in animals treated with both α-MSH 100 μg/kg (3.0 (3.0 to 4.0); P<0.001) and α-MSH 500 μg/kg (2.5 (2.3 to 3.0); P<0.001) than in control animals (4.0 (4.0 to 4.0)) (Figure 3B). For animals subjected to 3 hours MCAO, neither the infarct size nor the neurologic scores differed significantly between controls and those receiving low- (100 μg/kg) or high-dose (500 μg/kg) α-MSH (Figure 3).
Figure 3.
Treatment with α-MSH at 500 μg intraperitoneal resulted in decreased infarct volume in animals undergoing 2 hours (but not 3 hours) MCAO. (A) α-MSH at a dose of 100 μg/kg intraperitoneal was not protective for either duration of ischemia. (B) In animals undergoing the shorter duration of ischemia (2 hours), the higher dose of α-MSH (500 μg/kg) also improved neurologic scores at 24 hours. Box plots show median, IQR, outliers (error bars), and extreme outliers (open circles). Differs at *P<0.05 or **P<0.001 by Kruskal–Wallis H test. α-MSH, α-melanocyte-stimulating hormone; IQR, interquartile range; MCAO, middle cerebral artery occlusion.
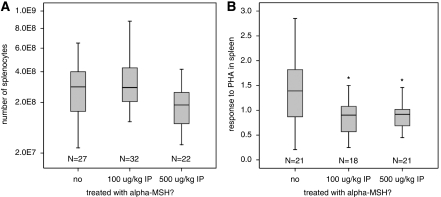
There was no difference in the number of splenocytes at 24 hours after MCAO in animals treated with α-MSH at either the 100 or the 500 μg/kg dose (Figure 4A). However, treatment with α-MSH at either dose was associated with a less robust response of splenocytes to the T-cell mitogen PHA (Figure 4B). For animals receiving α-MSH 500 μg at the time of MCAO (both 2 and 3 hours), plasma concentrations of α-MSH were significantly higher than in saline-treated animals at 24 hours (Table 1 ). Interestingly, we observed that administration of α-MSH 100 μg/kg decreased concentrations of plasma α-MSH at 24 hours. Table 1 also shows that plasma concentrations of α-MSH in control animals tends to be lower in those undergoing 3 hours versus 2 hours MCAO.
Figure 4.
Treatment with α-MSH decreases splenocyte responsiveness to PHA. (A) There were no significant differences in the numbers of splenocytes at 24 hours after stroke among the different treatment groups. (B) Animals treated with either dose of α-MSH, however, had decreased responsiveness of the splenocytes to PHA at this time point. Box plots show median, IQR, and outliers. Differs from control group at *P<0.05 by Mann–Whitney U-test. α-MSH, α-melanocyte-stimulating hormone; IQR, interquartile range.
Table 1. Plasma α-MSH concentrations (pg/mL) 24 hours after MCAO and intraperitoneal administration of α-MSH or saline.
| Dose of α-MSH | Saline | 100 μg/kg | 500 μg/kg | P-value |
|---|---|---|---|---|
| Two hours MCAO (median, IQR) | 27.4 (15.4–49.6) | 10.3 (1.9–16.9) | 38.1 (22.5–82.5) | <0.001 |
| Three hours MCAO (median, IQR) | 9.6 (6.0–44.4) | 4.8 (3.4–5.9) | 38.2 (29.1–52.1) | 0.002 |
α-MSH, α-melanocyte-stimulating hormone; IQR, interquartile range; MCAO, middle cerebral artery occlusion.
Statistics are by the Kruskal–Wallis H-test.
As proof of concept, we explored the potential of administering α-MSH by inhalation. In animals subjected to 2 hours MCAO, intranasal administration of α-MSH (100 μg/kg) at 2 hours after MCAO was associated with lower (better) neurologic outcome at 24 hours (neurologic scores=3.0 (2.3 to 3.0) versus 4.0 (3.0 to 4.0); P=0.016). The percentage of the hemisphere infarcted was also less in these animals as compared with saline-treated animals (42.5% (36.0% to 46.1%) versus 55.7% (48.3% to 72.2%); P=0.003). Plasma concentrations of α-MSH tended to be less in α-MSH-treated animals than in control animals (18.1 (7.3 to 25.3) pg/mL versus 27.4 (15.4 to 49.6) pg/mL; NS), although the difference was not as dramatic as seen in animals that received intraperitoneal α-MSH (Table 1).
Long-Term Neurologic Outcomes
Given the lack of effect on low-dose α-MSH (100 μg/kg) on infarct size, only the higher dose was used in long-term experiments. There was no difference in 1-month mortality among animals treated with α-MSH 500 μg/kg or saline irrespective of the duration of MCAO. For animals subjected to 3 hours MCAO, mortality was 7/19 (37%) in α-MSH-treated animals and 8/29 (28%) in saline-treated animals (NS); for animals subjected to 2 hours MCAO, mortality was 2/16 (13%) in α-MSH-treated animals and 3/15 (20%) in saline-treated animals (NS). Furthermore, there were no significant differences between α-MSH- and saline-treated animals in body temperature, weight, or any of the neurologic outcomes at any time point after stroke for either duration of MCAO.
Immunologic Outcomes
In general, longer durations of MCAO were associated with less robust responses to PHA in the brain (Table 2 ). For animals treated with α-MSH, the longer duration of MCAO was also associated with a reduction in splenocyte numbers and an increase in the number of mononuclear cells in the ischemic brain (similar trends were seen in control animals). Among those animals subjected to 2 hours MCAO (but not 3 hours), the magnitude of the T1 response to MBP in the spleen was reduced by α-MSH (Table 3 ); the decrease in the magnitude of the T1 response to PLP in the spleen among α-MSH-treated animals did not reach statistical significance. T1 responses to both MBP and PLP in the brain were similar among α-MSH- and saline-treated animals.
Table 2. Differences in immunologic parameters at 1 month based on the duration of MCAO.
| Duration of occlusion |
Control |
α-MSH |
||||
|---|---|---|---|---|---|---|
| 2 hours (N=12) | 3 hours (N=12) | P-value | 2 hours (N=12) | 3 hours (N=−12) | P-value | |
| Brain | ||||||
| Response to PHA | 1.50 (1.29–1.87) | 1.16 (1.07–1.35) | 0.038 | 1.73 (1.27–2.44) | 1.30 (1.08–1.44) | 0.028 |
| Number of MNCs ( × 106) | 5.58 (4.26–9.21) | 7.45 (5.59–9.08) | NS | 5.28 (3.62–7.38) | 9.90 (6.7–12.3) | 0.023 |
| TH1 response to MBP | 1.15 (0.71–1.47) | 0.98 (0.76–1.11) | NS | 1.13 (0.94–2.04) | 0.98 (0.80–1.09) | NS |
| TH1 response to PLP | 0.84 (0.51–1.28) | 0.93 (0.74–1.24) | NS | 1.26 (0.87–1.89) | 0.92 (0.68–1.16) | 0.083 |
| Spleen | ||||||
| Response to PHA | 1.06 (0.84–1.48) | 1.15 (0.98–1.52) | NS | 1.03 (0.71–1.26) | 0.98 (0.83–1.22) | NS |
| Number of MNCs ( × 108) | 3.82 (3.32–5.44) | 2.75 (2.43–3.90) | NS | 3.83 (3.44–5.45) | 2.63 (2.15–3.36) | 0.003 |
| TH1 response to MBP | 1.22 (0.80–2.71) | 1.01 (0.83–1.24) | NS | 0.76 (0.56–1.02) | 1.02 (0.89–1.09) | NS |
| TH1 response to PLP | 2.46 (0.84–4.32) | 1.02 (0.67–1.92) | 0.065 | 1.05 (0.87–1.29) | 1.10 (0.90–1.22) | NS |
α-MSH, α-melanocyte-stimulating hormone; IQR, interquartile range; MCAO, middle cerebral artery occlusion; MNC, mononuclear cell; NS, nonsignificant; PHA, phytohemagglutinin.
All data are presented as median (IQR); statistics are by the Mann–Whitney U-test.
Bold and italic values indicate statistically significant.
Table 3. Magnitude of T1 responses to MBP and PLP at 1 month based on the duration of MCAO and α-MSH treatment status.
| Duration of occlusion |
2 hours |
3 hours |
||||
|---|---|---|---|---|---|---|
| Saline (N=12) | α-MSH (N=12) | P-value | Saline (N=12) | α-MSH (N=12) | P-value | |
| TH1 response to MBP | ||||||
| Brain | 1.15 (0.71–1.47) | 1.13 (0.94–2.04) | NS | 0.98 (0.76–1.11) | 0.97 (0.80–1.09) | NS |
| Spleen | 1.22 (0.80–2.71) | 0.76 (0.56–1.02) | 0.021 | 1.01 (0.83–1.24) | 1.02 (0.89–1.09) | NS |
| TH1 response to PLP | ||||||
| Brain | 0.84 (0.51–1.28) | 1.26 (0.87–1.89) | NS | 0.93 (0.74–1.24) | 0.92 (0.68–1.16) | NS |
| Spleen | 2.46 (0.84–4.32) | 1.05 (0.87–1.29) | NS | 1.02 (0.67–1.92) | 1.10 (0.90–1.22) | NS |
α-MSH, α-melanocyte-stimulating hormone; IQR, interquartile range; MCAO, middle cerebral artery occlusion; NS, nonsignificant; PLP, proteolipid protein.
All data are presented as median (IQR); statistics are by the Mann–Whitney U-test.
Bold and italic values indicate statistically significant.
Effect of Immune Status on Outcome
If the entire cohort of animals is considered together, there is a significant inverse correlation between performance on the rotarod and robustness of the T1 response to brain antigens. These relationships are more apparent among control animals; for the T1 response to MBP in the spleen, the correlation coefficient is −0.540 (P=0.006), and for the T1 response to PLP in the spleen, the correlation coefficient is −0.723 (P<0.001). If one considers only the control animals subjected to 2 hours MCAO, the relationships are even more significant (r2=−0.782, P=0.003 and r2=−0.796, P=0.002, for MBP and PLP, respectively). Furthermore, the more robust the T1 response to PLP in the spleen, the more foot faults are made (r2=0.628, P=0.029).
Among animals subjected to 2 hours MCAO, 3 of 12 (25%) control animals and 7 of 12 (58%) α-MSH-treated animals had complete recovery on the rotarod at 1 month (P=0.098). The number of animals with complete recovery on the foot-fault test and sticky tape test were similar among α-MSH-treated and control animals. The T1 response to PLP in the spleen was less in those that fully recovered on the rotarod (0.89 (0.66 to 1.14) versus 2.46 (1.18 to 4.25); P=0.010); T1 responses to MBP and PLP were similar among animals that recovered on the foot-fault test and sticky tape test and those that did not. It is of interest that we observed that some of the animals that initially improved after MCAO experienced delayed worsening with shorter latencies to fall from the rotarod; such worsening occurred in 5 of 12 (42%) control animals and in 1 of 12 (8%) α-MSH-treated animals (P=0.076); treatment with α-MSH did not affect the tendency toward delayed worsening on the foot-fault test (only one animal that initially recovered on the sticky tape test subsequently worsened). There was a trend toward an increase in the T1 response to MBP in the spleen among those that worsened on the rotarod (2.46 (0.85 to 3.8) versus 1.17 (0.88 to 3.87); P=0.093); in the brain, this increase was significant (1.48 (0.96 to 1.72) versus 0.92 (0.75 to 1.31); P=0.030). Similar trends were not seen for the foot-fault test. For animals subjected to 3 hours MCAO, there was no difference in the proportion of control and α-MSH-treated animals experiencing full recovery on the rotarod, and none of the animals that recovered experienced subsequent worsening.
Discussion
Alpha-MSH is a 13 amino-acid neuropeptide produced primarily in the intermediate lobe of the pituitary; it is also produced by other cells within the central nervous system and by cells of the immune system (Brzoska et al, 2008). It is an attractive candidate for stroke therapy, given its multiplicity of actions, all of which suggest it should lessen ischemic brain injury. In fact, experimental studies have shown improved outcome associated with α-MSH treatment in models of global (Forslin Aronsson et al, 2006; Giuliani et al, 2006b) and focal cerebral ischemia (Chen et al, 2008; Giuliani et al, 2007). The attractiveness of α-MSH as a therapeutic agent is further enhanced by its potential ease of administration—neuropeptides similar to α-MSH are absorbed through the nasal mucosa rapidly after administration (Born et al, 2002).
In this study, we documented a rapid decrease in plasma α-MSH concentrations after MCAO. To our knowledge, there are no published data addressing endogenous changes in α-MSH after cerebral ischemia. In a clinical study of patients with brain injury (either traumatic or resulting from subarachnoid hemorrhage), however, systemic decreases in α-MSH were documented; furthermore, lower concentrations of α-MSH were associated with worse neurologic outcomes (Magnoni et al, 2003). Similar decreases in circulating α-MSH were observed in critically ill patients without neurologic injury (Catania et al, 2000; Todd et al, 2009). Secretion of α-MSH from the pars intermedia is under hypothalamic control; release is tonically inhibited by dopamine and γ-amino butyric acid (Saland, 2001). There are a multitude of possible feedback loops which might result in decreased α-MSH secretion, but little attention has been paid to the feedback inhibition by α-MSH itself. Despite the fact that the half-life of α-MSH in circulation is only minutes, we found that animals treated with α-MSH 100 μg at the time of stroke actually had lower α-MSH concentrations of the neuropeptide at 24 hours after MCAO than did animals treated with saline, suggesting that this small dose somehow affected the regulation of peptide process and secretion (Reith and Neidle, 1981). Conversely, treatment with α-MSH 500 μg/kg at the time of stroke was associated with elevated plasma concentrations of the neuropeptide at 24 hours after MCAO, indicating that such a large dose is not as rapidly metabolized or affects the regulation of endogenous α-MSH secretion in a different way. If the degree of brain injury alters endogenous α-MSH secretion, it is also possible that the relative increase in plasma α-MSH at 24 hours after MCAO reflects the fact that a higher dose of α-MSH decreased infarct size.
Exogenous administration of α-MSH at both doses improves stroke outcome at 24 hours in animals subjected to 2 hours MCAO, although an effect on infarct size could only be seen with the 500 μg/kg dose. However, this same dose was ineffective in improving short-term outcome or decreasing infarct size in animals subjected to 3 hours MCAO. The lack of benefit in animals with longer duration of ischemia is likely related to the fact that the degree of injury is more significant and thus less likely to be affected by therapeutic interventions. These short-term neuroprotective properties of α-MSH have been documented previously (Chen et al, 2008; Forslin Aronsson et al, 2006; Giuliani et al, 2006b, 2007). Moreover, although the immunomodulatory properties of α-MSH are clear, we are the first to explore their contribution to long-term stroke outcome. Similar to our findings in other studies (Gee et al, 2008, 2009; Zierath et al, 2010), the data in this study show an inverse relationship between the T1 immune response to brain antigens in the spleen and performance on the rotarod in animals subjected to 2 hours MCAO. Treatment with α-MSH 500 μg/kg was associated with a decreased T1 response to MBP (and a trend toward a decreased response to PLP), but did not translate into a clear benefit in neurologic outcome at 1 month. If the effects of α-MSH on the immune response could be accentuated, it is possible that this intervention may prove to have long-term benefits. Given the short half-life of α-MSH (Redding et al, 1978), it would be reasonable to use repeated dosing of this neuropeptide over the initial days after MCAO.
This study did not directly assess the mechanism of action of α-MSH. It is possible that the tendency toward decreased immune response to α-MSH-treated animals resulted from a decrease in infarct size/severity, either by direct neuroprotective properties or by its effect on the immediate inflammatory response. In addition to repeated dosing of α-MSH in future experiments, delayed administration of α-MSH at a time point when it would have no effect on infarct volume, but could still affect the immune response (i.e., 24 hours after MCAO), is warranted and might help to better determine whether long-term benefits are immunologically mediated.
Cerebral ischemia seems to lead to a sympathetically mediated depression of immune function that predisposes to infection (Prass et al, 2003). An important observation in this study is that an additional hour of cerebral ischemia significantly affects the immune response; more mononuclear cells are found in the brains of animals undergoing 3 hours MCAO, but these cells are less reactive to PHA than animals undergoing 2 hours MCAO. The response to PHA is blunted in splenocytes irrespective of the duration of ischemia, and longer periods of ischemia are associated with decreased numbers of splenocytes. In a mouse model of stroke, increased concentrations of α-MSH were found in bronchial alveolar fluid after MCAO, and administration of α-MSH was shown to worsen the immune dysfunction leading to propagation of pulmonary bacterial infection with increased mortality (Schulte-Herbruggen et al, 2008). Despite the fact that we observed a decrease in the response of splenocytes to PHA in α-MSH-treated animals 24 hours after MCAO, we did not see an increase in mortality in α-MSH-treated animals in longer-term experiments. Furthermore, we found that there was an endogenous decrease in the circulating concentrations of α-MSH after MCAO.
In summary, we found that systemic α-MSH concentrations decrease after ischemic stroke and that a single dose of α-MSH administered 2 hours after stroke onset decreased infarct size and improved neurologic outcome at 24 hours and decreased the T1 response to MBP in the spleen (as assessed at 1 month after MCAO). Our data further suggest that attempts to therapeutically modulate the immune response after stroke onset in models of severe stroke may be difficult; shorter periods of ischemia may allow for more effective immunotherapy. Finally, we observed that animals with more robust T1 immune responses to MBP and PLP experience worse outcomes after experimental stroke and are more likely to deteriorate after a period of initial recovery, suggesting that the immune response to these antigens influences outcome. Given these observations and the fact that the neuropeptide can be safely and effectively administered through the nasal mucosa, we believe that α-MSH could have an important role in the treatment of stroke.
The authors declare no conflict of interest.
Footnotes
This work was supported by grants from the National Institutes of Neurological Disorders and Stroke (NINDS) (1RO1NS056457) and the American Heart Association Pacific Mountain Affiliate (0455505Z).
References
- Bai XF, Li HL, Shi FD, Liu JQ, Xiao BG, Van der Meide PH, Link H. Complexities of applying nasal tolerance induction as a therapy for ongoing relapsing experimental autoimmune encephalomyelitis (EAE) in DA rats. Clin Exp Immunol. 1998;111:205–210. doi: 10.1046/j.1365-2249.1998.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury. Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, Hallenbeck JM. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci USA. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Blanas E, Carbone FR, Allison J, Miller JF, Heath WR. Induction of autoimmune diabetes by oral administration of autoantigen. Science. 1996;274:1707–1709. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29:581–602. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- Catania A, Cutuli M, Garofalo L, Airaghi L, Valenza F, Lipton JM, Gattinoni L. Plasma concentrations and anti-L-cytokine effects of alpha-melanocyte stimulating hormone in septic patients. Crit Care Med. 2000;28:1403–1407. doi: 10.1097/00003246-200005000-00024. [DOI] [PubMed] [Google Scholar]
- Chen G, Frokiaer J, Pedersen M, Nielsen S, Si Z, Pang Q, Stodkilde-Jorgensen H. Reduction of ischemic stroke in rat brain by alpha melanocyte stimulating hormone. Neuropeptides. 2008;42:331–338. doi: 10.1016/j.npep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, Hallenbeck JM. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci USA. 2003;100:15107–15112. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Zhou Y, Lai Q, Li J, Park H, Diaz FG. Impaired motor activity and motor learning function in rat with middle cerebral artery occlusion. Behav Brain Res. 2002;132:29–36. doi: 10.1016/s0166-4328(01)00405-3. [DOI] [PubMed] [Google Scholar]
- Forslin Aronsson S, Spulber S, Popescu LM, Winblad B, Post C, Oprica M, Schultzberg M. Alpha-melanocyte-stimulating hormone is neuroprotective in rat global cerebral ischemia. Neuropeptides. 2006;40:65–75. doi: 10.1016/j.npep.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic D, Hancock W, Moskowitz M, Weiner H. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Zierath D, Hadwin J, Savos A, Kalil A, Thullbery M, Becker KJ. Long term immunologic consequences of experimental stroke and mucosal tolerance. Exp Transl Stroke Med. 2009;1:3. doi: 10.1186/2040-7378-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, Linington C, Raine CS, Hauser SL. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996;274:2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Leone S, Mioni C, Bazzani C, Zaffe D, Botticelli AR, Altavilla D, Galantucci M, Minutoli L, Bitto A, Squadrito F, Guarini S. Broad therapeutic treatment window of [Nle(4), D-Phe(7)]alpha-melanocyte-stimulating hormone for long-lasting protection against ischemic stroke, in Mongolian gerbils. Eur J Pharmacol. 2006a;538:48–56. doi: 10.1016/j.ejphar.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Mioni C, Altavilla D, Leone S, Bazzani C, Minutoli L, Bitto A, Cainazzo MM, Marini H, Zaffe D, Botticelli AR, Pizzala R, Savio M, Necchi D, Schioth HB, Bertolini A, Squadrito F, Guarini S. Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology. 2006b;147:1126–1135. doi: 10.1210/en.2005-0692. [DOI] [PubMed] [Google Scholar]
- Giuliani D, Ottani A, Mioni C, Bazzani C, Galantucci M, Minutoli L, Bitto A, Zaffe D, Botticelli AR, Squadrito F, Guarini S. Neuroprotection in focal cerebral ischemia owing to delayed treatment with melanocortins. Eur J Pharmacol. 2007;570:57–65. doi: 10.1016/j.ejphar.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Han D, Tian Y, Zhang M, Zhou Z, Lu J. Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated alpha-melanocyte-stimulating hormone-transduced PLP139-151-specific T cells. Gene Ther. 2007;14:383–395. doi: 10.1038/sj.gt.3302862. [DOI] [PubMed] [Google Scholar]
- Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- Huh SK, Lipton JM, Batjer HH.1997The protective effects of alpha-melanocyte stimulating hormone on canine brain stem ischemia Neurosurgery 40132–139.discussion 9–40 [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, Parsons AA. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Magnoni S, Stocchetti N, Colombo G, Carlin A, Colombo A, Lipton JM, Catania A. Alpha-melanocyte-stimulating hormone is decreased in plasma of patients with acute brain injury. J Neurotrauma. 2003;20:251–260. doi: 10.1089/089771503321532833. [DOI] [PubMed] [Google Scholar]
- Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J Leukoc Biol. 2002;72:946–952. [PubMed] [Google Scholar]
- Ng TF, Kitaichi N, Taylor AW. In vitro generated autoimmune regulatory T cells enhance intravitreous allogeneic retinal graft survival. Invest Ophthalmol Vis Sci. 2007;48:5112–5117. doi: 10.1167/iovs.07-0175. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding TW, Kastin AJ, Nikolics K, Schally AV, Coy DH. Disapearance and excretion of labeled alpha-MSH in man. Pharmacol Biochem Behav. 1978;9:207–212. doi: 10.1016/0091-3057(78)90166-1. [DOI] [PubMed] [Google Scholar]
- Reith ME, Neidle A. Breakdown and fate of ACTH and MSH. Pharmacol Ther. 1981;12:449–461. doi: 10.1016/0163-7258(81)90092-9. [DOI] [PubMed] [Google Scholar]
- Saland LC. The mammalian pituitary intermediate lobe: an update on innervation and regulation. Brain Res Bull. 2001;54:587–593. doi: 10.1016/s0361-9230(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Quarcoo D, Brzoska T, Klehmet J, Meisel A, Meisel C. Alpha-MSH promotes spontaneous post-ischemic pneumonia in mice via melanocortin-receptor-1. Exp Neurol. 2008;210:731–739. doi: 10.1016/j.expneurol.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Taylor A, Namba K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) Immunol Cell Biol. 2001;79:358–367. doi: 10.1046/j.1440-1711.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Kitaichi N. The diminishment of experimental autoimmune encephalomyelitis (EAE) by neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) therapy. Brain Behav Immun. 2008;22:639–646. doi: 10.1016/j.bbi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW, Cousins SW. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation. 1994;1:188–194. doi: 10.1159/000097167. [DOI] [PubMed] [Google Scholar]
- Todd SR, Kao LS, Catania A, Mercer DW, Adams SD, Moore FA. Alpha-melanocyte stimulating hormone in critically injured trauma patients. J Trauma. 2009;66:465–469. doi: 10.1097/TA.0b013e31818b1e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao BG, Link H. Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85:119–128. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- Zierath D, Thullbery M, Hadwin J, Gee JM, Savos A, Kalil A, Becker KJ. CNS immune responses following experimental stroke. Neurocrit Care. 2010;12:274–284. doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]