Abstract
Neural and oligodendrocyte progenitor cells in the adult brain express Ascl1 (also known as Mash1), a basic helix-loop-helix transcription factor. We examined the progeny and fate of this progenitor population in adult male Ascl1-CreERTM;R26R-stop-yellow fluorescent protein mice subjected to right middle cerebral occlusion over 60 days after stroke using inducible Cre recombination to label Ascl1-expressing cells at poststroke days 2 to 6 in vivo. Seven days after stroke, a substantial increase in Ascl1 lineage cells was detected in the ipsilateral subventricular zone (SVZ), striatum, and corpus callosum. These cells exhibited proliferating progenitor cell phenotypes (Sox2+, BrdU+, and Ki67+). Although Ascl1 lineage cells in the ipsilateral SVZ gradually decreased during 14 to 60 days after stroke, Ascl1 lineage cells in the ischemic striatum revealed a remarkable increase during this period. Thirty and sixty days after stroke, Ascl1 lineage cells in the ischemic striatum gave rise to GABAergic neurons and mature oligodendrocytes. In contrast, none of the Ascl1 lineage cells in the contralateral striatum exhibited neuronal and oligodendrocyte phenotypes. Moreover, Ascl1 lineage cells in the corpus callosum were only fated to become mature oligodendrocytes. Our data suggest that Ascl1 lineage cells contribute to stroke-induced neurogenesis and oligodendrogenesis in the adult ischemic brain.
Keywords: Ascl1, bHLH transcription factor, neural stem cells, oligodendrocyte progenitor, stroke, subventricular zone (SVZ)
Introduction
The adult rodent subventricular zone (SVZ) of the lateral ventricle is composed of migratory neuroblasts, actively proliferating progenitor cells, and quiescent neural stem cells (Lois and Alvarez-Buylla, 1994; Doetsch et al, 1997). Neuroblasts in the SVZ travel the rostral migratory stream to the olfactory bulb where they differentiate into granule and periglomerular neurons throughout adult life (Morshead et al, 1994). Subventricular zone neural stem cells generate oligodendrocyte progenitor cells and oligodendrocytes that distribute to the corpus callosum (Menn et al, 2006). Cerebral ischemia promotes proliferation of actively proliferating SVZ cells and recruits SVZ neuroblasts to the ischemic boundary regions (Jin et al, 2001; Zhang et al, 2001; Arvidsson et al, 2002; Parent et al, 2002). In contrast, the contribution of neural progenitor cells to oligodendrogenesis in the ischemic brain is understudied, although stroke triggers oligodendrogenesis (Gregersen et al, 2001; Dewar et al, 2003; Zhang et al, 2009). Moreover, molecular mechanisms underlying stroke-induced neurogenesis and oligodendrogenesis have not been extensively investigated.
Ascl1 (also known as Mash1) is a basic helix-loop-helix transcription factor, which is transiently expressed in neural progenitor cells (Guillemot et al, 1993; Battiste et al, 2007). During development, Ascl1 directs neurogenesis and oligodendrogenesis (Parras et al, 2007). In vivo lineage tracing of Ascl1-expressing cells in the SVZ of the adult mouse reveals that actively proliferating SVZ cells express Ascl1 and that Ascl1 lineage cells are fated to become neurons in the olfactory bulb and oligodendrocytes in the corpus callosum, but not astrocytes (Kessaris et al, 2006; Battiste et al, 2007; Kim et al, 2007). New neurons and oligodendrocytes have promising potential to facilitate ischemic brain repair (Lindvall et al, 2004; Zhang and Chopp, 2009). As Ascl1 is a transcription factor with essential regulatory functions in directing both neurogenesis and oligodendrogenesis (Battiste et al, 2007; Parras et al, 2007), we investigated in vivo the generation of Ascl1 lineage progenitor cells and their ultimate fate in ischemic brain by means of the inducible Ascl1-CreERTM mouse.
Materials and methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Henry Ford Hospital.
Bigenic Ascl1-CreERTM; R26R-Stop-Yellow Fluorescent Protein Mice
A pair of Ascl1-CreERTM mice was kindly provided by Dr Johnson (University of Texas Southwestern Medical Center) (Kim et al, 2007). The Ascl1-CreERTM mouse is a bacterial artificial chromosome transgenic mouse in which sequences encoding a Cre recombinase fused with a modified estrogen receptor replace the Ascl1 coding sequence (Battiste et al, 2007; Kim et al, 2007). A pair of R26R-stop-yellow fluorescent protein (YFP) mice, a Cre recombinase reporter strain (Srinivas et al, 2001), was purchased from Jackson Laboratory (Bar Harbor, ME, USA). Bigenic Ascl1-CreERTM; R26R-stop-YFP mice were generated by breeding the Ascl1-CreERTM mouse with the YFP reporter mouse. Mice were genotyped by PCR using genomic DNA and primers previously published for Ascl1-CreERTM (Battiste et al, 2007; Kim et al, 2007) and R26R reporter (Srinivas et al, 2001) mice.
Animal Model of Stroke and Tamoxifen Treatment
Adult male Ascl1-CreERTM; R26R-stop-YFP mice aged 2 to 3 months with the genotype Ascl1-CreERTM/+R26R-stop-YFP/+ were used in this study. The right middle cerebral artery (MCA) was permanently occluded by inserting a 6-0 nylon filament as described previously (Zhang et al, 1998). These mice received intraperitoneal injection of tamoxifen (300 mg/kg, Sigma Chemical, St Louis, MO, USA) in sunflower seed oil daily for 5 consecutive days starting 48 hours after stroke. The 48 hours time point was selected because we previously showed a significant increase in proliferating SVZ cells 2 days after stroke (Zhang et al, 2004). The dose of tamoxifen was selected on the basis of published studies (Kim et al, 2007). These animals were killed 7, 14, 30, and 60 days after stroke (Figure 1A). Nonischemic mice with the genotype Ascl1-CreERTM/+R26R-stop-YFP/+ or the genotype Ascl1-CreERTM/−R26R-stop-YFP/− mice received the same tamoxifen treatment and were killed after 5 days of injection and used as controls.
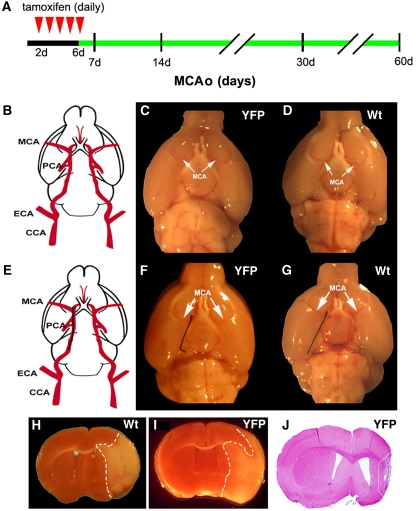
Figure 1.
Experimental protocol and ischemic lesion. (A) A diagram of experimental design for analysis of the progeny and fate of Ascl1-expressing cells. Tamoxifen was injected (intraperitoneally) daily for 5 consecutive days starting 2 days after the onset of stroke (red arrowheads) and Ascl1-CreERTM;R26R-stop-YFP mice were killed 7, 14, 30, and 60 days after stroke. (B) A schematic drawing of mouse carotid and cerebral arteries. (C and D) Gross structure of the MCA (arrows) in Ascl1-CreERTM;R26R-stop-YFP (panel C, YFP) and wild-type mice (panel D, Wt). (E) Schematic drawing of the mouse right internal carotid artery with a filament (black). (F and G) A nylon filament within the right intracranial segment of the internal carotid artery of Ascl1-CreERTM;R26R-stop-YFP (panel F, YFP) and wild-type mice (panel G, Wt). (H and I) Brain coronal sections show ischemic lesion (outlined by white dash line) in the territory supplied by the right MCA assayed by triphenyltetrazolium chloride staining 48 hours after MCAo in wild-type (panel H, Wt) and Ascl1-CreERTM;R26R-stop-YFP (panel I, YFP) mice. (J) A coronal section stained by H&E of an infarction 1 month after MCAo in Ascl1-CreERTM;R26R-stop-YFP mouse (YFP). CCA, common carotid artery; ECA, external carotid artery; H&E, hematoxylin and eosin; MCA, middle cerebral artery; PCA, posterior cerebral artery; MCAo, middle cerebral artery occlusion; YFP, yellow fluorescent protein.
Bromodeoxyuridine Labeling
Bromodeoxyuridine (BrdU), the thymidine analog that is incorporated into the DNA of dividing cells during the S phase, was used for mitotic labeling (Sigma Chemical). Ischemic mice were intraperitoneally injected with BrdU (100 mg/kg) daily for 5 consecutive days starting 48 hours after stroke.
Brain Tissue Preparation and Immunohistochemistry
Animals were transcardially perfused with heparinized saline, followed by 4% paraformaldehyde. The brains were removed from the skull, fixed further in 4% formaldehyde for 4 hours at 4°C, and then transferred into 30% sucrose in phosphate-buffered saline for 24 hours. The brains were embedded and frozen in optimal cutting temperature compound. A series of 30-μm-thick brain coronal sections were cut in a cryostat from the bregma (1.18 mm to −0.82 mm) for the mouse (Franklin and Paxinos, 1997).
Every fifth section was used for immunohistochemistry, as described previously (Zhang et al, 2001). The following antibodies were used in this study: sheep anti-BrdU (1:100, Abcam, Cambridge, MA, USA), mouse anti-nestin (1:100, BD Bioscience, Franklin, NJ, USA), rabbit anti-Ki67 (1:300, Thermo, Fremont, CA, USA), goat anti-doublecortin (DCX, 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), got anti-Sox2 (1:500, Santa Cruz Biotechnology), mouse anti-NeuN (1:500, Chemicon/Millipore, Billerica, MA, USA), chicken anti-green fluorescent protein (1:500, Aves Labs, Tigard, OR, USA), rabbit anti-DARPP32 (1:200, Cell Signaling Technology, Danvers, MA, USA), mouse anti-calbindin (1:800, Swant/Fisher Scientific, Waltham, MA, USA), rabbit anti-GAD-47 (1:500, Sigma Chemical), rabbit-anti-GFAP (glial fibrillary acidic protein) (1:10,000, Dako, Carpinteria, CA, USA), rabbit anti-NG2 (1:800, Chemicon/Millipore), and mouse anti-2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase, 1:200, Chemicon/Millipore). Cell nuclei were stained with DAPI (4′, 6′-diamidino-2-phenylindole).
Double immunofluorescent images were acquired using a Zeiss (Zeiss, Thornwood, NY, USA) LSM 510 Meta-NLO system with Coherent Chameleon Ti:Sa laser. Three-color images were scanned using 488 nm argon, 543 HeNe, and Chameleon (750 nm for DAPI) lasers.
Quantification of Yellow Fluorescent Protein+ Cells
Stereological unbiased estimates of the total numbers of YFP+ cells within the regions of interest were obtained using a microcomputer imaging device stereology software (3D Fractionator, InterFocus Imaging, Cambridge, England), which drives the Ludl X-Y-Z-motorized stage of the actual microscope stage (Olympus BX61, Olympus, Center Valley, PA, USA) focus position. The YFP+ cells were identified with antibodies against green fluorescent protein. In brief, using the automated optical fractionator method, we drew the corpus callosum, striatal, and SVZ areas on coronal sections at a × 4 objective. A higher power (a × 60 objective, NA 1.4) was then selected, and the system used random systematic sampling to sample 30% of the defined region. When the system moved to the first location within the region of interest, a counting frame was placed over the selected area. We then counted the number of immunostained cells by focusing up and down and marking targets within the counting frame. Data are presented as an estimate of the total number of green fluorescent protein+ cells in defined regions.
Statistical Analysis
Data were evaluated for normality. Data transformation would be considered if data were abnormal. The average of the two measurements was taken for each mouse at each location at each time point. One-way analysis of variance was used to study the time effect on each region of interest. The analysis began with testing for the overall time effect, followed by pairwise group comparisons. All data are presented as mean±s.e. Statistical significance was set at P<0.05.
Results
Occlusion of the Middle Cerebral Artery Results in Ischemic Lesion
To examine whether Ascl1-CreERTM;R26R-stop-YFP mice have any gross cerebral vascular abnormality, we compared gross cerebral vessels in Ascl1-CreERTM;R26R-stop-YFP mice aged 2 to 3 months with age-matched wild-type mice. The gross structure of the circle of Willis and the MCA in Ascl1-CreERTM;R26R-stop-YFP mice (Figures 1B and 1C) were comparable with the structure in wild-type mice (Figures 1B and 1D). Occlusion of the right MCA (MCAo) with a nylon filament in these mice resulted in an ischemic lesion in the territory supplied by the MCA assayed by triphenyltetrazolium chloride staining (Figures 1E to 1I). These data indicate that MCAo in Ascl1-CreERTM;R26R-stop-YFP mice generates an ischemic lesion.
Stroke did not Alter the Ascl1-Expressing Cell Profile in the Contralateral Hemisphere
Before tracking the progeny of Ascl1-expressing cells in the ischemic brain, we verified tamoxifen-inducible Cre-mediated recombination targeted at Ascl1-expressing cells in the young adult mice without MCAo. Ascl1-CreERTM;R26R-stop-YFP mice aged 2 to 3 months were administered tamoxifen daily for 5 five consecutive days and the brains were harvested 1 day after the last injection. The YFP+ cells were detected in the SVZ of the lateral ventricles, the corpus callosum, and striatum (Figure 2). Using unbiased stereology analysis, we estimated YFP+ cells. We found that the number of YFP+ cells in the SVZ, corpus callosum, and striatum was 2,032±79, 3,351±133, and 614±83, respectively (Table 1). In contrast, in the absence of tamoxifen, Ascl1-CreERTM;R26R-stop-YFP mice did not exhibit any YFP+ cells in their brains (data not shown). These data indicate successful recombination in Ascl1-expressing cells.
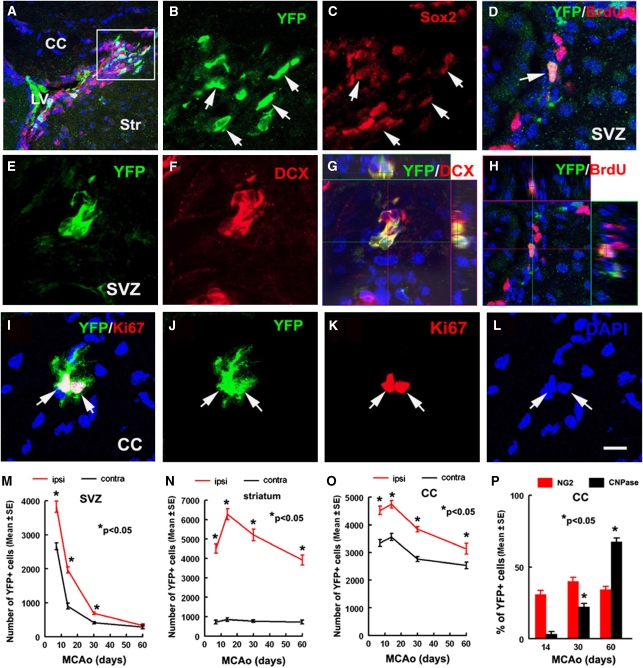
Figure 2.
YFP+ cells are proliferating progenitor cells or neuroblasts in the SVZ and corpus callosum of nonischemic Ascl1-CreERTM;R26R-stop-YFP mice. Double immunostaining shows that YFP+ cells (A, B, arrows) were Sox2+ (panels A, C, arrows), YFP+ cells (E, G, arrows) were DCX+ (panels F, G, arrows), or YFP+ cells (D, H, arrow) were BrdU+ (panels D, H, arrow). (Panels G and H) Orthogonal views. A pair of YFP+ cells (I, J, and L, arrows) was Ki67+ (panels I and K, arrows) in the corpus callosum. (Panels M–P) Line graphs show that the estimated number of YFP+ cells in the SVZ (panel M), striatum (panel N), and corpus callosum (panel O) 7, 14, 30, and 60 days after stroke. (Panel P) Percentage of NG2+ and CNPase+ cells in the ipsilateral corpus callosum 14, 30, and 60 days after stroke. n=6 mice for 7, 30, and 60 days and n=5 mice for 14 days. *P<0.05 versus contralateral for panels A–C, *P<0.05 versus 14 days for panel D. Bars=10 μm. Panels B and C were from a box area in panel A. BrdU, bromodeoxyuridine; CC, corpus callosum; DCX, doublecortin; MCAo, middle cerebral artery occlusion; SVZ, subventricular zone; YFP, yellow fluorescent protein.
Table 1. Percentage of double immunoreactive YFP+ cells in nonischemic and ischemic brains.
| SVZ |
Striatum |
CC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Contra | Ipsi | Normal | Contra | Ipsi | Normal | Contra | Ipsi | |
| Number of YFP+ cells | 2,032±78 | 2,261±99 | 3,825±166*# | 614±83 | 764±98 | 4,514±237*# | 3,351±133 | 3,346±122 | 4,527±150*# |
| % (Cells counted) | |||||||||
| BrdU/YFP | 24.1±2.2 (874) | 23.2±1.8 (964) | 29.7±2.1*# (1,016) | 0.38±0.1 (412) | 0.37±0.2 (437) | 20.01±1.4# (892) | 21.6±1.7 (1,012) | 22.8±1.2 (972) | 26.3±1.2# (948) |
| KI67/YFP | 58.5±3.1 (865) | 60.2±4.4 (1,022) | 71.1±2.2*# (1,236) | 0.33±0.2 (478) | 0.38±0.1 (502) | 33.47±1.9*# (967) | 35.3±2.4 (986) | 36.4±2.4 (832) | 41.4±2.9 (884) |
| SOX2/YFP | 83.1±2.8 (1,078) | 86.6±2.7 (1,112) | 87.3±2.4 (1,621) | 0.58±0.2 (503) | 0.53±0.2 (476) | 47.94±3.8*# (864) | 76.6±2.6 (1,168) | 78.6±3.5 (874) | 82.1±2.0 (983) |
CC, corpus callosum; contra, contralateral; ipsi, ipsilateral; SVZ, subventricular zone; YFP, yellow fluorescent protein.
Data are presented as mean±s.e. Normal indicates data obtained from nonischemic rats killed 1 day after 5 five consecutive day injections of tamoxifen.
* and # are P<0.05 versus contralateral and normal regions, respectively. There were no significant differences between normal and contralateral regions.
Double immunostaining revealed that many (86%) of the YFP+ cells in the SVZ were SOX2+ (Table 1, Figures 2A to 2C), a marker of neural progenitor cells, and ∼63% of YFP+ cells were DCX+ (62.7±3.7, Figures 2E to 2G), a marker of neuroblasts. The YFP+ cells were also BrdU+ after 24 hours exposure (Table 1, Figures 2D and 2H). These data indicate that Ascl1-expressing cells comprise transient amplifying cells and neuroblasts in the SVZ of the lateral ventricles, which is consistent with the published studies (Kim et al, 2007, 2008).
In the corpus callosum, many YFP+ cells appeared as doublets or clusters and were Ki67+ (Figures 2I to 2L, Table 1), suggesting that these YFP+ cells are actively proliferating in situ. Approximately 77% of YFP+ cells were SOX2+ and some YFP+ cells were NG2+ (Table 1). The YFP+/DCX+ or YFP+/GFAP+ cells were not detected in the corpus callosum (data not shown). These data are consistent with published studies that the adult corpus callosum contains many Ascl1-expressing oligodendrocyte progenitor cells (Kim et al, 2007, 2008). In contrast to the SVZ and corpus callosum, <1% of YFP+ cells in the striatum were proliferating (Table 1).
To examine whether stroke affects Ascl1-expressing cells in the contralateral hemisphere, Ascl1-CreERTM;R26R-stop-YFP mice were subjected to MCAo and tamoxifen was administered daily for 5 five days starting 2 days after MCAo. These mice were killed 1 day after tamoxifen treatment (7 days after MCAo). We estimated YFP+ cells in the contralateral hemisphere (nonischemic hemisphere) 7 days after stroke and found that the number of YFP+ cells in the SVZ, corpus callosum, and striatum of the contralateral hemisphere was 2,261±99, 3,346±122, and 764±98, respectively, which is comparable with the numbers in the corresponding regions of nonischemic mouse (Table 1). In the contralateral SVZ, YFP+ cells exhibited phenotypes of transient amplifying cells and neuroblasts (Tables 1 and 2), whereas in the contralateral corpus callosum, YFP+ cells exhibited phenotypes of oligodendrocyte progenitor cells 7 days after stroke (Table 1). The YFP+ cells were not actively proliferating in the contralateral striatum (Table 1). Collectively, these data indicate that stroke does not change the number and phenotypes of Ascl1-expressing cells in the contralateral hemisphere, although recombination was induced 1 day after stroke. Therefore, we used an Ascl1-expressing cell profile in the contralateral hemisphere as a reference for the following experiments.
Table 2. Percentage of YFP+ cells coexpressing neuronal and oligodendrocyte phenotypes in the ischemic striatum.
| Days (sample size) |
Percentages (counted cells) |
||||
|---|---|---|---|---|---|
| DCX/YFP | NN/YFP | Calretinin/YFP | NG2/YFP | CNPase/YFP | |
| 7 (n=6) | 9.5±1.0 (1,021) | 0 | 0 | 21.9±1.9 (774) | 0 |
| 14 (n=5) | 21.2±1.8*(1,154) | 8.4±1.7 (1,012) | 0 | 30.9.4±2.8*(1,024) | 3.4±1.7 (984) |
| 30 (n=6) | 12.7±1.2*(1,062) | 23.5±2.5# (1,226) | 17.7±2.3 (1,124) | 43.5±2.7*(1,134) | 22.4±2.2# (972) |
| 60 (n=6) | NC | 26.3±2.9# (1,092) | 20.7±3.3 (1,118) | 32.9±2.1*(1,232) | 36.7±3.2# (1,022) |
CNPase, 2′,3′-cyclic nucleotide 3′-phosphodiesterase; DCX, doublecortin; NC, not counted; NN, NeuN; YFP, yellow fluorescent protein.
Data are presented as mean± s.e.
* and # P<0.05 versus 7 and 14 day groups, respectively.
Stroke Increases Ascl1 Lineage Cells in the Ipsilateral Subventricular Zone
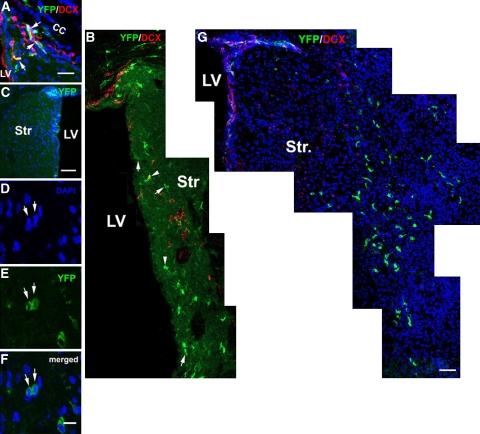
To follow the Ascl1-expressing cells in the ischemic brain, Ascl1-CreERTM;R26R-stop-YFP mice were subjected to MCAo and tamoxifen was administered daily for 5 five days starting 2 days after MCAo. These mice were killed 7, 14, 30, and 60 days after MCAo (Figure 1A). Stereology analysis revealed that 7 days after stroke, the number of YFP+ cells in the ipsilateral SVZ increased by 70% (3,825±166, P<0.05) compared with that in the contralateral SVZ (Figure 2M). Among these YFP+ cells, 30, 71, and 87% were BrdU+, Ki67+, and Sox2+ (Table 1), respectively, whereas 65% were DCX+ (65.4%±3.4%, Figure 3A). These data suggest that after stroke, Ascl1-expressing cells remain as transient amplifying cells and neuroblasts in the SVZ. Fourteen days after stroke, the number of YFP+ cells in the ischemic SVZ was reduced by 49% compared with the number 7 days after stroke (Figure 2M), which was still significantly (P<0.05) higher than the number in the contralateral SVZ where YFP+ cells decreased by 60% (Figure 2M). Many YFP+ cells extended from the ipsilateral dorsal and ventral SVZ toward the ischemic striatum and these cells exhibited bipolar morphology with a long leading process or a cell body with several small processes (Figure 3B), suggesting that YFP+ cells in the ipsilateral SVZ migrate to the ischemic striatum. The number of YFP+ cells in the contralateral and ipsilateral SVZs continuously decreased 30 and 60 days after stroke (Figure 2M). Interestingly, ∼9 and 12% of YFP+ cells remained in the ipsilateral and contralateral SVZ, respectively, 60 days after stroke compared with the number 7 days after stroke (Figure 2M). The YFP+ cells that remained in the SVZ were GFAP negative (data not shown).
Figure 3.
Distribution of YFP+ cells in the ischemic striatum. Double immunostaining shows YFP+/DCX+ cells (A, arrows) in the ipsilateral SVZ 7 days after stroke. Many YFP+ cells with bipolar (B, arrows) and multiple branches (panel B, arrowheads) were distributed in the ipsilateral dorsal and ventral SVZ, whereas YFP+ cells were localized within the contralateral SVZ (C) 14 days after stroke. (D, E) A YFP+ cell in telophase (arrows) during mitosis in the ischemic striatum. (G) Thirty days after stroke, YFP+ cells were distributed to the ischemic boundary region. It must be noted that there were some YFP+ cells that remained in the SVZ. Bars=20 μm in panels A and G, 100 μm in panel C, and 10 μm in panel F. CC, corpus callosum; DCX, doublecortin; LV, lateral ventricle; Str, striatum; SVZ, subventricular zone; YFP, yellow fluorescent protein.
Stroke Increases Ascl1 Lineage Cells that Become Neurons and Oligodendrocyte Progenitor Cells in the Ischemic Striatum
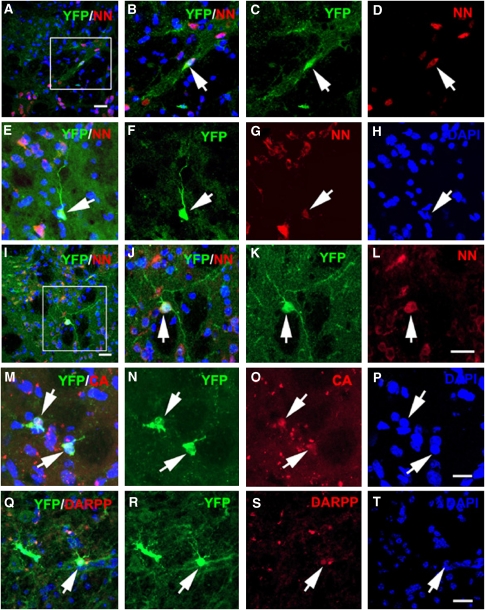
In the ischemic striatum, 7 days after stroke, the number of YFP+ cells increased by 4.6-fold (P<0.01) from 764±98 in the contralateral striatum to 4,514±237 in the ipsilateral striatum (Figure 2N). Approximately 20, 34, and 48% YFP+ cells were BrdU+ (20%±1.46%), Ki67+ (33.5%±1.9%), and Sox2+ (47.9%±3.8%), respectively, indicating that the majority of Ascl1-expressing cells in the ischemic striatum are actively proliferating progenitor cells. Less than 10% of YFP+ cells in the ischemic striatum were DCX+ (Table 2), and none of the YFP+ cells were colabeled with a pan-neuronal marker NeuN+ at this time point. In contrast to the SVZ where YFP+ cells decreased, 14 days after stroke, the number of YFP+ cells in the ischemic striatum further increased (P<0.05) compared with 7 days after stroke (Figures 2N and 3B). The DCX+ cell population also substantially increased to 21% of YFP+ (P<0.05, Table 2), suggesting that Ascl1 lineage neuroblasts in the SVZ migrate to the ischemic striatum during this period. Some of the YFP+ cells formed doublets and exhibited mitosis (Figures 3D to 3F), indicating that these cells are actively proliferating in the ischemic striatum. Previous studies have indicated that ∼80% or more of stroke-induced new neurons die in the ischemic striatum during 2 to 6 weeks after stroke (Arvidsson et al, 2002). We found that the number of YFP+ cells in the ischemic striatum did not decrease during 30 to 60 days compared with the number 7 days after stroke (Figures 2N and 3G). Within the ischemic striatum, YFP+ cells distributed to the ischemic boundary (Figure 3G). The YFP+/NeuN+ cells were observed in the striatal ischemic boundary starting 14 days after stroke (8%, Table 2, Figures 4A to 4D) and the number of YFP+/NeuN+ cells increased by 30 (24%, Table 2, Figures 4E to 4H) and 60 (26%, Table 2, Figures 4I to 4L) days after stroke. The YFP+/NeuN+ cells exhibited multiple long processes (Figures 4E to 4L). Approximately 20% of YFP+ cells exhibited the phenotype of calretinin (Table 2, Figures 4M to 4P), a calcium-binding protein that has been used as a marker of GABAergic interneurons (Gabbott and Bacon, 1996). Few YFP+ cells were DARPP32+ (Figures 4Q to 4T), a marker of medium-sized spiny neurons (Ivkovic and Ehrlich, 1999). However, calbindin and glutamic decarboxylase 67 (GAD-67) immunoreactivity was not detected in YFP+ cells (data not shown). Studies in the adult rodent brain show that a restricted dorsomedial region of the striatum adjacent to the SVZ generates new neurons (Dayer et al, 2005). However, we did not detect YFP+/NeuN+ cells in the contralateral striatum at the time points examined in this study. Our results suggest that Ascl1 lineage cells are destined to become neurons in the ischemic striatum.
Figure 4.
Neuronal phenotypes of YFP+ cells in the ischemic striatum. Double immuostaining shows YFP+/NN+ cells in the ischemic striatum 14 (A–D, arrow), 30 (E–H, arrow), and 60 (I–L, arrow) days after stroke. Some YFP+ cells (M and N, arrows) were calretinin+ (O, arrows) and some (panel Q and R, arrow) were DARPP 32+ (Q and panel S, arrow). (Panel H, panel P, and T) are DAPI nuclei. Panels B–D were from a box area in panel A. Panels J–L) from a box area in panel I. . Bars=20 μm in panels A, I, L, and T; 10 μm in P. CB, calbindin; DARPP, DARPP 32; NN, NeuN; YFP, yellow fluorescent protein.
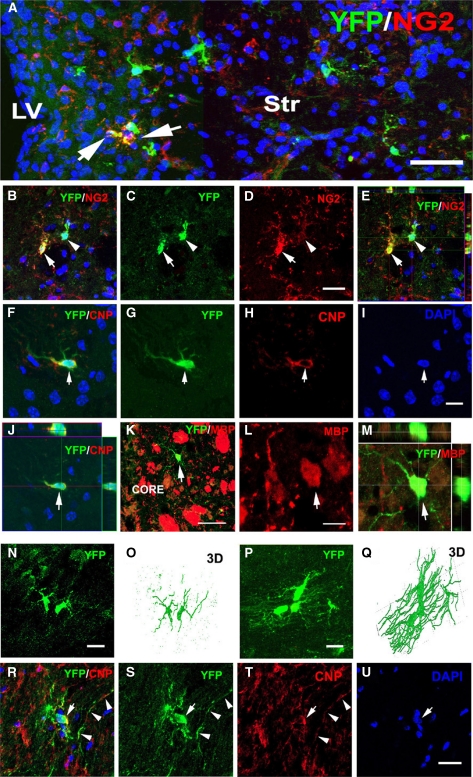
In the ischemic striatum, some of the YFP+ cells exhibited morphologic characteristics of oligodendrocyte progenitor cells and their cell bodies and processes were immunoreactive for the chondroitin sulfate proteoglycan NG2 (Table 2, Figure 5A). Some YFP+ cells exhibited faint NG2 immunostaining (Figures 5B to 5E) 30 days after stroke, and orthogonal reconstruction of confocal images revealed that the cell bodies of YFP+ cells with faint or strong NG2 immuostaining were within the imaged tissue (Figure 5E), indicating that cells with weak NG2 immunosignals are not owing to tissue cutting. This observation suggests that these YFP+ oligodendrocyte progenitor cells may differentiate into mature oligodendrocytes. By immunostaining mature oligodendrocytes with antibodies against CNPase and myelin basic protein (MBP), we found an increase in YFP+/CNPase+ and YFP+/MBP+ cells in the ischemic striatum during 30 to 60 days of stroke (Table 2, Figures 5F to 5M). These data indicate that in addition to neurons, Ascl1 lineage cells are fated to become oligodendrocyte progenitor cells and mature oligodendrocytes.
Figure 5.
Oligodendrocyte phenotypes of YFP+ cells in the ischemic corpus callosum and striatum. Double immunostaining shows that YFP+ cells in the ischemic striatum were NG2+ (A, arrows) 14 days after stroke. YFP+ cells (B, C, E,) with strong (panel B and D, arrow) or weak (panels B and D, arrowhead) were in the ischemic striatum 30 days after stroke. Panel E is an orthogonal view. (F–I) A YFP+ (panel F, panel G, panel I, and K, arrow) and CNPase+ (panels F and H, arrow) or MBP+ (L, arrow) cells in the ischemic striatum 30 days after stroke. (J and M) Orthogonal views. (N–Q) YFP+ cells (panels N and P) and confocal Z-stack 3D reconstructions of the YFP+ cells (panels O and Q) in the ipsilateral corpus callosum 14 (panels N and O) and 30 (panels P and Q) days after stroke. Double immunostaining shows that an YFP+ cell (R, S, and U, arrow) in the ipsilateral corpus callosum was CNPase+ in its cell body (T, arrow) and proximal processes (panel T, arrowheads). Bar=40 μm in panel A, 20 μm in panels D, I, N, P, and U, 50 μm in panel K, and 10 μm in panel L. CNPase, 2′,3′-cyclic nucleotide 3′-phosphodiesterase; core, ischemic core; LV, lateral ventricle; Str, striatum; YFP, yellow fluorescent protein; 3D, three dimensional.
Stroke Increases Ascl1 Lineage Cells that Become Oligodendrocytes in the Ischemic Corpus Callosum
Stroke significantly (P<0.05) increased the number of YFP+ cells in the ischemic corpus callosum compared with the number in the contralateral corpus callosum during 7 to 60 days after ischemia (Figure 2O). Immunostaining revealed that 7 days after stroke, 26 and 41% of YFP+ cells were BrdU+ and Ki67+, respectively, in the ipsilateral corpus callosum (Table 1) and that many YFP+ cells were SOX2+ (82%, Table 1), indicating that Ascl1 cells in the corpus callosum are actively proliferating progenitor cells. Three-dimensional confocal images revealed that 7 and 14 days after stroke, YFP+ cells had multiple radial processes and thin bushy branches (Figures 5N and 5O), which resemble oligodendrocyte progenitor cells (Marshall et al, 2005; Ness et al, 2005). However, 30 and 60 days after stroke, many YFP+ cells exhibited multiple parallel fine process sheaths aligned with white matter axonal fiber tracts (Figures 5P and 5Q) reminiscent of myelinating oligodendrocytes (Ness et al, 2005; Menn et al, 2006). During this period, we observed the cell bodies and proximal processes of YFP+ cells were CNPase+ (Figures 5R to 5U), indicating that they were synthesizing myelin. The number of YFP+/CNPase+ cells substantially increased 30 and 60 days after stroke (Figure 2P). We did not detect any YFP+ cells in the corpus callosum that were NeuN+ or GFAP+ (data not shown). These results suggest that Ascl1-expressing cells in the corpus callosum differentiate into oligodendrocyte progenitor cells and mature oligodendrocytes.
Discussion
This study provides in vivo fate-mapping evidence that stroke substantially increased Ascl1 lineage cells in the ipsilateral SVZ, striatum, and corpus callosum and that the increased Ascl1 lineage cells were fated to become neurons and oligodendrocytes in the ischemic striatum and oligodendrocytes only in the corpus callosum. These data indicate that Ascl1 lineage cells contribute to stroke-induced neurogenesis and oligodendrogenesis in the adult ischemic brain.
Ascl1 Lineage Cells Become Neurons in the Ischemic Striatum
Ascl1 is a pro-neural basic helix-loop-helix transcription factor that is transiently expressed in cells that are within the cell cycle (Guillemot et al, 1993; Battiste et al, 2007). Cells expressing Ascl1 in the Ascl1-CreERTM mouse can be labeled by tamoxifen-inducible Cre-mediated recombination (Kim et al, 2007, 2008). Cre recombination is detectable within 6 hours after tamoxifen treatment, and it persists for ∼24 hours (Hayashi and McMahon, 2002). In this study, we labeled cells expressing Ascl1 by tamoxifen-inducible Cre-mediated recombination for 5 days starting 2 days after stroke, which permitted us to follow the progeny and fate of Ascl1 progenitor cells arising within the first week of the onset of stroke. Our data show that stroke substantially augmented Ascl1 lineage cells in the ischemic striatum and the increased Ascl1 cells persisted at least for 60 days after stroke. A continuous increase in the number of YFP+ cells caused by additional Cre-mediated recombination is unlikely, because Cre recombination ceases 24 hours after tamoxifen injection (Hayashi and McMahon, 2002). Thus, migration of Ascl1 lineage cells in the SVZ toward the ischemic striatum and in situ proliferation of Ascl1 lineage cells likely contribute to an increase in YFP+ cells in the ischemic striatum. Previous studies have shown that stroke recruits SVZ neuroblasts to the ischemic striatum (Jin et al, 2001; Zhang et al, 2001; Arvidsson et al, 2002; Parent et al, 2002). Our lineage tracking experiments show that 7 days after stroke, there was already a 4.6-fold increase in Ascl1-expressing cells and that only 10% of Ascl1 lineage cells were neuroblasts (DCX positive). These findings suggest that in addition to recruitment of SVZ Ascl1-expressing cells, considerable in situ dividing Ascl1-expressing cells likely contribute to the augmentation of Ascl1 lineage cells in the ischemic striatum. During development, Ascl1 progenitor cells contribute to GABAergic interneurons in the striatum (Kim et al, 2007, 2008). However, in the adult brain, Ascl1 progenitor cells give rise to neurons only in the olfactory bulb and the dentate gyrus of the subgranular zone (Kim et al, 2007, 2008). Consistently, this study did not detect any Ascl1 lineage neurons in the contralateral striatum where Ascl1 cells were present. In marked contrast to observations in the contralateral striatum, Ascl1 cells in the ischemic striatum were fated to become mature neurons (NeuN positive) and some of the Ascl1 lineage neurons exhibited the GABAergic interneuron phenotype (calbindin positive). We previously showed that stroke triggers embryonic molecule expression in SVZ neural progenitor cells (Liu et al, 2007). Collectively, these data suggest that stroke may evoke embryonic molecular signals to redirect Ascl1 lineage cells into neurons in the striatum. A recent study has shown that adult Ascl1-expressing hippocampal neural progenitor cells exhibit substantial plasticity by changing their fate from neurons to oligodendrocytes (Jessberger et al, 2008). Our results suggest that adult Ascl1-expressing cells in the SVZ and striatum exhibit plasticity under ischemic conditions. The present data also suggest that there is a window for Ascl1 lineage cells to differentiate into neurons, because 30 days after stroke Ascl1 lineage neurons no longer increase. Further studies aimed to identify molecules that trigger differentiation of Ascl1 lineage cells into neurons that may amplify neurogenesis after stroke are warranted.
Ascl1 Lineage Cells Become Oligodendrocyte Progenitor Cells and Oligodendrocytes in the Corpus Callosum and Striatum
Oligodendrocytes are the myelin-forming glial cells in the adult brain (Levison and Goldman, 1993; Menn et al, 2006). Mature oligodendrocytes do not proliferate and new oligodendrocytes are derived from nonmyelinating oligodendrocyte progenitor cells (Carroll et al, 1990). The corpus callosum of the adult rodent contains heterogeneous oligodendrocyte progenitor cells (Gonzalez-Perez et al, 2009). Most NG2+ cells in the adult brain originate from Ascl1 lineage cells (Parras et al, 2007). Ascl1 could be the earliest oligodendrocyte marker in the corpus callosum based on its expression before Olig2 and Sox10 expression, two early markers of oligodendrocyte progenitor cells (Kim et al, 2007). Approximately 50% of oligodendrocyte progenitor cells in the corpus callosum are within the cell cycle (Rivers et al, 2008). This study confirms that Ascl1 lineage oligodendrocyte progenitor cells are actively proliferating and that these oligodendrocyte progenitor cells differentiate into myelinating oligodendrocytes. Oligodendrocytes are highly susceptible to ischemic insults and the loss of myelin leads to axonal damage after stroke (Pantoni et al, 1996). The contribution of progenitor cells to oligodendrogenesis in the ischemic brain has not been extensively investigated (Dewar et al, 2003). Oligodendrocyte progenitor cells exist in the corpus callosum, the striatum, and the SVZ (Levison and Goldman, 1993; Menn et al, 2006). Our lineage tracking experiments show that a considerable population of Ascl1 lineage cells contributes to NG2+ oligodendrocyte progenitor cells and mature oligodendrocytes in the ischemic corpus callosum and striatum, indicating that stroke augments oligodendrogenesis by Ascl1 lineage cells. We observed that in addition to the striatal Ascl1 lineage NG2+ cells, Ascl1-expressing cells extended from the SVZ to the ischemic striatum were NG2+, suggesting that stroke-induced oligodendrocytes in the striatum differentiate not only from the SVZ but also from parenchymal Ascl1 lineage NG2+ cells. These data indicate that both SVZ- and striatum-derived Ascl1 lineage cells are important for remyelination in the ischemic brain. However, oligodendrocytes in the corpus callosum likely differentiate from local Ascl1 lineage NG2+ cells. This study also revealed Ascl1 lineage oligodendrocytes in the ischemic striatum and corpus callosum, which is consistent with previous findings that Ascl1 lineage oligodendrocytes are heterogeneous and there are different sub-populations of oligodendrocytes in the brain (Parras et al, 2007). During stroke recovery, expansion of the white matter in the ischemic damaged areas has been observed (Li et al, 2009). It is possible that the newly formed Ascl1 lineage oligodendrocytes with myelin sheath observed in this study may function to remyelinate damaged and sprouting axons in the ischemic brain. Remyelination reinstates saltatory conduction and resolves functional deficits of axons (Franklin and Ffrench-Constant, 2008). Thus, amplification of Ascl1 lineage oligodendrogenesis could potentially promote axonal repair, leading to improvement in neurologic function.
The Ascl1 lineage cells gave rise to oligodendrocyte progenitor cells and to mature oligodendrocytes in the corpus callosum even after stroke, which is consistent with previous observations showing that the Ascl1 progeny do not differentiate into neurons in the corpus callosum (Kim et al, 2007, 2008). This is in contrast with Ascl1 lineage cells in the ischemic striatum that gave rise to neurons and oligodendrocytes, suggesting that differences in the local environment may be responsible. The fate determination of Ascl1 lineage cells is context dependent and additional molecular components are required for Ascl1 lineage cells to differentiate into neurons (Kim et al, 2007, 2008).
This study establishes the fate of Ascl1 lineage cells in the ischemic brain, which is consistent with essential regulatory functions of Ascl1 in directing both neurogenesis and oligodendrogenesis (Kim et al, 2007, 2008). Given the fact that recombination is only detected in ∼10% of Ascl1-expressing cells in Ascl1-CreERTM mice, this study indicates that Ascl1 lineage cells make a substantial contribution to neurogenesis and oligodendrogenesis in the ischemic brain. Amplification of Ascl1 expression has the potential to facilitate endogenous brain repair during stroke recovery.
Acknowledgments
The authors thank Dr Jane Johnson at the University of Texas Southwestern Medical Center for providing Ascl1-CreERTM mice, and Drs Jane Johnson and Euiseok Kim for critical comments on this paper.
The authors declare no conflict of interest.
Footnotes
This work was supported by NINDS grant nos PO1 NS23392 and RO1HL 64766.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Carroll WM, Jennings AR, Mastaglia FL. The origin of remyelinating oligodendrocytes in antiserum-mediated demyelinative optic neuropathy. Brain. 1990;113 (Pt 4:953–973. doi: 10.1093/brain/113.4.953. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp Brain Res. 2001;138:384–392. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Ehrlich ME. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J Neurosci. 1999;19:5409–5419. doi: 10.1523/JNEUROSCI.19-13-05409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Battiste J, Nakagawa Y, Johnson JE. Ascl1 (Mash1) lineage cells contribute to discrete cell populations in CNS architecture. Mol Cell Neurosci. 2008;38:595–606. doi: 10.1016/j.mcn.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, Panda S, Kapke A, Lu M, Ewing JR, Chopp M. MRI identification of white matter reorganization enhanced by erythropoietin treatment in a rat model of focal ischemia. Stroke. 2009;40:936–941. doi: 10.1161/STROKEAHA.108.527713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27:564–574. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25:7289–7298. doi: 10.1523/JNEUROSCI.1924-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Ness JK, Valentino M, McIver SR, Goldberg MP. Identification of oligodendrocytes in experimental disease models. Glia. 2005;50:321–328. doi: 10.1002/glia.20206. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA.1996Cerebral white matter is highly vulnerable to ischemia Stroke 271641–1646.discussion 1647 [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parras CM, Hunt C, Sugimori M, Nakafuku M, Rowitch D, Guillemot F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci. 2007;27:4233–4242. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Y, Zhang ZG, Lu M, Borneman J, Buller B, Savant-Bhonsale S, Elias SB, Chopp M. Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab. 2009;29:1166–1174. doi: 10.1038/jcbfm.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Goussev A, Powers C. Cerebral vessels express interleukin 1beta after focal cerebral ischemia. Brain Res. 1998;784:210–217. doi: 10.1016/s0006-8993(97)01317-6. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]