Abstract
Vascular risk factors affect cerebral blood flow (CBF) and cerebral vascular reactivity, contributing to cognitive decline. Hippocampus is vulnerable to both Alzheimer's disease (AD) pathology and ischemia; nonetheless, the information about the impact of vascular risk on hippocampal perfusion is minimal. Cognitively, healthy elderly (NL=18, 69.9±6.7 years) and subjects with mild cognitive impairment (MCI=15, 74.9±8.1 years) were evaluated for the Framingham cardiovascular risk profile (FCRP). All underwent structural imaging and resting CBF assessment with arterial spin labeling (ASL) at 3T magnetic resonance imaging (MRI). In 24 subjects (NL=17, MCI=7), CBF was measured after a carbon dioxide rebreathing challenge. Across all subjects, FCRP negatively correlated with hippocampal (ρ=−0.41, P=0.049) and global cortical (ρ=−0.46, P=0.02) vasoreactivity to hypercapnia (VRh). The FCRP–VRh relationships were most pronounced in the MCI group: hippocampus (ρ=−0.77, P=0.04); global cortex (ρ=−0.83, P=0.02). The FCRP did not correlate with either volume or resting CBF. The hippocampal VRh was lower in MCI than in NL subjects (Z=−2.0, P=0.047). This difference persisted after age and FCRP correction (F[3,20]=4.6, P=0.05). An elevated risk for vascular pathology is associated with a reduced response to hypercapnia in both hippocampal and cortical tissue. The VRh is more sensitive to vascular burden than either resting CBF or brain volume.
Keywords: atherosclerosis, cerebral blood flow, cognitive impairment, hippocampus, MRI
Introduction
Multiple imaging modalities corroborate the relationship between vascular risk factors and deficits in resting cerebral blood flow (CBF) (Claus et al, 1996; Novak et al, 2006; Selim et al, 2008; Beason-Held et al, 2007). Similarly, atherosclerosis risk factors impair vascular response to hypercapnia (Groschel et al, 2007; Settakis et al, 2003), as evidenced by Doppler studies.
Resting CBF reductions are also well documented by single photon emission tomography in individuals with mild cognitive impairment (MCI) (Staffen et al, 2006; Hirao et al, 2005; Caroli et al, 2007). Furthermore, both CBF reduction (Hirao et al, 2005; Caroli et al, 2007; Hansson et al, 2009; Johnson et al, 1998; Kitagawa et al, 2009; Chao et al, 2010) and deficits in the reactivity of cerebral vessels to hypercapnia (vasoreactivity to hypercapnia (VRh)) forecast cognitive decline (Silvestrini et al, 2006). It has been thus proposed that preexisting vascular risk factors mechanistically contribute to cognitive deterioration by directly compromising cerebral blood supply, especially in the states of increased demand.
Although many reports substantiate the link between risks for atherosclerosis and blood flow at a global cerebral or lobar level (Claus et al, 1996; Novak et al, 2006; Selim et al, 2008; Groschel et al, 2007; Settakis et al, 2003), there is only a limited information about the influence of these factors on CBF in structures directly related to memory function. Hippocampus is a major anatomical substrate of memory (Eichenbaum, 2004; Shapiro, 2001), and its structural and functional abnormalities predict Alzheimer's disease (AD) (Glodzik-Sobanska et al, 2005; Mosconi, 2005). As hippocampal cornu ammonis sector1 is highly sensitive to both AD pathology and ischemia (Zarow et al, 2005), the presence of vascular risk can strongly compound on AD-related damage. It hence follows that evaluation of the vascular risk–perfusion relationship in the hippocampus of nondemented individuals could be informative in assessing the contribution of atherosclerosis to cognitive decline. Although hypertension-related deficits in resting hippocampal CBF were previously described (Dai et al, 2008; Beason-Held et al, 2007), there are no hippocampal VRh data available. Moreover, previously used statistical parametric mapping procedures do not provide an accurate assessment of small structures like hippocampus because of smoothing and likely mismatch of coordinates (Mosconi et al, 2005).
Arterial spin labeling (ASL) is a noninvasive magnetic resonance imaging (MRI) perfusion technique in which labeled arterial blood is used as a tracer. The ASL does not involve radiation exposure or exogenous contrast material (Buxton, 2005). Unlike positron emission tomography or single photon emission tomography, ASL is capable of direct CBF examination at spatial resolution sufficient to resolve small structures of interest. Yet, hippocampal MRI perfusion studies are still challenging, as the often used ASL techniques based on echo-planar imaging may suffer from susceptibility artifacts. This can be particularly detrimental for regions located in the proximity of bone and sinuses. Moreover, conventionally used 64 × 64 echo-planar imaging matrix does not allow one to separate hippocampus from adjacent large vessels, including circle of Willis. To overcome these shortcomings, we used ASL based on true fast imaging in steady-precession (TrueFISP) sequence (Boss et al, 2007; Rusinek et al, 2010).
We compared perfusion characteristics in elderly subjects with normal cognition or MCI and investigated the hypothesis that an elevated Framingham cardiovascular risk profile (FCRP) score (NIH Publication No.02-5125 & US Department of Health and Human Services, 2002) is associated with a hemodynamic impairment of the hippocampus and neocortex.
Materials and methods
Subjects
In all, 33 individuals (age 71.2±7.6 years, range 59 to 88 years, 58% women) were studied at the NYU School of Medicine, Center for Brain Health and Alzheimer's Disease Center. All were active participants in longitudinal studies of aging and dementia. All gave written informed consent to an institutional review board-approved protocol. Subjects received medical, neurologic, psychiatric, neuropsychological, laboratory and MRI examinations. Patients with confounding brain conditions, for example tumor, depression, significant metabolic diseases, and neocortical infarction, were excluded. Also excluded were patients with contraindications for MRI scanning. The clinical assessment included a semistructured interview based on the Brief Cognitive Rating Scale, Global Deterioration Scale score (GDS) (Reisberg and Ferris, 1988), and Mini-Mental State Examination (MMSE) (Folstein, 1983). All subjects were diagnosed as normal (NL; GDS=1 or 2, n=18) or MCI (GDS=3, n=15) (Reisberg et al, 1993). GDS=1 indicated no subjective memory complaint, and GDS=2 indicated awareness of memory change over the lifespan, in the absence of objective evidence of memory or functional problems on clinical interview. The MCI was defined as global functioning in the no-dementia range and evidence of cognitive impairment on clinical interviews (Reisberg et al, 1993). All diagnoses were assigned at a consensus case conference.
Cardiovascular risk was determined with the Framingham risk equation (NIH Publication No.02-5125 & US Department of Health and Human Services, 2002), which accounts for age, gender, systolic blood pressure, antihypertensive treatment, smoking, total cholesterol, and HDL levels. For each subject, we computed a score representing the percentage risk for coronary heart disease in the next 10 years.
Imaging
Brain imaging was performed within 6.7±7.0 months of the clinical assessment. Imaging was performed on a 3T Siemens TIM Trio scanner equipped with high-performance gradient coils (45 mT/m maximum gradient strength, 200 mT/m per milliseconds slew rate) and a 12-element head coil.
Structural imaging
High-resolution T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) images were acquired with the following parameters: repetition time (TR)/echo time (TE)/inversion time (TI)=2300/2.8/1100 milliseconds, flip angle (FA)=9°, 208 1 mm slices, bandwidth (BW)=220 Hz/pixel, field of view (FOV)=25 cm, isotropic voxel size=1 × 1 × 1 mm3, 5 minutes 4 seconds acquisition time.
White matter hyperintensities
The fluid attenuated inversion recovery (FLAIR) images were available for 90% of the subjects. They were acquired during a separate session on average within 16 months of the ASL study. They were used to assess the extent of periventricular white matter hyperintensities (PWMH) and deep white matter hyperintensities (DWMH) on the 0 to 3 Fazekas scale (Fazekas et al, 1987).
Perfusion imaging
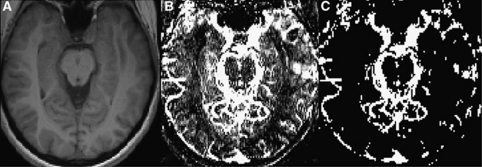
A pulsed ASL sequence which combines a flow-sensitive alternating inversion-recovery (FAIR) labeling scheme with segmented TrueFISP readout was used. Imaging parameters are: TR/TE/TI=3.4/1.7/1200 milliseconds, flip angle=50°, bandwidth=975 Hz/pixel, 256 × 168 matrix, 171 phase-encoding steps, 30 × 19.7 cm2 FOV, voxel size=1.2 × 1.2 × 6 mm3, number of excitations (NEX)=8. The TrueFISP acquisition is repeated without the FAIR preparation (with NEX=4) to estimate the steady-state magnetization M0. Total acquisition time was approximately 2:40 minutes (Rusinek et al, 2010). Selecting acquisition plane included (1) selecting the sagittal slice through the long axis of the right hippocampus, (2) drawing the line segment H–H' through this axis of the right hippocampus, and (3) adjusting the axial plane by rotating it about the H–H' axis to encompass the left hippocampus (Figure 1).
Figure 1.
(A) Anatomical axial T1 image of the brain (section through the hippocampus), (B) flow image with overlaid vessels, (C) vessels separated from tissue flow image.
Imaging the Vasoreactivity to Hypercapnia
To estimate the VRh, blood CO2 level was increased using a rebreathing protocol. Subjects were asked to breathe through a mouthpiece and a respiratory tube. The rebreathing apparatus included a high efficiency particulate air (HEPA) bacterial/viral filter and a standard gas-anesthesia tube (VitalSigns, Totowa, NJ, USA) of 35 mm diameter and a custom-adjusted length. Nose was clamped to force inspiration of partially exhaled air. The protocol was demonstrated to the patients who practiced rebreathing during a 15-minute training session, before the MRI examination. Oxygen saturation, heart rate, and CO2 content in the expired air were monitored in the training room and in the scanner using a multigas monitor (Medrad (R) 9500, Warendale, PA, USA). Expired air was sampled continuously by an infrared capnometer via a 3-m long cannula.
Postprocessing
Segmentation of structural images
Segmentation of high-resolution MPRAGE images to gray matter (GM), white matter (WM), and cerebrospinal fluid volumes was performed using automated statistical parametric mapping (SPM2) procedures (Ashburner and Friston, 2000). Left and right hippocampus were manually outlined on reformatted coronal MPRAGE images (Convit et al, 1997).
Image coregistration
As both perfusion and MPRAGE images were collected during one short session, coregistration of perfusion and structural images was based on ‘Image Orientation Patient' and ‘Image Position Patient' digital imaging and communications in medicine (DICOM) parameters preserved by the scanner console. The accuracy of coregistration was then checked individually.
Calculation of Blood Flow
Calculation was based on the following equation:
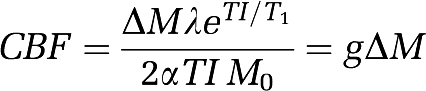 |
where ΔM stands for the adjusted difference between tagged and untagged data, λ is the blood–tissue water partition coefficient (assumed 0.9 mL/g), α is the inversion efficiency, TI is the inversion delay (1200 milliseconds), and M0 the steady-state magnetization. Since at TI=1200 milliseconds, most of the magnetization remains in the blood (Buxton, 2005), the relaxation time of the arterial blood T1=1930 milliseconds (Stanisz et al, 2005) was uniformly used across all regions. There was no smoothing of ASL images. The following steps were subsequently applied:
1. Calibration of tagged and untagged signal with WM: We used the WM to adjust ΔM. This correction is needed due to the violation of the ASL measurement assumption (equal signal of tagged and untagged image in the absence of flow) by FAIR preparation pulses. Given that WM perfusion is significantly lower than cortical flow (Law et al, 2000), we used the signal difference measured in WM ΔMWM to adjust CBF, where g is the factor from the ‘standard ASL equation' (as above).
On the basis of prior studies, we assume 25 mL per 100 g per minute for CBFWM (Law et al, 2000).
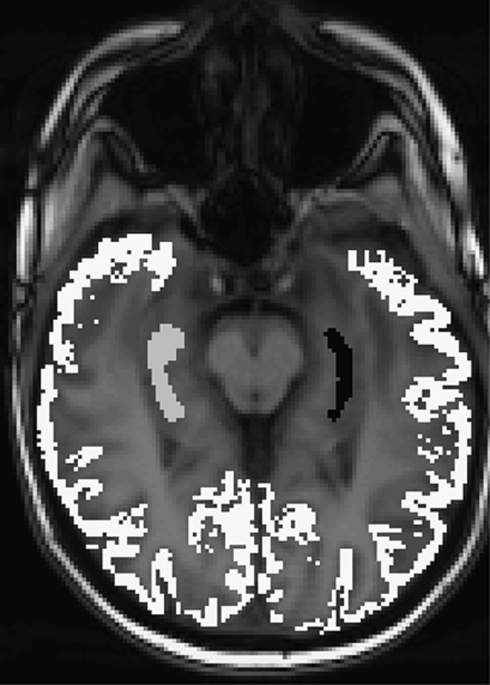
2. Correction for atrophy and tissue inhomogeneity: TrueFISP images were coregistered with MPRAGE volumes (GM and WM) and regions of interest (ROIs). Each voxel in each ROI is then examined in relation to its SPM2-derived GM and WM. For cortical and hippocampal ROIs (Figure 2), voxels were deleted if their probability of being GM was <75%. Similarly, WM voxels were not included if their probability of being WM was <75%.
Figure 2.
Cortical and hippocampal regions of interest (ROIs) overlaid on arterial spin labeling (ASL) image.
3. Exclusion of blood vessels: To avoid error introduced by labeled arterial blood within the ROIs, all voxels with CBF>150 mL per 100 g per minute were deemed to contain large blood vessels and were excluded from ROIs. This is possible due to distinctive ability of segmented TrueFISP to visualize blood vessels of diameter larger than ∼1 mm (Figure 1).
Sampling of regional cerebral blood flow
This was performed using manually (hippocampus) or automatically SPM-segmented (global GM) volumes, coregistered with the TrueFISP images (Figure 2). The CBF was calculated for cortical GM (an integral of all cortical regions visible on the imaged slice), the right and left hippocampus separately (Figure 2).
Calculating the response to CO2
The VRh response to an increase in blood CO2 was calculated as:
where CBFCO2 indicates CBF calculated during the session when subjects breathed through a respiratory tube, CBFrest indicates CBF calculated during the imaging session without the tube; ΔCO2 indicates the difference in end tidal CO2 between the two sessions.
Statistical Analysis
Demographic measures were examined using t-test for continuous variables and χ2 for categorical variables. Between-group comparisons of global volumes, CBF and VR data were performed with t-tests. When covariates were necessary, analysis of covariance was used. Correlations between variables were examined with Spearman coefficient. When accounting for confounding variables was necessary, partial correlations were used. Normality was checked using the Shapiro–Wilk test. When the data did not meet the assumptions of normality, the Mann–Whitney U-test was used to compare groups (Z values are given). When covariates were needed, dependent variables were converted to ranks and analyzed in analysis of covariance models. All volumes (global GM, hippocampus left, right, and averaged) were divided by total intracranial volume determined by manual tracing (Convit et al, 1997) and presented as intracranial volume percentage. The CBF and VRh data for the right and left hippocampus were analyzed separately and averaged. Analyses were performed with SPSS 16, Chicago, IL, USA, with P values <0.05 declared statistically significant.
Results
Normal Versus Mild Cognitive Impairment
General description
Characteristics of both groups are presented in Table 1. The MCI subjects had lower MMSE scores (Z=−2.3, P=0.03) and tended to be older (t=−1.8, P=0.08) than controls. After accounting for age, MMSE difference was no longer significant. Although, the groups did not differ in FCRP, the percentage of subjects with high-FCRP score (>10%) was significantly increased in the MCI group (53% versus 11%, χ2=6.9, P=0.009).
Table 1. Study variables by cognitive diagnosis (NL versus MCI).
| Variable | NL (n=18) | MCI (n=15) | P |
|---|---|---|---|
| Age | 69.9±6.7 | 74.9±8.1 | 0.07 |
| Gender (% female) | 56 | 60 | NS |
| MMSE | 29.2±1.0 | 27.5±2.4 | 0.02 |
| FCRP | 7.4±4.3 | 10.3±8.6 | NS |
| % With FCRP>10% | 11 | 53 | 0.009 |
| % With CVD | 0 | 7 | NS |
| % With LVH | 6 | 20 | NS |
| % With DB | 0 | 7 | NS |
| PWMH score | (n=17) 0.90±0.85 | (n=17) 1.40±0.65 | NS |
| DWMH score | (n=13) 0.90±0.70 | (n=13) 0.80±0.60 | NS |
| Baseline CO2 (mm Hg) | 39.5±3.3 | 39.3±4.9 | NS |
| Volume (% of ICV) | |||
| GM | 54±3 | 53±4 | NS |
| Right hippocampus | 0.20±0.02 | 0.18±0.03 | NS |
| Left hippocampus | 0.20±0.03 | 0.18±0.03 | NS |
| Averaged hippocampus | 0.20±0.02 | 0.18±0.03 | NS |
| CBF (mL per 100 g per minute) | |||
| Global cortical | 60.4±7.6 | 58.2±11.3 | NS |
| Right hippocampus | 58.1±9.1 | 58.8±15.3 | NS |
| Left hippocampus | 57.1±11.6 | 55.7±14.6 | NS |
| Averaged hippocampus | 57.6±8.8 | 57.3±12.7 | NS |
CBF, cerebral blood flow; CVD, cardiovascular disease; DB, diabetes mellitus; DWMH, deep white matter hyperintensities; FCRP, Framingham cardiovascular risk profile; GM, gray matter; ICV, intracranial volume; LVH, left ventricular hypertrophy (on ECG); MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PWMH, periventricular white matter hyperintensities.
Values represent mean±s.d.
PWMH and DWMH were measured on the 0–3 Fazekas scale (Fazekas et al, 1987).
Volumetrics
The groups did not differ in hippocampal or cortical volumes (age corrected).
Cerebral blood flow
The CBF did not differ between NL and MCI groups in any examined region (age corrected) (Table 1).
Cerebral vasoreactivity to hypercapnia
In all, 24 subjects (NL=17 and MCI=7; Table 2) underwent the rebreathing challenge. Overall, the mean CO2 increase was 6.8±1.7 mm Hg. The mean increase in global cortical, right hippocampal, left, and averaged hippocampal CBF was 2.7, 3.3, 3.1, and 3.2 mL per 100 g per minute, respectively. The nine subjects with no VRh data did not differ in age, gender, or FCRP score from subjects with VRh data available, they had, however, lower MMSE score (26.7±2.7 versus 29.0±1.1; Z=−2.6, P<0.05). The MCI group had lower average hippocampal VRh than NL subjects (Z=−2.0, P=0.047). This association still tended to be significant after age and FCRP correction (F[3,20]=4.6, P=0.05).
Table 2. Study variables by cognitive diagnosis (NL versus MCI) in the groups with VRh data available.
| Variable | NL (n=17) | MCI (n=7) | P |
|---|---|---|---|
| Age | 69.8±6.9 | 73.4±8.2 | NS |
| Gender (% female) | 53 | 71 | NS |
| MMSE | 29.2±1.0 | 28.6±1.4 | NS |
| FCRP | 7.6±4.4 | 8.0±5.4 | NS |
| % With FCRP >10% | 12 | 57 | 0.02 |
| % With LVH | 6 | 0 | NS |
| PWMH score | (n=16) 0.90 ± 0.85 | (n=6) 1.20±0.40 | NS |
| DWMH score | (n=16) 0.90±0.70 | (n=6) 0.70±0.50 | NS |
| Baseline CO2 (mm Hg) | 39.2±3.1 | 38.7±5.9 | NS |
| mean CO2 increase (mm Hg) | 7.1±1.8 | 6.2±1.4 | NS |
| Volume (% of ICV) | |||
| GM | 54±3 | 54±3 | NS |
| Right hippocampus | 0.20±0.02 | 0.19±0.03 | NS |
| Left hippocampus | 0.20±0.03 | 0.20±0.03 | NS |
| Averaged hippocampus | 0.20±0.02 | 0.19±0.03 | NS |
| CBF (mL per 100 g per minute) | |||
| Global cortical | 60.6±7.7 | 57.8±4.2 | NS |
| Right hippocampus | 58.1±9.4 | 60.3±13.4 | NS |
| Left hippocampus | 57.4±11.8 | 55.3±7.8 | NS |
| Averaged hippocampus | 57.8±9.0 | 57.8±9.4 | NS |
| VRh (% ΔCBF/1 mm Hg CO2) | |||
| Global cortical | 1.0±1.5 | 0.31±1.2 | NS |
| Right hippocampus | 1.4±2.7 | 0.23±2.5 | NS |
| Left hippocampus | 1.5±3.3 | −0.22±1.5 | 0.099 |
| Averaged hippocampus | 1.4±2.7 | −0.05±1.7 | 0.047 |
CBF, cerebral blood flow; DWMH, deep white matter hyperintensities; FCRP, Framingham cardiovascular risk profile; GM, gray matter; ICV, intracranial volume; LVH, left ventricular hypertrophy (on ECG); MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PWMH, periventricular white matter hyperintensities; VRh, vasoreactivity to hypercapnia.
Values represent mean±s.d.
None of the subjects in the subset had diabetes mellitus or cardiovascular disease.
PWMH and DWMH were measured on the 0–3 Fazekas scale (Fazekas et al, 1987).
Framingham Cardiovascular Risk Profile and Cerebral Perfusion
General description
The mean FCRP score was 8.7%±6.7%. The FCRP score correlated with age in the entire group (ρ=0.46, P=0.007) and in the MCI subjects (ρ=0.61, P=0.02). The FCRP did not correlate with any examined brain volumes in the entire group, nor in NL or MCI subgroups (age corrected). The FCRP correlated with PWMH (ρ=0.48, P<0.01) but not DWMH.
Cerebral blood flow
In the whole group, FCRP score did not correlate with CBF in any region. Similarly, no correlations were found in NL or MCI groups (age corrected).
Cerebral vasoreactivity to hypercapnia
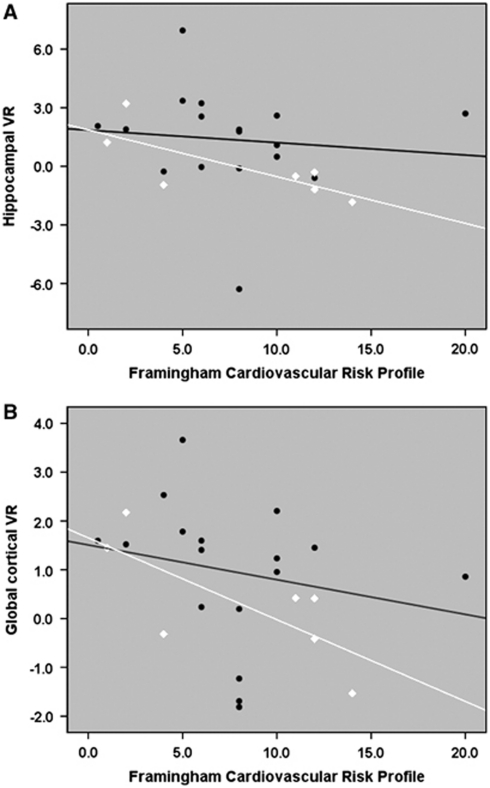
In the entire group, the average hippocampal VRh was related to FCRP (ρ=−0.41, P=0.049). Global cortical VRh also correlated with FCRP (ρ=−0.46, P=0.02). Further analyses revealed that in the MCI subjects, FCRP was strongly related to hippocampal (ρ=−0.77, P=0.04) and global cortical VRh (ρ=−0.83, P=0.02) (Figure 3). Relationships in the NL group were weaker with (ρ=−0.25, P=0.30) for the average hippocampal and (ρ=−0.45, P=0.07) for global cortical VRh, respectively. Cortical VRh was also associated with PWMH (ρ=−0.45, P=0.04). There was also a similar trend for PWMH and hippocampal VRh (ρ=−0.31, P=0.16). After correcting for PWMH, the relationship between FCRP and VRh was still at the trend level: ρ=−0.36, P=0.1 for the cortex; and ρ=−0.33, P=0.15 for the hippocampus.
Figure 3.
Correlations between Framingham cardiovascular risk profile (FCRP) and average hippocampal (A) and cortical (B) vasoreactivity to hypercapnia (VRh). The units of VRh are % ΔCBF/1 mm Hg CO2. Values on x axis represent the percentage risk for coronary heart disease in the next 10 years. Black circles represent normal (NL) subjects and white diamonds represent subjects with mild cognitive impairment (MCI). CBF, cerebral blood flow.
Cerebral Perfusion and Other Measures
Cerebral blood flow
Resting CBF was not related to age in any examined region. This was true for the entire group, and within NL and MCI subsets. Regionally, the CBF was not related to the brain volumes in the entire groups nor in subsets of NL and MCI subjects. There was no association between resting flow and baseline CO2 levels, PWMH, or DWMH.
In the entire group, global CBF was related to MMSE score (ρ=0.40, P=0.027, age corrected). Additional corrections did not change this association (ρ=0.40, P=0.031, age, gender, and education corrected). When examined within groups, this relationship was found only in MCI subjects (ρ=0.52, P=0.045). In the smaller MCI subgroup, MMSE did not correlate with education or age and did not differ between men and women, thus corrections were not necessary.
Cerebral vasoreactivity to hypercapnia
The VRh was not related to age, regional brain volumes, or MMSE, in either the entire group or in NL and MCI subgroups.
Discussion
Our study yielded two major findings: (1) cerebral VRh, as measured with ASL combined with CO2-rebreathing procedure, was negatively related to the level of cardiovascular risk and (2) VRh was lower in the hippocampus of MCI subjects as compared with normal controls. Our result expands previous observations from Doppler (Groschel et al, 2007) and ASL (Last et al, 2007) studies, where decreased response to hypercapnia was found in association with vascular risk factors. Specifically, among all the subjects, both hippocampal and global cortical VRh were affected by vascular risk, corroborating the contribution of impaired vessel function to the damage of brain regions strategic for cognition.
Our observation of reduced hippocampal VRh in the MCI group also add to recent results of diminished vessel function in dementia subjects (Silvestrini et al, 2006; Vincenzini et al, 2007). The FCRP–VRh association was also found in the MCI subjects. As the number of subjects with increased vascular load (risk>10%) was higher among MCI subjects, vascular contribution explains at least in part lower VRh in this group. Recent evidence indicates that patient with hypertension have increased parahippocampal neurofibrillary tangles (Sparks et al, 1995), supporting the role of cardiovascular factors in AD. Still, the VRh difference between NL and MCI persisted after age and FCRP score correction. We offer that preexisting vessel impairment (independent of vascular risk, but possibly related to AD) had to be present to potentiate the influence of vascular risk factors on vessel expandability.
Opposite to earlier findings (Dai et al, 2009; Staffen et al, 2006; Hirao et al, 2005; Caroli et al, 2007), CBF was not different between NL and MCI. Global CBF was, however, related to the global cognitive abilities as assessed with MMSE. As MCI subjects were highly functioning (an average MMSE score was above 27) between-group differences in CBF were subtle. However, a stronger relationship emerged when cognition–CBF relationship was examined across the entire spectrum with MMSE.
Differing from recent reports of reduced resting hippocampal CBF (Dai et al, 2008; Beason-Held et al, 2007) in hypertensive nondemented subjects, we did not observe a relationship between FCRP and resting cortical or hippocampal flow. It is noteworthy that in our group, the impact of arthrosclerosis risk factors on vessel dilatation capacity was stronger than their impact on volume or resting CBF. One possible explanation for a lack of a relationship between volume or CBF and FCRP is low burden of vascular risk in our group. The mean risk for cardiovascular event in our group was 9%, substantially lower than 20% considered being high risk (NIH Publication No.02-5125 & US Department of Health and Human Services, 2002). Conceivably, a mild stage of vascular damage may not affect volumes or CBF, and the influence of vascular burden is only evident in a challenge condition. The intrinsically variable nature of resting CBF, dependent on such factors as heart rate, hematocrit, subject alertness, and stress level could also explain our negative findings. It is possible that changes in resting perfusion and brain volume reductions occur only in the late stage of atherosclerosis or with more advanced cognitive impairment. We provide, however, a strong evidence for a relationship between vascular burden and a challenge that demands additional blood supply. This is in agreement with previous observations that FCRP correlated with impaired endothelium-dependent vasodilatation in the skin (Ijzerman et al, 2003).
Decreases in VRh could reflect hippocampal or cortical volume reductions. However, neither CBF nor VRh in the hippocampus or in the cortex were related to their volumes. In our study, the flow was calculated after exclusion of non-GM voxels, which we believe, accounted for the effects of atrophy and related bias. Thus, the presented values are likely to reflect perfusion of neuronal tissue without partial voluming that is unavoidable in positron emission tomography or single photon emission tomography imaging.
Not surprisingly in our group, vascular burden, VRh, and PWMH were intercorrelated. The association between reduced response to hypercapnia and WM lesions, especially PWMH, is well known (Bakker et al, 1999; Bonoczk et al, 2004; Fu et al, 2006; Isaka et al, 1994). Nonetheless, it still is not completely clear what is a cause what is an effect. Vascular risk factors can be particularly harmful for periventricular WM located in the borderline zone. Conceivably, by compromising blood flow they can contribute to the development of WMH. However, another explanation cannot be ruled out: WMH once present can contribute to the impairment in vascular reactivity, as periventricular zone harbors receptor involved in cerebral vasoregulation (Sandor et al, 1986). Our work cannot give a definite answer.
To our knowledge, this is one of the first studies to examine regional VRh in the hippocampus. The technique we used is less sensitive to susceptibility artifacts than echo-planar imaging (Boss et al, 2007), and has improved spatial resolution, enabling separation of vascular signal from tissue blood flow. In addition, the 3T field strength provides a unique advantage for ASL because of longer blood T1 relaxation times, making this technique a promising quantitative approach for CBF and VR measurement (Petersen et al, 2006). Many arteries ∼1 mm in diameter can be visualized (Figure 1). Consequently, large vessels can be automatically excluded from tissue ROIs, and no crusher gradient pulse is needed to dephase fast-moving vascular flow. The voxel size of 1.2 × 1.2 × 6 mm3 translates into 8.6 mm3 volume resolution, enabling sampling of small anatomical structures like hippocampus. Finally, we did not smooth images, and we used manually traced coregistered ROIs, which contributed to the reliability of hippocampal assessment.
This study had several limitations. We calibrated the tagged and untagged signal with WM. Although there is a potential bias due to WM disease, especially in subjects with high FCRP, the estimated low rates of WM perfusion are less than the increased CBF variability associated with uncorrected flow. Second, based on the segmentation procedure, all WM voxels containing <75% of WM (see below) are removed from the WM ROI, which is used to correct cortical flow. We believe this reduced the WM disease-related bias.
Arterial stenosis is known to reduce both the CBF (Bokkers et al, 2009) and the capacity to respond to hypercapnia (Bricic et al, 2008; Markus and Harrison, 1992). However, most of the studies examined this issue in patients with symptomatic stenosis. None of our subjects fell into this category. Nonetheless, we cannot account for the influence of stenotic lesions, as such information was not available. The prevalence of asymptomatic carotid stenosis ranges from 0.5% in people under 60 to up to 10% in subjects over the age of 80 years (Benavente et al, 1998). Stenosis is more common among patients with hypertension with prevalence reported to reach 25% (Sutton-Tyrrell et al, 1993). With a mean age of around 70 years and about 40% of subjects meeting criteria for hypertension, we can estimate that the probable prevalence of asymptomatic carotid stenosis in our group could not be higher than 5% to 10%. We consider it unlikely that this will affect our results.
The circulatory response to hypercapnia consists of increases in heart rate and arterial pressure. Unfortunately, we were not able to record blood pressure measurements during rebreathing procedure. For few reasons, we do not believe that these phenomena could significantly affect the results. First, hypercapnia induced during our study can be classified as mild. Second, heart rate (positively correlated with blood pressure) increased during the rebreathing challenge only by few percent.
Finally, the group with missing VRh data could also represent a bias. These subjects had lower MMSE, pointing out to possible challenges in planning the study with impaired individuals. Our sample was small and perfusion was examined only in one slab. However, the hippocampus appeared to be sampled consistently in each case, because of careful slice positioning technique.
In conclusion, vascular risk, as measured with FCRP, correlates with both hippocampal and cortical vessels' ability to expand in response to a mild hypercapnia. The VRh is more sensitive to vascular burden than either resting CBF or brain volume. It is compromised already in the MCI stage but vascular risk does not fully explain this impairment. It remains to be determined in longitudinal design how these effects contribute to cognitive deterioration. Further evaluation with bigger cohort, larger brain coverage, and more extensive assessment of vascular risk is crucial.
Acknowledgments
The authors thank Ms. Anna Hemraj for her assistance with study coordination.
The authors declare no conflict of interest.
Footnotes
This study was supported by the following grants: NIH-NIA AG08051, AG12101, AG022374, and the Alzheimer's Association Grants: IIRG-08-91038 and NIRG-09-132490.
References
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bakker SLM, de Leeuw FE, de Groot JC, Hofman A, Koudstaal PJ, Breteler MMB. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52:578. doi: 10.1212/wnl.52.3.578. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- Benavente O, Moher D, Pham B. Carotid endarterectomy for asymptomatic carotid stenosis: a metaanalysis. Br Med J. 1998;317:1477–1480. doi: 10.1136/bmj.317.7171.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokkers RPH, van der Worp HB, Mali WPTM, Hendrikse J. Noninvasive MR imaging of cerebral perfusion in patients with a carotid artery stenosis. Neurology. 2009;73:869–875. doi: 10.1212/WNL.0b013e3181b7840c. [DOI] [PubMed] [Google Scholar]
- Bonoczk P, Panczel G, Nagy Z. Vasoreactivity in patients with periventricular white matter lucency. Acta Neurologica Scandinavica. 2004;110:254–259. doi: 10.1111/j.1600-0404.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Boss A, Martirosian P, Klose U, Nagele T, Claussen CD, Schick F. FAIR-TrueFISP imaging of cerebral perfusion in areas of high magnetic susceptibility differences at 1.5 and 3 Tesla. J Magn Reson Imaging. 2007;25:924–931. doi: 10.1002/jmri.20893. [DOI] [PubMed] [Google Scholar]
- Bricic I, Horner S, Thaler D, Demarin V, Klein GE, Niederkorn K. Improved cerebral vasoreactivity following percutaneous transluminal angioplasty with stenting of high-grade internal carotid artery stenosis. Cerebrovasc Dis. 2008;25:555–560. doi: 10.1159/000132201. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Quantifying CBF with arterial spin labeling. J Magn Reson Imaging. 2005;22:723–726. doi: 10.1002/jmri.20462. [DOI] [PubMed] [Google Scholar]
- Caroli A, Testa C, Geroldi C, Nobili F, Barnden LR, Guerra UP, Bonetti M, Frisoni GB. Cerebral perfusion correlates of conversion to Alzheimer's disease in amnestic mild cognitive impairment. J Neurol. 2007;254:1698–1707. doi: 10.1007/s00415-007-0631-7. [DOI] [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Dis. 2010;24:19–27. doi: 10.1097/WAD.0b013e3181b4f736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus JJ, Breteler MMB, Hasan D, Krenning EP, Bots ML, Grobbee DE, van Swieten JC, van Harskamp F, Hofman A. Vascular risk factors, atherosclerosis, cerebral white matter lesion and cerebral perfusion in a population based study. Eur J Nucl Med. 1996;23:675–682. doi: 10.1007/BF00834530. [DOI] [PubMed] [Google Scholar]
- Convit A, de Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, George AE. Specific hippocampal volume reductions in individuals at risk for Alzheimer's disease. Neurobiol Aging. 1997;18:131–138. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Folstein M.1983The Mini-Mental State Examination Assessment in Geriatric Psychopharmacology(Crook T, Ferris SH, Bartus R eds),New Canaan: Mark Powley Associates; 47–51. [Google Scholar]
- Fu JH, Lu CZ, Hong Z, Dong Q, Ding D, Wong KS. Relationship between cerebral vasomotor reactivity and white matter lesions in elderly subjects without large artery occlusive disease. J Neuroimaging. 2006;16:120–125. doi: 10.1111/j.1552-6569.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Rusinek H, Mosconi L, Li Y, Zhan J, De Santi S, Convit A, Rich KE, Brys M, de Leon MJ. The role of quantitative structural imaging in the early diagnosis of Alzheimer's disease. Neuroimaging Clin N Am. 2005;15:803–826. doi: 10.1016/j.nic.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Groschel K, Terborg C, Riecker A, Witte OW, Kastrup A. Effects of physiological aging and cerebrovascular risk factors on the hemodynamic response to brain activation: a functional transcranial Doppler study. Clin Neurophysiol. 2007;118:e35–e36. doi: 10.1111/j.1468-1331.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- Hansson O, Buchhave P, Zetterberg H, Blennow K, Minthon L, Warkentin S. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer's disease. Neurobiol Aging. 2009;30:165–173. doi: 10.1016/j.neurobiolaging.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Hirao K, Ohnishi T, Hirata Y, Yamashita F, Mori T, Moriguchi Y, Matsuda H, Nemoto K, Imabayashi E, Yamada M, Iwamoto T, Arima K, Asada T. The prediction of rapid conversion to Alzheimer's disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005;28:1014–1021. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Ijzerman RG, de Jongh RT, Beijk MAM, van Weissenbruch MM, Delemarre-van De Waal HA, Serne EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest. 2003;33:536–542. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- Isaka Y, Okamoto M, Ashida K, Imaizumi M. Decreased cerebrovascular dilatory capacity in subjects with asymptomatic periventricular hyperintensities. Stroke. 1994;25:375–381. doi: 10.1161/01.str.25.2.375. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Jones K, Holman BL, Becker BS, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer′s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- Kitagawa T, Oku N, Kimura Y, Yagita Y, Sakaguchi M, Hatazawa J, Sakoda S. Relationship between cerebral blood flow and later cognitive decline in hypertensive patients with cerebral small vessel disease. Hypertension Res. 2009;32:816–820. doi: 10.1038/hr.2009.100. [DOI] [PubMed] [Google Scholar]
- Last D, de Bazelaire C, Alsop DC, Hu K, Abduljalil AM, Cavallerano J, Marquis RP, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law I, Iida H, Holm S, Nour S, Rostrup E, Svarer C, Paulson OB. Quantitation of regional cerebral blood flow corrected for partial volume effect using O-15 water and PET: II. Normal values and gray matter blood flow response to visual activation. J Cerbral Blood Flow Metab. 2000;20:1237–1251. doi: 10.1097/00004647-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. 1992;23:668–673. doi: 10.1161/01.str.23.5.668. [DOI] [PubMed] [Google Scholar]
- Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. Eur J Nucl Med. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, De Santi S, Rusinek H, Li J, Convit A, Li Y, de Leon MJ. Reduced hippocampal metabolism in mild cognitive impairment and Alzheimer′s disease: automated FDG-PET image analysis. Neurology. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- NIH Publication No.02-5125 & US Department of Health and HUman Services, N.I.o.H.N.H.L.a.B.I Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high cholesterol in adults (Adult Treatment panel III) final report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, Cavallerano J, Lipsitz LA. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29:1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen ET, Zimine I, Ho Y-CL, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79:688–701. doi: 10.1259/bjr/67705974. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH. The brief cognitive rating scale (BCRS) psychopharmacology. Bulletin. 1988;24:629–636. [PubMed] [Google Scholar]
- Reisberg B, Sclan SG, Franssen EH, de Leon MJ, Kluger A, Torossian CL, Shulman E, Steinberg G, Monteiro I, McRae T, Boksay I, Mackell JA, Ferris SH. Clinical stages of normal aging and Alzheimer's disease: the GDS staging system. Neurosci Res Commun. 1993;13 (Suppl 1:551–554. [Google Scholar]
- Rusinek H, Glodzik L, Brys M, Haas F, Mcgorty KA, Chen Q, de Leon MJ.2010Hippocampal Blood Flow and Vascular Reactivity in Normal AgingProceedings of the 18th scientific meeting ISMRM, Stockholm, Sweden, 1–7 May
- Sandor P, De Jong W, De Wied D. Endorphinergic mechanisms in cerebral blood flow autoregulation. Brain Res. 1986;386:122–129. doi: 10.1016/0006-8993(86)90148-4. [DOI] [PubMed] [Google Scholar]
- Selim M, Jones R, Novak P, Zhao P, Novak V. The effects of body mass index on cerebral blood flow velocity. Clin Auton Res. 2008;18:331–338. doi: 10.1007/s10286-008-0490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settakis G, Pall D, Molnar C, Bereczki D, Csiba L, Fulesdi B. Cerebrovascular reactivity in hypertensive and healthy adolescents: TCD with vasodilatory challenge. J Neuroimaging. 2003;13:106–112. [PubMed] [Google Scholar]
- Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Arch Neurol. 2001;58:874–881. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37:1010–1015. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JCI. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131:162–169. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]
- Staffen W, Schonaueer U, Zauner H, Spindler I, Mair A, Iglseder B, Bernroider G, Ladurner G. Brain perfusion SPECT in patients with mild cognitive impairment and Alzheimer's disease: comparison of a semiquantitative and a visual evaluation. J Neural Trans. 2006;113:195–203. doi: 10.1007/s00702-005-0321-5. [DOI] [PubMed] [Google Scholar]
- Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Resonance Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Alcorn HG, Wolfson SK, Jr, Kelsey SF, Kuller LH. Predictors of carotid stenosis in older adults with and without isolated systolic hypertension. Stroke. 1993;24:355–361. doi: 10.1161/01.str.24.3.355. [DOI] [PubMed] [Google Scholar]
- Vincenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, Lenzi GL. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. Eur Neurol. 2007;58:84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, Chui HC. Correlates of hippocampal neuron number in Alzheimer's disease and ischemic vascular dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]