Abstract
Erythropoietin (EPO) enhances angiogenesis in the ischemic brain. Stroke induces secretion of tumor necrosis factor α (TNF-α). We investigated the effect of TNF-α on EPO-induced in vitro angiogenesis in cerebral endothelial cells. Using a capillary-like tubular formation assay, we found that transient incubation of primary rat cerebral microvascular endothelial cells (RECs) with TNF-α substantially upregulated EPO receptor (EPOR) expression and addition of EPO into TNF-α-treated RECs significantly augmented the capillary-like tube formation. Blockage of TNF receptor 1 (TNFR1) suppressed TNF-α-upregulated EPOR expression and abolished EPO-induced tube formation. Attenuation of endogenous EPOR with small interfering RNA (siRNA) also inhibited EPO-enhanced tube formation. Treatment of RECs with EPO activated nuclear factor-kappa B (NF-κB) and Akt. Incubation of the TNF-α-treated endothelial cells with EPO activated vascular endothelial growth factor (VEGF), VEGF receptor 2 (VEGFR2), angiopoietin 1 (Ang1), and Tie2. Blockage of VEGFR2 and Tie2 resulted in reduction of EPO-augmented tube formation. These data indicate that interaction of TNF-α with TNFR1 sensitizes cerebral endothelial cells for EPO-induced angiogenesis by upregulation of EPOR, which amplifies the effect of EPO on activation of the VEGF/VEGFR2 and Ang1/Tie2 pathways. Our results provide the evidence for crosslink between TNF and EPOR to coordinate the onset of angiogenesis in cerebral endothelial cells.
Keywords: angiogenesis, endothelial cell, EPO, EPO receptor, TNF-α
Introduction
Tumor necrosis factor α (TNF-α), a major inflammatory cytokine, has a critical role in many pathological and physiological events (Ashkenazi et al, 1991). The TNF-α is induced within 1 hour in the ischemic brain, reaches a peak 6 to 12 hours, and then subsides 1 to 2 days after stroke (Leibovich et al, 1987; Liu et al, 1994). Elevated TNF-α contributes to both neurotoxic and neuroprotective effects after stroke (Barone et al, 1997; Wilde et al, 2000). The TNF interacts with two types of cell surface receptors, TNF receptor 1 (TNFR1) and TNFR2 (Tacchini-Cottier et al, 1998).
Erythropoietin (EPO) is a hematopoietic cytokine and promotes proliferation and differentiation of erythroid progenitors and the survival of maturing erythroid cells (Watowich, 1999; Wojchowski et al, 1999). The EPO has been shown to have neuroprotective and neurorestorative effects after stroke (Ruscher et al, 2002; Wang et al, 2004a). Administration of recombinant human EPO (rhEPO) promotes interaction with the EPO receptor (EPOR) and augments angiogenesis and neurogenesis in the ischemic brain (Liu et al, 2008; Todokoro et al, 1987; Wang et al, 2004a, 2004b). Mechanisms underlying exogenous EPO-enhanced angiogenesis have not been investigated in the brain. Studies in neuroprotection have shown that TNFR1 targets EPOR and vascular endothelial growth factor (VEGF) to reduce cortical neuronal damage after cerebral ischemia (Taoufik et al, 2008). The TNFR1 and TNFR2 mediate hindlimb ischemia-induced angiogenesis (Luo et al, 2006). Adult cerebral endothelial cells express EPOR (Pillai and Mahadik, 2006; Siren and Ehrenreich, 2001). Therefore, in this study, using a capillary-like tubular formation assay, we tested the hypothesis that TNF-α sensitizes cerebral endothelial cells for EPO-induced angiogenesis by upregulation of EPOR.
Materials and methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital. Male Wistar rats (6 to 8 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Carbamylated EPO (CEPO) is manufactured by Lundbeck A/S (Valby, Denmark) under GMP and released for use in human clinical trials. rhEPO was purchased from AMGEN (Thousand Oaks, CA, USA).
Culture of Rat Brain Microvascular Endothelial Cells
Rat brain microvascular endothelial cells (RECs) were isolated from normal adult rats (n=10), according to published protocols (Wu et al, 2003). Briefly, rats were killed and their brains were collected in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1% penicillin and streptomycin (Invitrogen). Cerebella, white matter, meninges, and visible blood vessels of the brain were removed under a microscope. The cerebral cortex and subcortex of normal rats were cut into small pieces and homogenized. Homogenates were suspended in 15% dextran (Sigma, St Louis, MO, USA) and centrifuged at 6000 g for 15 minutes at 41°C. Pellets were resuspended and digested with 0.1% collagenase/dispase (Roche Applied Science, Penzberg, Germany) and 2% fetal bovine serum albumin (Invitrogen) in RPMI1640. Digested microvessels were separated with 45% Percoll (Sigma) (20,000 g, 10 minutes, 41°C) and plated into Collagen I (BD Biosciences, Bedford, MA, USA) coated plates. Cultures were maintained in endothelial growth medium described by Wu et al (2003). Passage 2 to 4 endothelial cells were used in this study.
Capillary-Like Tube Formation Assay
The RECs (2 × 104 cells) were incubated in Matrigel (BD Biosciences) for 5 hours and capillary-like tube formation was measured. All assays were performed in n=6/group. For quantitative measurements of capillary tube formation, each well was digitized under a × 10 objective (Olympus BX40, Artisan Scientific Corporation, Champaign, IL, USA) for measurement of total tube length of capillary tube formation using a video camera (Sony DXC-970MD, Ampronix Incorporated, Irvine, CA, USA) interfaced with the MCID image analysis system (Imaging Research, St. Catharines, Canada). Tracks of endothelial cells organized into networks of cellular cords (tubes) were counted and averaged in randomly selected five microscopic fields (Rikitake et al, 2002).
Transfection of Rat Brain Microvascular Endothelial Cells with Small Interfering RNA Against Erythropoietin Receptor
The RECs were transfected with small interfering RNA (siRNA)-EPOR (Dharmacon, Inc., Lafayette, CO, USA) using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Briefly, RECs were plated out in six-well plates and cultured until they were 80% confluent. They were then transfected with 100 nmol/L siRNA-EPOR in serum-free medium for 6 hours. Afterward, growth medium +20% fetal bovine serum was added. mRNA and protein expression were measured 48 and 72 hours after transfection.
Immunocytochemistry and Quantification
The RECs were incubated with recombinant human TNF (rhTNF) (0, 2.5, 5, 10, and 50 ng/mL) for 24 hours. Apoptotic cells were labeled by Apoptag In Situ Apoptosis Detection Kit (CHEMICON International, Inc., Temecula, CA, USA) following the manufacture's instruction. The number of apoptotic cells and total cell number were counted and the percentage of apoptotic cells was determined.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using SYBR Green real-time PCR method (Wang et al, 2004a, 2004b). Total RNA was isolated from RECs cultures using the Stratagene Absolutely RNA MicroRNA isolation kit (Stratagene, La Jolla, CA, USA). Quantitative RT-PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, USA) using three-stage program parameters provided by the manufacturer, as follows: 2 minutes at 50°C, 10 minutes at 95°C, and then 40 cycles of 15 seconds at 95°C, and 1 minutes at 60°C. Specificity of the produced amplification product was confirmed by examination of dissociation reaction plots. A distinct single peak indicated that a single-DNA sequence was amplified during PCR. Each sample was tested in triplicate and samples obtained from three independent experiments were used for analysis of relative gene expression using the 2−ΔΔCT method (Livak and Schmittgen, 2001). The following primers for real-time PCR were designed using Primer Express software (ABI): Glyceraldehyde-3-phosphate dehyrogenase (FWD: AGA GAG AGG CCC TCA GTT GCT, REV: TTG TGA GGG AGA TGC TCA GTG T), EPOR (FWD: GAC CCC AGC TCT AAG CTC CT, REW: AGC CCC CTG AG C TGT AAT CT), VEGF (FWD: TGC CTC GTG GGA CTG GAT, REW: CCG GGC TTG GCG ATT T), VEGF receptor 2 (VEGFR2) (FWD: GCA CTT GCA GGC TCC TAA TGA, REW: AGC AAC CTG GGA AGC ATC AC), angiopoietin 1 (Ang1) (FWD: TCT GTT GTC GGT TTT TG GC, REW: GCT TGG CAT CAT AG T GCT GA), Tie2 (FWD: AAG GGC CTA GAG CCA GAG AC, REW: AAG GTC TTT AGG GG C TGG AA).
Western Blot Analysis
Western blots were performed according to published methods (Wang et al, 2006). Briefly, RECs were lysed and sonicated for 10 seconds and centrifuged at 10,000 g for 10 minutes. Protein concentration in the supernatants of cell extract was determined using a BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Equal amounts of proteins were loaded on 10% SDS-polyacrylamide gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes, and the blots were subsequently probed with the following primary antibodies: rabbit polyclonal anti-EPOR (1:1000, Santa Cruz, Santa Cruz, CA, USA), phospho-NF-κB (1:1000, Cell Signaling Technology, Danvers, MA, USA), phospho-Akt (1:1000, Cell Signaling Technology), rabbit polyclonal anti-VEGF (3 μg/mL, Abcam Inc., Cambridge, MA, USA), rabbit polyclonal anti-VEGFR2 (1:1000, Santa cruz), rabbit polyclonal anti-Ang1 (1:1000, Abcam Inc.), rabbit polyclonal anti-Tie2 (1:1000, Santa cruz), and β-actin (1:5000, Abcam Inc.). For detection, horseradish peroxidase-conjugated secondary antibodies were used (1:2000) followed by enhanced chemiluminescence development (Pierce Biotechnology, Inc.). Normalization of results was ensured by running parallel Western blots with the β-actin antibody used as an internal control. The optical density was quantified using an image processing and analysis program (Scion image, Ederick, MA, USA).
Experimental Protocol
(1) To examine the effect of TNF-α, EPO, and CEPO on angiogenesis, RECs were incubated with rhTNF-α (0, 2.5, 5, and 10 ng/mL, R&D system, Minneapolis, MN, USA), rhEPO (0, 1, 10, or 100 ng/mL, epoietin α, AMGEN), or CEPO (0, 1, 10, 100 ng/mL, H Lundbeck AS) for 24 hours. The same volume of saline that was used to dilute rhEPO and rhTNF was used as a control. (2) To examine the effect of TNF-α on EPO-induced angiogenesis, RECs were preincubated with rhTNF at 5 ng/mL for 24 hours and then incubated with rhEPO at 10 ng/mL or CEPO at 1 ng/mL for other 24 hours. (3) To examine whether TNFR1, TNFR2, NF-κB, and Akt signaling pathway are involved in the upregulation of EPOR by TNF, RECs were preincubated with or without the TNFR1 or TNFR2 neutralizing antibodies (10 μg/mL, R&D system) or NF-κB inhibitor SN50 (50 mg/mL, US Biological, Swampscott, MA, USA) or phosphatidylinositol 3-kinase inhibitor LY294002 (10 μmol/L, CALBIOCHEM, Gibbstown, NJ, USA) for 30 minutes and then treated with rhTNF-α (5 ng/mL) for 24 hours. (4) To examine whether EPOR mediates TNF-α and EPO-induced angiogenesis and VEGF and Ang1 expression, RECs were preincubated with TNFR1 neutralizing antibodies (10 μg/mL) or transfected with siRNA-EPOR or scrambled siRNA with rhTNF-α (5 ng/mL) for 24 hours, and then incubated with rhEPO (10 ng/mL) for an additional 24 hours.
Statistical Analysis
Data were evaluated for normality. Two-way or one-way analysis of variance was used to test either the two treatment combination effect or group effect. Analysis started testing the treatment interaction or main effect of the factor, followed by a subgroup analysis. Statistical significance was set at P<0.05. All data are presented as mean±s.e.
Results
Tumor Necrosis Factor-α Enhances Erythropoietin-Induced In Vitro Angiogenesis
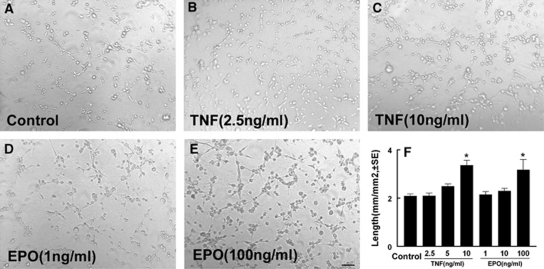
To examine the effect of TNF-α on EPO-induced angiogenesis, we first examined the effect of TNF-α and EPO on angiogenesis by means of a capillary tube formation assay. The RECs were incubated with rhTNF (0, 2.5, 5, and 10 ng/mL) or rhEPO (0, 1, 10, and 100 ng/mL) for 24 hours and capillary-like tube formation was measured. Incubation of RECs with rhTNF or rhEPO induced capillary-like tube formation in a dose-dependent manner (Figure 1). A dose at 5 and 10 ng/mL of rhTNF-α and rhEPO, respectively, did not significantly increase the capillary-like tube formation compared with the control group (Figures 2B, 2C, and 2J). However, preincubation of RECs with rhTNF at 5 ng/mL for 24 hours and then incubation with rhEPO at 10 ng/mL for 24 hours significantly increased the capillary-like tube formation compared with the rhTNF and rhEPO alone groups (Figure 2D and 2J). Interestingly, using the same condition, when we incubated RECs first with rhEPO and then added rhTNF or incubated RECs with both rhEPO and rhTNF at the same time, we found that neither preincubation with rhEPO and then rhTNF (2.6±0.1 mm/mm2) nor rhEPO and rhTNF together (2.5±0.1 mm/mm2) significantly increased the capillary-like tube formation compared with rhTNF alone (2.5±0.1 mm/mm2) and rhEPO alone (2.3±0.2 mm/mm2) groups. Together, these data suggest that rhTNF sensitizes the response of RECs to EPO. The TNF induces apoptosis, whereas EPO reduces apoptotic cell death (Celik et al, 2002; Polunovsky et al, 1994). To examine the effect of TNF and EPO on endothelial cell apoptosis, a terminal deoynucleotidyl transferase-mediated 2'-deoxyuridine 5' triphosphate-biotin-nick end labeling assay was performed. Treatment of RECs with rhTNF did not significantly increase apoptotic cells until the rhTNF dose reached 50 ng/mL (Figure 3A), which is 10 times higher than the dose to sensitize RECs.
Figure 1.
The effect of recombinant human tumor necrosis factor (rhTNF) and recombinant human erythropoietin (rhEPO) on the capillary-like tube formation. (A–E) Representative images of the capillary-like tube formation of rat brain microvascular endothelial cells (RECs) in control (A), rhTNF alone at a dose of 2.5 ng/mL (B), or 10 ng/mL (C), and rhEPO alone at a dose of 1 ng/mL (D), or 100 ng/mL (E). (F) Quantitative data of total lengths of the tube in mm/mm2 (n=6/group). *P<0.05 versus the control group. Bar=100 μm.
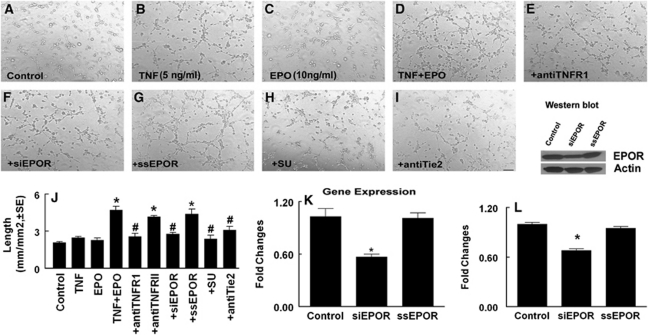
Figure 2.
Recombinant human tumor necrosis factor (rhTNF) sensitizes rat brain microvascular endothelial cells (RECs) for recombinant human erythropoietin (rhEPO)-induced capillary tube formation. (A–I) Representative images of the capillary-like tube formation of RECs in control (A), rhTNF alone (B), rhEPO alone (C), rhTNF and rhEPO (D), and rhTNF and rhEPO with antibody against TNF receptor 1 (TNFR1) (E), small interfering RNA (siRNA)-EPO receptor (EPOR) (F), scramble control (G), SU4832 (H), or antibody against anti-Tie2 (I). (J) Quantitative data of total lengths of the tube in mm/mm2. (K, L) Real-time RT-PCR (K) and Western blot (L) analysis of EPOR mRNA and protein levels in RECs transfected with siRNA-EPOR (siEPOR) and scramble control (ssEPO). Glyceraldehyde-3-phosphate dehyrogenase (GAPDH) and β-actin were used as internal controls for mRNA and proteins, respectively. Bar=100 μm. *P<0.05 and #P<0.05 versus the control and TNF+EPO groups, respectively (n=6/group).
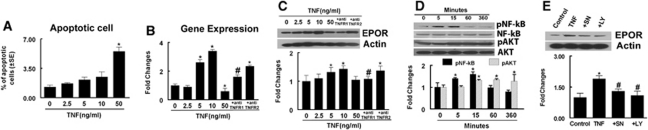
Figure 3.
Effects of recombinant human tumor necrosis factor (rhTNF) on apoptotic cells and erythropoietin receptor (EPOR), NF-κB, and Akt levels. (A) Quantitative data of the percentage of apoptotic cells. Real-time RT-PCR (B) and Western blot (C) show EPOR expression in rat brain microvascular endothelial cell (REC) treated with rhTNF at different concentrations, rhTNF with the antibody against TNFRI (+anti-TNFRI) or against TNFRII (+anti-TNFRII). (D) Western Blot analysis of phosphorylated NF-κB and phosphorylated Akt in RECs treated with rhTNF at different times. (E) Western Blot analysis of EPOR levels in REC treated with rhTNF, rhTNF with the NF-κB inhibitor, SN50 (+SN) or with the phosphatidylinositol 3-kinase (PI3K) inhibitor, LY294002 (+LY). Glyceraldehyde-3-phosphate dehyrogenase (GAPDH) and β-actin were used as internal controls for mRNA and proteins, respectively. *P<0.05 and #P<0.05 versus the control and rhTNF groups, respectively (n=6/group).
Interaction of Recombinant Human Tumor Necrosis Factor with Tumor Necrosis Factor Receptor 1 Induces Erythropoietin Receptor Expression Through the NF-κB and Akt Signaling Pathways
To examine whether EPOR is required for this sensitizing process, we measured EPOR levels in RECs by real-time RT-PCR and Western blot. Pretreatment of RECs with rhTNF at 5 and 10 ng/mL but not 50 ng/mL substantially induced EPOR mRNA and protein levels (Figures 3B and 3C). Blockage of TNFR1 but not TNFR2 with neutralizing antibodies against TNFR1 and TNFR2, respectively, suppressed upregulation of EPOR expression (Figure 3B and 3C), which is consistent with the observation that rhTNF primarily acts on TNFR1 (Tartaglia et al, 1991). These data indicate that interaction of rhTNF with TNFR1 upregulates EPOR expression. To examine whether NF-κB and Akt signaling pathways are involved in the upregulation of EPOR by TNF (Ozes et al, 1999), we assessed levels of phospho-NF-κB and phospho-Akt. Western blot analysis revealed that rhTNF significantly increased phospho-NF-κB at 5 and 15 minutes after incubation, whereas a significant increase in phospho-Akt was detected starting at 15 minutes and persists for at least 6 hours after incubation (Figure 3D). Either an NF-κB inhibitor, SN50 (50 mg/mL) or a phosphatidylinositol 3-kinase inhibitor, LY294002 (10 μmol/L) suppressed TNF-upregulated EPOR expression (Figure 3E), suggesting that activation of NF-κB and Akt triggers upregulation of EPOR.
Erythropoietin Receptor Mediates Tumor Necrosis Factor-α and Erythropoietin-Induced Angiogenesis
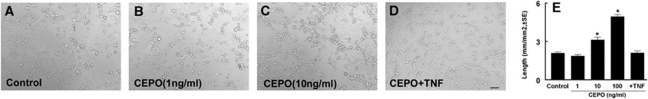
We then examined the cause effect of EPOR on the TNF-sensitizing process for tube formation by blocking EPOR. The RECs transfected with siRNA against EPOR (siRNA-EPOR) exhibited a significant reduction in EPOR expression assayed by real-time RT-PCR and Western blot compared with RECs transfected with scrambled siRNA (Figures 2K and 2L), indicating that the siRNA-EPOR effectively attenuates endogenous EPOR expression. Suppression of endogenous EPOR by siRNA-EPOR significantly blocked the effect of preincubation with rhTNF on EPO-induced capillary tube formation, whereas scrambled siRNA did not reduce the tube formation (Figures 2F, 2G, and 2J). In addition, blockage of TNFR1 but not TNFR2 with the neutralizing antibodies also abolished induction of the tube formation in the presence of rhEPO (Figures 2E and 2J), indicating that EPOR induced by rhTNF/TNFR1 mediates the sensitizing process. To further verify the EPOR effect, we used a CEPO, which does not bind to the EPOR homodimer or monomer (Leist et al, 2004). The CEPO at doses of 10 and 100 ng/mL, but not 1 ng/mL, significantly induced capillary-like tube formation (Figures 4A–4C and 4E). Preincubation of RECs with rhTNF and then incubation with CEPO at 1 ng/mL did not increase capillary-like tube formation compared with the control group (Figures 4A, 4D, and 4E), suggesting that EPOR upregulated by rhTNF does not enhance the CEPO effect on in vitro angiogenesis.
Figure 4.
Rat brain microvascular endothelial cells (RECs) treated with carbamylated erythropoietin (CEPO) promote capillary tube formation. (A–D) Representative images of the capillary-like tube formation of RECs in control (A), CEPO alone at a dose of 1 ng/mL (B), or 10 ng/mL (C), and CEPO (1 ng/mL) + recombinant human tumor necrosis factor (rhTNF) (5 ng/mL) (D). (E) Quantitative data of capillary tube formation. Bar=100 μm. *P<0.05 versus the control group (n=6/group).
Erythropoietin Receptor Upregulated by Tumor Necrosis Factor-α Triggers Expression of Vascular Endothelial Growth Factor and Angiopoietin 1 in Rat Brain Microvascular Endothelial Cells
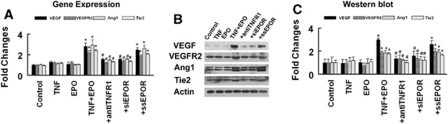
The TNFR1 targets VEGF and VEGF and Ang1 mediate angiogenesis (Shweiki et al, 1992; Zhang and Chopp, 2002). To investigate whether VEGF and Ang1 signaling contributes to TNF-α and EPO-induced angiogenesis, we assayed the expression of VEGF/VEGFR2 and Ang1/Tie2. Real-time RT-PCR and Western blot analysis revealed that treatment of RECs with rhTNF (5 ng/mL) or rhEPO (10 ng/mL) alone did not significantly upregulate expression of VEGF/VEGFR2 and Ang1/Tie2 (Figure 5). However, incubation of rhTNF-treated RECs with rhEPO dramatically increased mRNA and protein levels of VEGF/VEGFR2 and Ang1/Tie2 compared with the levels in the rhTNF or rhEPO alone group (Figure 5), suggesting that interaction of rhEPO with EPOR upregulated by rhTNF activates the VEGF/VEGFR2 and Ang1/Tie2 pathways. To verify this, we blocked TNFR1 with the neutralizing antibody and found that the anti-TNFR1 neutralizing antibody significantly suppressed VEGF and Ang1 expression in RECs treated with rhTNF and rhEPO (Figure 5). Attenuation of endogenous EPOR with siRNA-EPOR also significantly reduced VEGF and Ang1 expression (Figure 5). These data indicate that endogenous EPOR is required for the activation of the VEGF/VEGFR2 and Ang1/Tie2. We then inhibited VEGFR2 and Tie2 with a receptor tyrosine kinase inhibitor against VEGFA (SU1498) and a neutralizing antibody against Tie2, respectively, in the RECs treated with rhTNF and rhEPO. As expected, SU1498 and the neutralizing antibody suppressed the tube formation in the presence of rhEPO in RECs treated with rhTNF (Figure 2).
Figure 5.
Effect of recombinant human tumor necrosis factor (rhTNF) and recombinant human EPO (rhEPO) on expression of vascular endothelial growth factor (VEGF)/VEGFR2 and angiopoietin 1 (Ang1)/Tie2. Real-time RT-PCR (A) and Western blot (B and C) show VEGF/VEGFR2 and Ang1/Tie2 mRNA and protein levels, respectively, in rat brain microvascular endothelial cells (RECs) treated with rhTNF alone (5 ng/mL), rhEPO alone (10 ng/mL), rhTNF and rhEPO, rhTNF and rhEPO with the antibody against TNF receptor 1 (TNFR1) (+anti-TNFR1), rhTNF and rhEPO with small interfering RNA (siRNA)-EPO receptor (EPOR) (+siEPOR), or rhTNF and rhEPO with scramble control (+ssEPO). Glyceraldehyde-3-phosphate dehyrogenase (GAPDH) and β-actin were used as internal controls for real-time RT-PCR and Western blot analysis, respectively. *P<0.05 and #P<0.05 versus the control and TNF+EPO groups, respectively (n=6/group).
Discussion
This study shows that interaction of TNF-α with TNFR1 sensitizes cerebral endothelial cells for EPO-induced angiogenesis by upregulation of EPOR that amplifies the effect of EPO on activation of the VEGF/VEGFR2 and Ang1/Tie2 pathways. Activation of NF-κB and Akt-mediated TNF/TNFR1-upregulated EPOR expression. These data provide the evidence for crosstalk between TNF/TNFR1 and EPOR to coordinate the onset of angiogenesis in cerebral endothelial cells.
The EPO evokes its biological function through interaction with its receptor EPOR (Todokoro et al, 1987). Cerebral endothelial cells express EPOR that has 10 times higher affinity to EPO (2 nmol/L) than EPOR in neurons (20 nmol/L) (Brines and Cerami, 2005). The expression of EPOR is regulated by pro-inflammatory cytokines including TNFα, whereas hypoxia upregulates EPO (Chang and Stevenson, 2004). Our results show that TNF through TNFR1-upregulated endothelial EPOR, which is consistent with the recent studies that TNF-α markedly increases EPOR expression in human neurons and knockout of TNFR1 but not TNFR2 gene substantially suppresses EPOR expression (Taoufik et al, 2008). Recent studies suggest that in addition to an EPOR homodimer, an EPOR monomeric and a βCR homodimer constitute a heteromeric receptor complex (Leist et al, 2004). The neuroprotective effect of EPO is mediated by the heteromeric EPOR complex, whereas EPO induces erythropoiesis through the homodimeric EPOR (Leist et al, 2004). The CEPO does not bind monomeric or dimeric EPOR but has been suggested to interact with the heteromeric EPOR (Leist et al, 2004). Our data showed that rhEPO at a low concentration promoted angiogenesis in the cerebral endothelial cells that had upregulation of EPOR by TNF/TNFR1, whereas a low concentration of CEPO failed to enhance the tube formation in endothelial cells treated with TNF. The observed distinct effect of rhEPO and CEPO on the TNF-treated endothelial cells suggests that TNF/TNFR1 primes cerebral endothelial cells for EPO-induced angiogenesis through upregulation of the EPOR homodimer in cerebral endothelial cells, which is consistent with the view that endothelial cells predominantly express the EPOR homodimer (Brines and Cerami, 2005). However, because our data also showed that CEPO at higher concentrations promoted the tube formation, further studies are warranted to address the mechanism of action of CEPO on endothelial cells.
The TNF activates many signaling pathways including NF-κB and Akt (Ozes et al, 1999). Our data suggest that activation of NF-κB by TNF may trigger Akt activation, whereas both NF-κB and phosphatidylinositol 3-kinase/Akt pathways are required for TNF-upregulated EPOR expression.
The VEGF and Ang1 families have a predominant role for angiogenesis and vascular maturation, respectively. The VEGF through VEGFR2 induces endothelial cell proliferation and increases cerebral vascular permeability, whereas interaction of Ang-1 with its receptor, Tie2, protects against VEGF-induced brain–blood barrier leakage (Thurston et al, 1999, 2000; Wu et al, 2003). The TNF/TNFR1 can directly act on endothelial cells to induce angiogenesis through activation of the VEGF pathway (Sugano et al, 2004; Yoshida et al, 2004). Our data showed that both rhTNF and rhEPO, but not rhTNF or rhEPO alone, at the concentration used in this study upregulated VEGF/VEGFR2 and Ang1/Tie2, which was mediated by EPOR. Thus, EPOR in TNF-primed cerebral endothelial cells is required for activation of the VEGF and Ang1 pathways that lead to augmentation of in vitro angiogenesis. Our findings are consistent with published data showing that EPO/EPOR has an important role to induce angiogenesis by modulating the VEGF/VEGF receptor and Ang1/Tie2 (Kertesz et al, 2004; Nakano et al, 2007).
In vivo, we and others have shown that EPO enhances angiogenesis in the ischemic boundary region where the presence of gradients of TNF expression are reported (Leibovich et al, 1987; Li et al, 2007; Liu et al, 1994; Wang et al, 2004a). Thus, TNF released from inflammatory cells in ischemic brain likely sensitizes cerebral endothelial cells to EPO-induced angiogenesis. The EPO could activate multiple signaling pathways to induce angiogenesis (Carlini et al, 1995; Ribatti et al, 1999). This study offers a potential mechanism for crosslinking between TNF and EPOR in cerebral endothelial cells to induce angiogenesis.
The authors declare no conflict of interest.
Footnotes
This work was supported by NIH grants PO1 NS23393 and RO1HL 64766 and American Heart Association Grant 0835154N.
References
- Ashkenazi A, Marsters SA, Capon DJ, Chamow SM, Figari IS, Pennica D, Goeddel DV, Palladino MA, Smith DH. Protection against endotoxic shock by a tumor necrosis factor receptor immunoadhesin. Proc Natl Acad Sci USA. 1991;88:10535–10539. doi: 10.1073/pnas.88.23.10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995;47:740–745. doi: 10.1038/ki.1995.113. [DOI] [PubMed] [Google Scholar]
- Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KH, Stevenson MM. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int J Parasitol. 2004;34:1501–1516. doi: 10.1016/j.ijpara.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Kertesz N, Wu J, Chen TH, Sucov HM, Wu H. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276:101–110. doi: 10.1016/j.ydbio.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Liu XB, Wang JA, Yu SP, Keogh CL, Wei L. Therapeutic strategy of erythropoietin in neurological disorders. CNS Neurol Disord Drug Targets. 2008;7:227–234. doi: 10.2174/187152708784936617. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol. 2006;169:1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Satoh K, Fukumoto Y, Ito Y, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res. 2007;100:662–669. doi: 10.1161/01.RES.0000260179.43672.fe. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pillai A, Mahadik SP. Differential effects of haloperidol and olanzapine on the expression of erythropoietin and its receptor in rat hippocampus and striatum. J Neurochem. 2006;98:1411–1422. doi: 10.1111/j.1471-4159.2006.04057.x. [DOI] [PubMed] [Google Scholar]
- Polunovsky VA, Wendt CH, Ingbar DH, Peterson MS, Bitterman PB. Induction of endothelial cell apoptosis by TNF alpha: modulation by inhibitors of protein synthesis. Exp Cell Res. 1994;214:584–594. doi: 10.1006/excr.1994.1296. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell′Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Siren AL, Ehrenreich H. Erythropoietin—a novel concept for neuroprotection. Eur Arch Psychiatry Clin Neurosci. 2001;251:179–184. doi: 10.1007/s004060170038. [DOI] [PubMed] [Google Scholar]
- Sugano M, Tsuchida K, Makino N. Intramuscular gene transfer of soluble tumor necrosis factor-alpha receptor 1 activates vascular endothelial growth factor receptor and accelerates angiogenesis in a rat model of hindlimb ischemia. Circulation. 2004;109:797–802. doi: 10.1161/01.CIR.0000112579.61522.67. [DOI] [PubMed] [Google Scholar]
- Tacchini-Cottier F, Vesin C, Redard M, Buurman W, Piguet PF. Role of TNFR1 and TNFR2 in TNF-induced platelet consumption in mice. J Immunol. 1998;160:6182–6186. [PubMed] [Google Scholar]
- Taoufik E, Petit E, Divoux D, Tseveleki V, Mengozzi M, Roberts ML, Valable S, Ghezzi P, Quackenbush J, Brines M, Cerami A, Probert L. TNF receptor I sensitizes neurons to erythropoietin- and VEGF-mediated neuroprotection after ischemic and excitotoxic injury. Proc Natl Acad Sci USA. 2008;105:6185–6190. doi: 10.1073/pnas.0801447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Todokoro K, Kanazawa S, Amanuma H, Ikawa Y. Specific binding of erythropoietin to its receptor on responsive mouse erythroleukemia cells. Proc Natl Acad Sci USA. 1987;84:4126–4130. doi: 10.1073/pnas.84.12.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004a;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Zhang R, Hafner MS, Wong HK, Jiao Z, Chopp M. Erythropoietin up-regulates SOCS2 in neuronal progenitor cells derived from SVZ of adult rat. Neuroreport. 2004b;15:1225–1229. doi: 10.1097/01.wnr.0000127636.15181.c1. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Jiao ZX, Wang Y, Pourabdollah-Nejad DS, LeTourneau Y, Gregg SR, Chopp M. Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells. J Cereb Blood Flow Metab. 2006;26:556–564. doi: 10.1038/sj.jcbfm.9600215. [DOI] [PubMed] [Google Scholar]
- Watowich SS. Activation of erythropoietin signaling by receptor dimerization. Int J Biochem Cell Biol. 1999;31:1075–1088. doi: 10.1016/s1357-2725(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Wilde GJ, Pringle AK, Sundstrom LE, Mann DA, Iannotti F. Attenuation and augmentation of ischaemia-related neuronal death by tumour necrosis factor-alpha in vitro. Eur J Neurosci. 2000;12:3863–3870. doi: 10.1046/j.1460-9568.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- Wojchowski DM, Gregory RC, Miller CP, Pandit AK, Pircher TJ. Signal transduction in the erythropoietin receptor system. Exp Cell Res. 1999;253:143–156. doi: 10.1006/excr.1999.4673. [DOI] [PubMed] [Google Scholar]
- Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods. 2003;130:53–63. doi: 10.1016/s0165-0270(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Yoshida A, Ishibashi T. Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol. 2004;242:409–413. doi: 10.1007/s00417-004-0874-2. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]