Abstract
7,8-Dihydro-8-oxoguanine DNA glycosylase (OGG1) is a major DNA glycosylase involved in base-excision repair (BER) of oxidative DNA damage to nuclear and mitochondrial DNA (mtDNA). We used OGG1-deficient (OGG1−/−) mice to examine the possible roles of OGG1 in the vulnerability of neurons to ischemic and oxidative stress. After exposure of cultured neurons to oxidative and metabolic stress levels of OGG1 in the nucleus were elevated and mitochondria exhibited fragmentation and increased levels of the mitochondrial fission protein dynamin-related protein 1 (Drp1) and reduced membrane potential. Cortical neurons isolated from OGG1−/− mice were more vulnerable to oxidative insults than were OGG1+/+ neurons, and OGG1−/− mice developed larger cortical infarcts and behavioral deficits after permanent middle cerebral artery occlusion compared with OGG1+/+ mice. Accumulations of oxidative DNA base lesions (8-oxoG, FapyAde, and FapyGua) were elevated in response to ischemia in both the ipsilateral and contralateral hemispheres, and to a greater extent in the contralateral cortex of OGG1−/− mice compared with OGG1+/+ mice. Ischemia-induced elevation of 8-oxoG incision activity involved increased levels of a nuclear isoform OGG1, suggesting an adaptive response to oxidative nuclear DNA damage. Thus, OGG1 has a pivotal role in repairing oxidative damage to nuclear DNA under ischemic conditions, thereby reducing brain damage and improving functional outcome.
Keywords: BER, DNA repair, Drp1, focal ischemic stroke, mitochondria, 8-oxoG, OGG1, XRCC1
Introduction
Oxidative stress and accumulation of oxidative damage to cellular nucleic acids occur in aging and age-related neurodegenerative conditions (Nakabeppu et al, 2004; Weissman et al, 2007; Shao et al, 2008). Neurons encounter particularly high levels of oxidative stress because of the high metabolic rate required to support their electrical and synaptic activities. Thus, the integrity and capacity of systems that repair oxidative DNA damage would be expected to be critical for the survival and proper function of neurons, particularly under conditions of increased oxidative stress that occurs during catastrophic pathological conditions including ischemic stroke.
The DNA base-excision repair (BER) pathway is the major system for repairing oxidative DNA damage and involves the sequential actions of multiple DNA repair enzymes (see Wilson and Bohr, 2007 for review). The BER is initiated by the action of lesion-specific DNA glycosylases that recognize and excise the damaged DNA base, particularly 8-oxoG, which is an oxidized form of guanine and is a major oxidative DNA base lesion implicated in carcinogenesis and aging in many biological systems (Hamilton et al, 2001; Barja, 2004), including the nervous system (Nakabeppu et al, 2004). 7,8-dihydro-8-oxoguanine DNA glycosylase (OGG1) is the major glycosylase that recognizes and excises modified 8-oxoG base lesions in nuclear and mitochondrial DNA (mtDNA) (Dizdaroglu, 2003; Jensen et al, 2003). The OGG1 null (OGG1−/−) mice have been generated (Klungland et al, 1999) and they accumulate 8-oxoG in mtDNA in the liver in amounts 9- to 20-fold higher than wild-type (WT) mice (de Souza-Pinto et al, 2001). However, despite the presence of high levels of 8-oxoG, these mice do not exhibit an abnormal phenotype, and mitochondria isolated from livers and hearts of OGG1−/− mice appear functionally normal (Stuart et al, 2005). Nevertheless, there is an increase in mutation frequency in OGG1−/− mice when they are exposed to γ radiation, largely because of G:C to T:A transversions at oxidative lesions during replication (Larsen et al, 2006). Transcriptional mutagenesis is also possible because RNA polymerases can bypass these lesions; phenotypic changes can occur if the mutations are located in critical coding regions or if DNA repair capacity is impaired (Viswanathan et al, 1999).
The BER pathway might be particularly important in repairing oxidative DNA lesions during stroke, a common acute neurologic disorder (Li et al, 2006). During cerebral ischemia, reactive oxygen and nitrogen species are generated from several sources including the mitochondrial electron-transport chain, activation of oxidases such as endothelial NADPH oxidase (Chan, 2001; Infanger et al, 2006) and nitric oxide, which is generated in response to Ca2+ influx and activation of nitric oxide synthase. Oxidative DNA damage is markedly increased in brain cells soon after ischemia. Ischemia induces abundant DNA lesions consisting of hydroxyl radical-modified bases including 8-oxoG, apurinic/apyrimidinic abasic site (AP site) lesions, single-strand breaks (SSBs), and double-strand DNA breaks (DSBs) (Liu et al, 1999; Chan, 2001; Lan et al, 2003). Thus, the activities of DNA repair enzymes to mitigate the DNA damage may be a critical determinant of the fate of injured neurons after an ischemia episode. Indeed, such oxidative DNA lesions, when unrepaired, can trigger neuronal cell death (Oka et al, 2008).
In this study, the potential role of OGG1 in ameliorating the detrimental effect of oxidative DNA damage to neurons was evaluated in cortical cell cultures exposed to oxidative and metabolic insults, and in a mouse model of focal cerebral ischemic brain injury (middle cerebral artery occlusion; MCAO) performed on WT and OGG1−/− mice. As both mtDNA and nuclear DNA are targets for oxidative DNA damage, the relative changes and contributions of mitochondrial and nuclear OGG1 to neuronal survival against oxidative injury were studied. After cerebral ischemia, the accumulation of brain oxidative DNA base lesions was significantly greater in OGG1-deficient mice, and was associated with greater brain damage and poorer behavioral outcome. Studies of cultured primary cortical neurons showed that OGG1 levels increase in the nucleus in response to an oxidative insult, coincident with dysfunction and structural damage to mitochondria, suggesting that nuclear DNA may be selectively protected by OGG1. Our findings reveal an important role for OGG1 in brain BER capacity, which contributes to neuronal survival after experimental stroke.
Materials and methods
7,8-Dihydro-8-Oxoguanine DNA Glycosylase-Deficient Mice and Neuronal Cell Cultures
Mice with a targeted disruption of the Ogg1 gene were kindly provided by Dr Arne Klungland, University of Oslo (Klungland et al, 1999) and maintained in the NIA intramural research facility under standard laboratory conditions. Procedures for the preparation and maintenance of dissociated cerebral cortical cell cultures from E18 embryonic rats or WT and homozygous Ogg1−/− littermate E16 embryonic mice were performed as described previously (Mattson et al, 1995). Briefly, cerebral hemispheres were removed, pooled, cut into small pieces, and subjected to mild trypsinization and trituration. Dissociated neurons were seeded onto polyethylenimine-coated plastic culture dishes (for biochemical assays) or glass coverslips (for imaging analyses) in MEM medium with 15% serum for 4 hours; the medium was then changed to Neurobasal medium (Invitrogen Corporation, Carlsbad, CA, USA) containing B-27 supplements (Invitrogen), 1 mmol/L HEPES and 2 μmol/L gentamycin at 37°C (in a 6% CO2/94% air atmosphere). Experiments were performed using 5- to 14-day-old cultures as indicated. All animal experiments were approved by the National Institute on Aging Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals of the NIH.
Immunofluorescence and Immunoblot Analysis
Primary cortical cultures grown on glass coverslips were fixed in 4% paraformaldehyde in phosphate-buffered saline, washed and permeabilized with 0.2% Triton X-100 (Sigma, St Louis, MO, USA) in phosphate-buffered saline, then incubated overnight in the presence of primary antibodies against OGG1 (Novus Biologicals, Inc., Littleton, CO, USA), which detected a single band in rat cortical cultures and several bands in mouse cultures; some cells were costained with an antibody against the neuronal marker microtubule-associated protein 2 (MAP2) (Chemicon International, Temecula, CA, USA). The secondary antibodies were conjugated to either Texas Red or FITC. The fluorescent DNA-binding dye Hoechst 33258 was used to label the nuclei of all cells. The 8-oxoG was detected using antibody clone N45.1 (Oxis, Inc., Beverly Hills, CA, USA) in rat cortical neurons using methods described previously (Kemeleva et al, 2006). For immunoblots, proteins from cultured cell or brain tissue lysates were separated by electrophoresis in 4% to 12% SDS gradient NuPAGE Novex gels (Invitrogen), and then transferred to a nitrocellulose membrane. The membranes were blotted with antibodies against OGG1 (Novus), cytochrome c oxidoreductase subunit IV (COX IV) (Cell Signaling, Inc., Danvers, MA, USA), COX I (MitoSciences, Eugene, OR, USA) or Hsp60 (Enzo Life Sciences, Plymouth Meeting, PA, USA) overnight at 4°C. The blots were reprobed with a mouse β-actin antibody (Sigma) to establish relative levels of protein loading. Horse radish peroxidase (HRP)-conjugated secondary antibodies were used in all immunoblots (Vector Laboratories, Burlingame, CA, USA) and visualized by enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA).
Mitochondrial Mass, Morphology, and Membrane Potential
Mitochondrial mass and membrane potential (Δψm, Delta psi) were evaluated with the fluorescent probes MitoFluor Green (Invitrogen Corporation, Carlsbad, CA, USA) and tetramethylrhodamine ethyl ester (TMRE), respectively, as described previously (Liu et al, 2006). MitoFluor Green accumulates in all mitochondria independent of mitochondrial membrane potential, whereas TMRE is an indicator of Δψm. Briefly, cortical cell cultures were exposed to different treatments for designed time periods, and were then loaded with MitoFluor Green or TMRE (25 nmol/L); TMRE was maintained in the medium throughout the imaging period. Images of labeled cells were acquired at excitation and emission wavelengths of 490 and 516 nm for MitoFluor Green and 549 and 574 nm for TMRE (Zeiss LSM 510, Carl Zeiss, Inc., Thornwood, NY, USA). The average pixel intensities of fluorescence were quantified in at least 20 cells per culture for three to four separate cultures for each treatment group using software supplied by the manufacturer (Zeiss).
Assessments of Cell Survival
Cortical neuronal cultures were stained with DNA-binding dye Hoechst 33258 to allow visualization of nuclear morphology. Neurons with intact neurites and soma with a smooth round appearance were considered viable, whereas neurons with fragmented nuclei and neurites with vacuolated soma were considered nonviable. The percentages of cells with fragmented or condensed nuclei were counted before and at designated time points after exposure to oxidative insults. Analyses were performed in a masked manner on four to five separate cultures for each experimental group.
Focal Cerebral Ischemia Model
Adult (5 to 6 months old) male WT and OGG1−/− weighing 25 to 30 g were maintained on a 12-hour light/12-hour dark cycle with continuous access to food and water. Permanent focal cerebral ischemia was induced by cauterizing the left middle cerebral artery using methods described previously (Liu et al, 2002). Briefly, mice were anesthetized using isoflurane administered as a vapor; body temperature was maintained at 37°C throughout the surgical procedure and recovery period. The left middle carotid artery was exposed through a 1-cm vertical incision between the left eye and ear. The temporal muscle was split, and a portion of the skull at the junction of the zygomatic arch and squamous bone was removed. Focal ischemia was produced by permanent occlusion of the left middle cerebral artery by electrocoagulation. In this model, the regional cerebral blood flow (measured using Laser Doppler technology) of all mice was reduced by >80% as reported previously (Liu et al, 2002). Mice were euthanized 48 hours after MCAO, and the brains were removed immediately after and cut into 1 mm thick coronal sections. Brain sections were incubated in a solution of 2% 2,3,5-triphenyltetrazolium chloride, which stains viable cells. The infarct areas were quantified for each brain slice, and the infarct volume was calculated by integration of infarct areas for all slices of each brain. Relative infarct volume was expressed as the percentage of the contralateral hemisphere. To correct for brain swelling, the infarct area was determined by subtracting the area of undamaged tissue in the left hemisphere from that of the intact contralateral hemisphere.
Quantification of Levels of 8-OxoG, FapyAde, and FapyGua in Brain Tissue Samples
Mice were killed 24 hours after focal ischemia, and their brains were rapidly removed. The contralateral and ipsilateral hemispheres were dissected separately and stored at −80°C. Genomic DNA was extracted and digested as described previously (Hu et al, 2005). The levels of 8-hydroxyl-2′-deoxyguanosine, FapyAde, and FapyGua were measured using gas chromatography mass spectroscopy and liquid chromatography mass spectroscopy. The 8-oxo-G was measured as its nucleoside 8-oxo-dG by liquid chromatography/mass spectrometry with isotope dilution technique using 8-oxo-dG-15N5 as an internal standard as described previously (Hu et al, 2005). FapyGua and FapyAde were measured by gas chromatography/mass spectrometry as described previously (Jaruga et al, 2000).
Brain Tissue 8-oxodG Incision Activity Assay
The 8-oxodG incision activities were measured in homogenates prepared from frozen brain tissues of WT and OGG1−/− mice. Samples were chopped into small pieces in buffer A containing 10 mmol/L Tris-HCl and 200 mmol/L KCl (pH 7.8). Next, 0.4 mL buffer B containing 10 mmol/L Tris-HCl, 600 mmol/L KCl, 2 mmol/L dithiothrietol (DTT), 2 mmol/L ethylene diamine tetraacetic acid (EDTA), 40% glycerol, 0.2% NP-40, 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 1 × protease inhibitors (Roche Applied Science, Indianapolis, IN, USA) was added and the tissues were sonicated, agitated for 1.5 hours (4°C), and then centrifuged at 130,000g for 1 hour. The supernatant was mixed 1:1 (v/v) in buffer C (25 mmol/L HEPES-KOH (pH 8.0), 100 mmol/L KCl, 1 mmol/L DTT, 1 mmol/L EDTA, 17% glycerol, and 12 mmol/L MgCl2. The suspensions were centrifuged at 16,000g, and the supernatant was stored at −80°C. Incision activity of 8-oxodG was measured using a 28-mer radiolabeled double-stranded DNA oligonucleotide as described previously (Croteau et al, 1997). The reaction (10 μL) contained 40 mmol/L HEPES-KOH (pH 7.6), 5 mmol/L EDTA, 1 mmol/L DTT, 50 mmol/L KCl, 10% glycerol, 80 fmol of oligonucleotide, and 20 μg protein. Reactions were incubated for 16 hours at 32°C then terminated by adding 10 μL of formamide loading dye (80% formamide, 10 mmol/L EDTA, 1 mg/mL xylene cyanol FF, and 1 mg/mL bromophenol blue). Reaction products were resolved by 20% acrylamide and 7 mol/L urea gel electrophoresis at 15 W for 1 hour. Gels were visualized on a Typhoon and analyzed using ImageQuant (GE Healthcare, Piscataway, NJ, USA). Incision activity was determined as the intensity of product bands relative to the combined intensities of substrate and product bands.
Nick Labeling of DNA-Strand Breaks (TUNEL) and Brain Histology
A modified nick-end labeling using DNA polymerase I Klenow fragment was performed to detect nicks and SSBs in DNA on brain sections, as described previously (Liu et al, 1999). Briefly, cryosections of brain were fixed with 4% formaldehyde, permeabilized, and incubated with deoxynucleotide triphosphate, biotinylated deoxyuridine triphosphate, and DNA polymerase. Labeled DNA fragments were visualized with avidin-fluorescein isothiocyanate conjugate. Cells and brain sections were double stained with neuronal markers. Immunohistological staining with OGG1 antibody (Novus) was performed on coronal sections of rat brains (because the OGG1 antibody recognizes a single band in rat brain cells, but not in mouse cells), and from sham control mice or mice that had been subjected to MCAO. The sections were costained with Hoechst dye.
Behavioral Tests
The Rota-rod behavioral test was used to assess motor coordination and endurance. The rotadrum was filled with warm water to a level just below the bottom of the rod. The treading surface consisted of four rotating drums divided by flanges and adjustable speed through changes of rod diameter. Mice were trained before MCAO to adapt to the apparatus; the speed of rotation was adjusted by the diameter of rotating drum, and pretested and selected based on the performance and tolerance of mice during total 5 minutes period on the rod, and the times of falling off the rod were recorded. Mice were dried with a towel and immediately placed back onto the rod until the total time on the rod was 5 minutes. The total number of falls during the 5-minute test period was recorded for each mouse. Performance based on motor coordination and limb strength in this test was assessed before and after MCAO (24 hours to 7 days before stroke) and 48 hours after stroke (7 to 9 mice per group).
Statistics
All data are presented as means±s.d. and P<0.05 was considered significant. If data were obtained from samples collected from paired groups (such as wt and OGG1-deficient mice), paired t-test was performed. For multiple regional analyses, analysis of variance and with appropriate post hoc comparisons were performed.
Results
Expression and Distribution of 7,8-Dihydro-8-Oxoguanine DNA Glycosylase in Neurons After Oxidative and Metabolic Stress
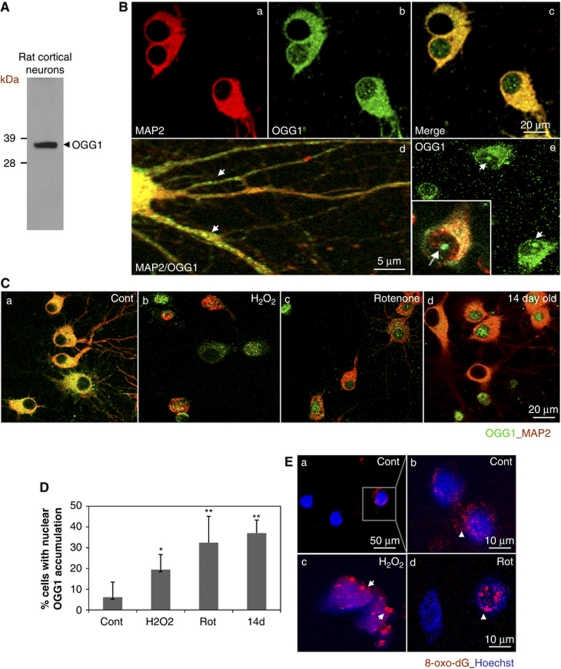
The expression and subcellular localization of OGG1 were examined in primary rat cortical neurons by immunoblotting and immunofluorescence microscopy (Figure 1). The OGG1 antibody (Novus) is targeted to the N-terminal 100 amino acids of human OGG1, which shares 85% homology with rat OGG1 and 90% homology with mouse OGG1 (Genebank). The OGG1 immunoblot revealed a predominant band around 39 kDa in cultured rat cortical neurons (Figure 1A). The OGG1 immunoreactivity was detected in both the cytosol and nuclei of neurons, including dendrites (Figure 1Bd) and in the nucleolus (Figure 1Be).
Figure 1.
Cortical neurons subjected to oxidative and metabolic insults exhibit increased nuclear localization of 7,8-dihydro-8-oxoguanine DNA glycosylase (OGG1) in association with oxidatively modified DNA. (A) Immunoblot revealed a single band of 39 kDa in protein lysate from cultured rat cortical neurons. (B) Representative confocal images of OGG1 immunoreactivity in primary cortical neurons. The OGG1 immunofluorescence signal (green) is present in both the cytosol and nucleus of 10-day-old cultured cortical neurons (panels b–e). The cells were costained with an antibody against the neuron-specific protein microtubule-associated protein 2 (MAP2) (red, a, c, d, e). The OGG1 signal is also observed in neuronal processes and nucleolus of neurons (d, e). Panels c and d and the inset in panel e are merged images. (C) Representative confocal images revealing cellular distribution of OGG1 (green) in young cultured rat cortical neurons (7 days in culture) after exposure to vehicle (Cont) or oxidative insults (H2O2, 40 μmol/L; rotenone, 1 μmol/L) for 6 hours, or in old neurons (14 days in culture) as indicated. Cells were costained with MAP2 (red). Note that OGG1 is located predominantly in the cytoplasmic compartment in control cultures (left), whereas after exposure to oxidative and metabolic insults, and in very old neurons, OGG1 levels are greatly diminished in the cytoplasm and increased in the nucleus. (D) Percentages of cells with OGG1 staining in the nucleus were quantified. Values are the mean and s.d. from 4 to 6 cultures (100 neurons analyzed in each culture) for each treatment group. *P<0.05; **P<0.01 compared with vehicle-treated control group. (E) 8-oxoG immunostaining (red) in cortical neurons from control cultures and cultures exposed to H2O2 or rotenone (Rot) for 6 hours. The cells were counterstained with Hoechst dye to label the nuclei (blue). The color reproduction of this figure is available on the html full text version of the manuscript.
The OGG1 immunofluorescence was examined in young (5 to 7 days in culture) and older (14 days in culture) rat cortical neurons exposed to oxidative (40 μmol/L H2O2) and metabolic (1 μmol/L rotenone; an inhibitor of mitochondrial complex I) insults. We observed increased OGG1 immunoreactivity in the cytosol and mitochondria of neurons exposed to H2O2 for 4 hours, whereas at 6 hours after exposure, there was an overall decrease in OGG1 in the cell body of neurons, but an increase in OGG1 immunofluorescence was observed in the nucleus (Figure 1Cb and c). The OGG1 levels were also elevated in nuclei of 14-day-old neurons (Figure 1Cd), presumably as the result of the accumulation of oxidative nuclear DNA damage in the older neurons (Aksenova et al, 1999). The percentages of cells with enhanced nuclear OGG1 staining at 6 hours after oxidative and metabolic insults were significantly greater than control vehicle-treated cultures (Figure 1D). The percentage of neurons with nuclear OGG1 staining was significantly greater in old (14 days) compared with young (7 to 10 days) neurons (Figure 1D). The changes of cellular OGG1 localization and levels might be a response to oxidative DNA damage induced under conditions of oxidative stress. We therefore immunostained neurons with an 8-oxoG antibody. Only a small portion of cells under control conditions showed a fluorescent signal for 8-oxoG, which was localized mainly in punctate regions in the cytosol with little 8-oxoG immunoreactivity in the nucleus (Figure 1Ea and b). After oxidative and metabolic insults, however, a notable 8-oxoG signal in the nucleus of neurons was observed (Figure 1Ec and d).
Neurons from 7,8-Dihydro-8-Oxoguanine DNA Glycosylase-Deficient Mice Exhibit Increased Vulnerability to Oxidative Stress, Mitochondrial Impairment and Nuclear DNA Damage
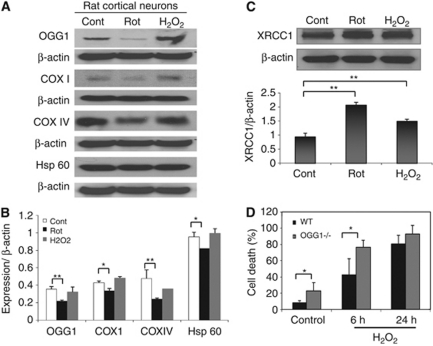
As both mitochondrial and nuclear DNA are susceptible to oxidative damage, and as OGG1 localizes to both compartments, we investigated the effects of oxidative and metabolic insults on the mitochondria and nucleus of neurons expressing or lacking OGG1. The levels of OGG1, and the mitochondrial cyclooxygenases COX IV and COX I, and Hsp60, were examined by immunoblot analysis of lysates of cortical neurons from cultures that had been treated for 6 hours with vehicle, H2O2, or rotenone. In rotenone-treated cells, the levels of OGG1, COX I, COX IV, and Hsp60 were significantly reduced compared with vehicle-treated control cells within 4 hours of exposure (Figures 2A and 2B). However, levels of OGG1, COX I, COX IV, and Hsp60 were not changed significantly exposure to H2O2 (Figures 2A and 2B). Immunoblot analysis of the extracts revealed increased levels of XRCC1 in cortical neurons exposed to H2O2 and rotenone (Figure 2C), indicating the activation of the BER pathway (Kulkarni et al, 2008) by these insults.
Figure 2.
Expression of 7,8-dihydro-8-oxoguanine DNA glycosylase (OGG1), XRCC1, and mitochondria proteins in neurons after oxidative stress. (A) Western blot analysis of OGG1 and the mitochondrial proteins, COX I, COX IV, and Hsp60 in cortical neurons exposed to vehicle (Cont), H2O2 (40 μmol/L), or rotenone (1 μmol/L) for 6 hours. (B) Quantification of relative levels of OGG1 and mitochondrial proteins normalized to β-actin levels. (C) Immunoblot showing that the XRCC1, another enzyme in base-excision repair (BER) pathway, is upregulated in rat cortical cells exposed to metabolic and oxidative insults. (D) Cortical cells were isolated from wild-type (WT) and OGG1−/− mice and cultured on dishes for 6 days. Cells were exposed to H2O2 (40 μmol/L) for 6 and 24 hours, or to vehicle for 24 hours (Control) and were stained with Hoechst dye. The percentages of neurons that were dead (fragmented or condensed nuclei, and degenerative changes as described in the text) were determined. Values are the mean and s.d. from 4 to 6 cultures prepared for each treatment group. *P<0.05, **P<0.01.
We next quantified the vulnerability of cortical neurons isolated from WT and OGG1-deficient mice to oxidative stress. Cells were cultured in 35 mm dishes for 6 days, then were exposed to H2O2 (40 μmol/L) or vehicle (saline) for 24 hours. Neuronal viability was assessed by morphological criteria and Hoechst nuclear DNA staining (see Materials and methods) at 6 and 24 hours after exposure to H2O2. At the 6-hour time point, significantly more neurons had died in cultures from OGG1-deficient mice compared with cultures from WT mice (Figure 2D). At the 24-hour time point, >80% of the neurons had died in cultures from WT and OGG1−/− mice (Figure 2D).
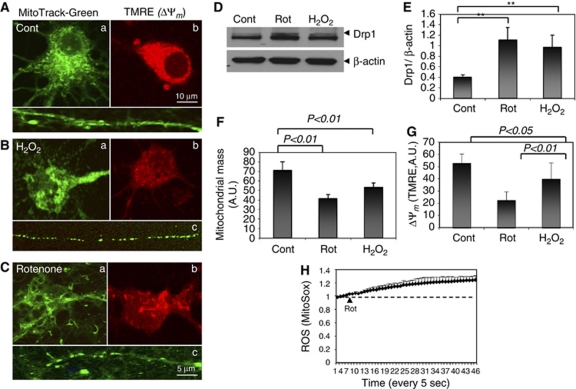
Mitochondria and mtDNA are believed to be prone to oxidative damage because of the relatively large amounts of superoxide generated by the mitochondrial electron-transport chain. Rat cortical cells were loaded with MitoTracker Green to label all mitochondria and the mitochondrial membrane potential (Δψm) indicator TMRE at 6 hours after exposure to H2O2 or to rotenone (Figures 3A–3C). Morphological changes of mitochondria appeared in cells treated with these insults, including fragmentation and aggregation (Figures 3B and 3C). The levels of dynamin-related protein 1 (Drp1), a protein that has a pivotal role in mitochondrial fission, were significantly elevated in cortical cells exposed to both oxidative insults (Figures 3D and 3E), consistent with previous reports linking Drp1 to neuronal injury (see Mattson et al, 2008 for review). Reduced mitochondrial mass and mitochondrial membrane potential (Δψm) were detected before death in cells exposed to oxidative insults (Figures 3F and 3G), as quantified from images of MitoTracker Green and TMRE fluorescence, respectively. Time-lapse confocal imaging confirmed the increased reactive oxygen species (ROS) production in cortical neurons exposed to rotenone (1 μmol/L) as indicated by levels of MitoSox fluorescence (Figure 3H). The extensive damage to mitochondria during a time period after exposure to oxidative/metabolic insults (4 to 6 hours) when total cellular OGG1 levels decrease and nuclear OGG1 levels increase, suggests a failed ability of the neurons to repair mtDNA and a continuing attempt to repair nuclear DNA.
Figure 3.
Evidence that mitochondria become damaged and dysfunctional within 6 hours of exposure to metabolic and oxidative insults. (A–C) Cortical cells from control, rotenone, or H2O2-treated groups were labeled with the fluorescent indicators MitoTracker Green for mitochondrial morphology (a, c) or TMRE (b) for mitochondrial membrane potential (Δψm). The images show typical morphological changes of mitochondria after exposure to H2O2 or rotenone including fragmentation in cell bodies (a) and processes (c) from control and cells treated with oxidative insults as indicated. (D, E) Immunoblot analysis of dynamin-related protein 1 (Drp1) levels in cortical cells after 6 hours of exposure to oxidative insults as indicated. (F, G) Quantification of mitochondrial mass and Δψm from multiple images collected from control cells and cells exposed to oxidative insults for 4 to 6 hours. Values are the mean and s.d. from determinations made in four separate cultures for each treatment group. **P<0.01. (H) Cells were loaded with fluorescence dye MitoSox, an indicator of reactive oxygen species (ROS). Time-lapse imaging showing increased fluorescence intensity after exposure to rotenone (1 μmol/L).
7,8-Dihydro-8-Oxoguanine DNA Glycosylase-Deficient Mice Develop Larger Lesions in the Brain in Response to Focal Cerebral Ischemia Compared with Age-Matched Wild-Type Mice
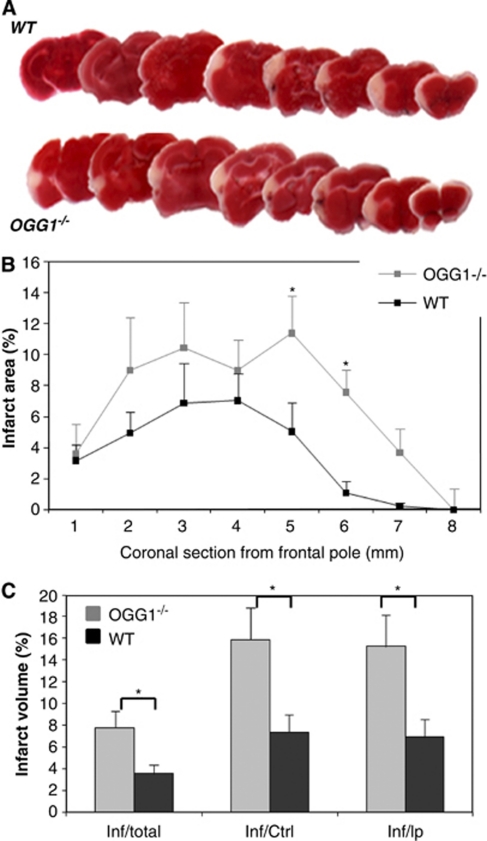
We next used a mouse model of focal ischemic stroke to determine the functional roles of OGG1 and the BER pathway in neuronal survival under conditions of metabolic and oxidative stress. Wild-type mice and OGG1−/− mice were subjected to unilateral permanent MCAO and 48 hours later, the mice were euthanized and brain sections were immunostained with 2,3,5-triphenyltetrazolium chloride. The representative 2,3,5-triphenyltetrazolium chloride-stained coronal sections from brains of WT and OGG1−/− mice 48 hours after MCAO are shown in Figure 4A. The OGG1-deficient mice showed larger cortical lesions compared with WT mice (Figure 4A). Quantification of infarct areas measured in each brain section established a significantly greater amount of cell damage in OGG1−/− mice compared with WT mice (Figure 4B). Average infarct volumes for OGG1−/− mice were significantly greater than for WT mice, when calculated as either a percentage of total brain volume, contralateral hemisphere volume, or ipsilateral hemisphere volume (Figure 4C).
Figure 4.
Mice lacking 7,8-dihydro-8-oxoguanine DNA glycosylase (OGG1) exhibit increased cortical damage in permanent middle cerebral artery occlusion (MCAO) model of focal ischemic stroke. The MCAO was performed on wild-type (WT) and OGG1−/− mice and 48 hours later, brains were processed for 2,3,5-triphenyltetrazolium chloride (TTC) staining to quantify brain damage. (A) Representative TTC-stained coronal sections from brains of a WT mouse and an OGG1−/− mouse. (B) Infarct areas for brain sections at the indicated rostro-caudal levels. (C) Infarct volumes in WT and OGG1−/− mice. Values are the mean and s.d. (six mice in each group). *P<0.05. Paired t-test.
7,8-Dihydro-8-Oxoguanine DNA Glycosylase Deficiency Results in Greater Stroke-Induced Accumulation of Oxidative DNA Base Lesions
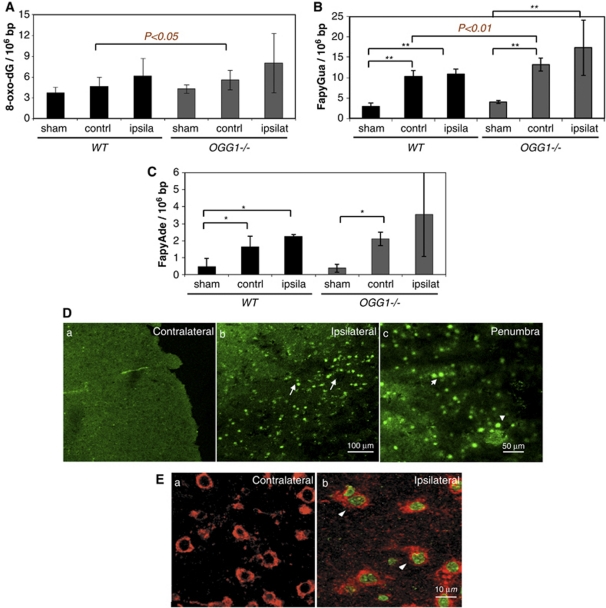
The levels of oxidative DNA base lesions in total DNA isolated from the brains of sham, WT, and OGG1−/− mice 24 hours after surgeries were measured as described in the Materials and methods section. Analysis of the total DNA extracted revealed an accumulation of 8-oxo-dG, FapyAde, and FapyGua as a result of the stroke and, interestingly, increased levels of oxidative DNA damage were detected in both the ipsilateral hemisphere and the contralateral hemisphere of WT and OGG1−/− mice (Figures 5A–5C). Stroke-induced oxidative DNA base lesions were more numerous in DNA obtained from the OGG1−/− mouse brains compared with lesions in DNA from WT mouse brains after MCAO, with the differences being statistically significant for 8-oxo-dG and FapyGua in the contralateral hemisphere. There was a very large interanimal variability in the amount of DNA damage in the ipsilateral hemisphere of OGG1−/− mice compared with WT mice, possibly as a result of the more complicated pathological changes in the ischemic hemisphere including various degrees of damage to multiple cell types and infiltration and activation of inflammatory cells at 24 hours after MCAO (Liu et al, 1999), as well as a possible differential compensation for lack of OGG1 among individual mice.
Figure 5.
Ischemia-induced oxidative DNA base lesions in genomic DNA are elevated in cerebral cortical cells in mice lacking 7,8-dihydro-8-oxoguanine DNA glycosylase (OGG1). (A–C) Levels of 8-oxodG were quantified by HPLC–MS/MS (A) and levels of FapyGua (B) and FapyAde (C) were measured by GC–MS/MS (see Materials and methods). The values are presented as number of lesions per 106 bases in the DNA extracted from the brains. Values are the mean and s.d. (three mice per group). *P<0.05, **P<0.01. contrl, contralateral cerebral cortex; ipsila, ipsilateral cerebral cortex. (D, E) Cells exhibiting nuclear DNA-strand breaks are abundant in neurons in the ischemic cerebral cortex, but not in the contralateral cerebral cortex. (D) Nick-end labeling (green) with DNA polymerase I Klenow fragment (for single-strand break (SSB)) was performed in mouse brain coronal sections at 24 hours after middle cerebral artery occlusion (MCAO). (E) Most nick-end labeling positive cells were neurons as indicated by costaining with neuronal protein marker TG2 (red) (arrowheads). The color reproduction of this figure is available on the html full text version of the manuscript. LC–MS, liquid chromatography–mass spectroscopy.
Increased DNA-strand breaks are hallmarks of cell damage after cerebral ischemia in stroke models. DNA-strand breaks detected by nick translation with DNA polymerase I for SSBs or terminal transferase (TUNEL) for DSB were abundant in the ipsilateral cortex in the penumbral regions at 24 to 48 hours after MCAO (Figure 5D). Most nick-labeled and TUNEL-positive cells were neurons that costained with neuronal markers by double labeling (Figure 5E; see also Liu et al, 1999).
8-oxoG Incision Activity Is Reduced in Brains of 7,8-Dihydro-8-Oxoguanine DNA Glycosylase-Deficient Mice Under Basal Conditions and After Cerebral Ischemia
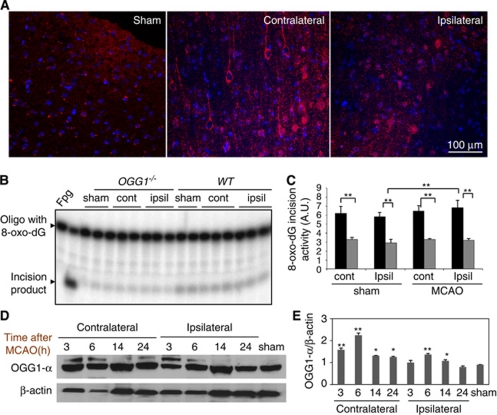
Cultured cortical neurons express OGG1 (Figure 1). To confirm that OGG1 is also expressed in the cortical neurons affected by ischemic stroke in vivo, we immunostained brain tissue sections from sham and ischemic (14 hours after stroke) brains. The OGG1 immunoreactivity was present in cells throughout the cortex including neurons where its levels appeared to be elevated in the contralateral cortex of ischemic animals compared with the ipsilateral cortex of ischemic animals and to sham controls (Figure 6A). Many cells in the ipsilateral ischemic penumbra exhibited nuclear OGG1 immunoreactivity, in contrast to cells in sham controls or the contralateral cortex of ischemic animals (Figure 6A).
Figure 6.
Cells in the cerebral cortex of mice lacking 7,8-dihydro-8-oxoguanine DNA glycosylase (OGG1) exhibit a large reduction in 8-oxoG incision activity under basal conditions and after a stroke. (A) The OGG1 immunoreactivity (red) in the cerebral cortex of a rat subjected to sham surgery, and in the contralateral and ipsilateral cerebral cortex 14 hours after permanent middle cerebral artery occlusion (MCAO). Cell nuclei are stained blue (Hoechst counterstaining). Note that levels of OGG1 immunoreactivity are elevated in neurons in the contralateral and to a lesser extent the ipsilateral, cerebral cortex after the stroke. (B) Representative gel scan showing 8-oxoG incision activity in brain extracts from the contralateral (cont) and ipsilateral (ipsi) cerebral cortex of OGG1−/− and wild-type (WT) mice 24 hours after MCAO or sham surgery. (C) Quantification of 8-oxoG incision activities in brain extracts from the contralateral (cont) and ipsilateral (ipsi) hemispheres of OGG1−/− and WT mice 24 hours after MCAO or sham surgery. (D) Immunoblot analysis of OGG1 protein levels in mouse brains from sham control mice, and from the contralateral and ipsilateral cerebral cortex from mice killed at the indicated poststroke time points. (E) Quantification of protein band intensity, normalized to β-actin levels. Values are the mean and s.d. (three to four mice per group). *P<0.05; **P<0.01. The color reproduction of this figure is available on the html full text version of the manuscript.
The elevated levels of oxidative DNA damage in the brains of OGG1-deficient mice might reflect a decrease in the capacity to repair the lesions or a greater amount of oxidative stress after a stroke. Although OGG1−/− mice lack the major DNA glycosylase, which initiates repair of oxidized purines, it is possible that other repair enzymes could compensate for the loss of OGG1 glycosylase activity in brain cells. We therefore measured 8-oxo-dG incision activity in brain extracts of WT and OGG1−/− mice 24 hours after sham surgery or MCAO. There were highly significant reductions of 8-oxo-dG incision activity in cortical tissue samples from all OGG1−/− mice examined including sham controls, and the ipsilateral and contralateral cortex of ischemic mice (Figures 6B and 6C). There was a significant, albeit small elevation of 8-oxo-dG incision activity in cortical tissue samples from the ipsilateral hemispheres of WT mice that had been subjected to a stroke compared with sham controls (Figures 6B and 6C). The latter elevated incision activity may occur in cells in the peri-infarct region that survive the ischemic insult.
Immunoblot analysis of proteins in cortical brain tissue samples from WT mice that had been killed at different time points after a stroke revealed time-dependent changes in OGG1 protein levels (Figures 6D and 6E). Consistent with previous studies (Szczesny et al, 2003), two immunoreactive mouse OGG1 bands were detected, with a major band around 37 kDa that corresponds to OGG1-α (nuclear form). We suspect that the other OGG1 immunoreactive band represents another isoform of OGG1, such as OGG1-β. Densitometric analysis of OGG1 immunoreactive bands (normalized to actin levels) showed that levels of OGG1-α were elevated at 6 and 14 hours after stroke and then reduced by 24 hours in the ipsilateral cortex. The OGG-1α levels in the contralateral cortex were also elevated post-MCAO, particularly at 3 and 6 hours (Figures 6D and 6E).
7,8-Dihydro-8-Oxoguanine DNA Glycosylase Deficiency Impairs Functional Recovery from a Stroke
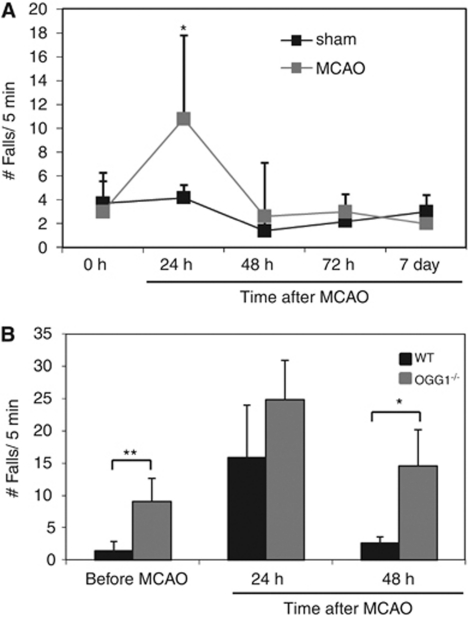
To determine the consequences of OGG1 deficiency on the outcome after stroke, we evaluated the functional recovery of WT and OGG1−/− mice during a 2-day poststroke time period (Figure 7). Performance was evaluated using a rota-rod test for motor coordination and limb strength. For WT mice, 24 hours after the stroke, there was a significant doubling of the frequency of falls during a 5-minute test period on the rota-rod apparatus. However, their motor performance returned to levels similar to that of sham-operated control mice by 48 hours after stroke (Figure 7A). The OGG1-deficient mice exhibited a poorer basal (prestroke) performance on the rota-rod compared with WT mice, and had a higher number of falls at 24 and 48 hours after stroke compared with WT mice (Figure 7B).
Figure 7.
7,8-Dihydro-8-oxoguanine DNA glycosylase (OGG1)-deficient mice exhibit poorer functional outcome after a focal ischemic stroke. (A) The performance of wild-type (WT) sham-operated control mice and mice subjected to permanent middle cerebral artery occlusion (MCAO) at the indicated poststroke time points. (B) The performance of WT and OGG1-deficient mice on the rota-rod before and at 24 and 48 hours after a focal ischemic stroke. Values are the mean and s.d. (five to nine mice per group). *P<0.05, **P<0.01.
Discussion
Our findings suggest that the capacity to repair oxidative DNA damage is a significant factor that determines the vulnerability of neurons to metabolic and oxidative stress, and functional outcome after stroke. We found that cortical neurons from OGG1-deficient mice are more vulnerable than WT neurons to metabolic and oxidative stress. The accumulation of oxidative DNA base lesions (8-oxoG, FypyAde, and FypyGua) after experimental stroke was greater in cortical brain tissue from OGG1-deficient mice than in WT mice. The OGG1-deficient mice also exhibited a worse behavioral outcome after a stroke, as indicated by protracted motor impairment in the rota-rod test, compared with WT mice. Our results indicate that the BER pathway, which is initiated by glycosylases, including OGG1, has a critical role in the survival of neurons under conditions of severe metabolic and oxidative stress. With regards to brain damage from a stroke, our findings suggest that neurons that are better able to repair oxidative DNA lesions have an increased chance of survival. This conclusion is consistent with the results of previous studies of BER activity in models of ischemic stroke (Li et al, 2006). Moreover, mice deficient in uracil-DNA glycosylase, another DNA glycosylase that initiates BER of uracil lesions, also exhibit increased neuronal vulnerability to experimental stroke (Endres et al, 2004).
The mechanism(s) by which increased oxidative base lesions, especially under conditions of defective oxidative DNA repair capacity, contribute to neuronal death after brain ischemia is unclear. As oxidative damage occurs in both mitochondrial and nuclear DNA, and because OGG1 is involved in the repair of both nuclear and mtDNA, we examined pathological changes of mitochondria and nuclei in cultured cortical neurons after oxidative and metabolic stress. In response to both oxidative and metabolic stress, cortical neurons exhibited multiple mitochondrial morphological alterations, which were associated with increased levels of the mitochondrial fission protein Drp1, decreased mitochondrial mass, and mitochondrial membrane depolarization. Mitochondrial impairment, including fragmentation and fission, has previously been observed in experimental models of neurodegenerative disorders (Jahani-Asl et al, 2007; Knott and Bossy-Wetzel, 2008). Mitochondrial impairment could result in cellular energy depletion, increased ROS production, and impaired mitochondrial dynamics and trafficking, thus aggravating the vulnerability of neurons to metabolic and oxidative stress under ischemic conditions. We observed a decrease in nonnuclear OGG1 and an increase in levels of nuclear OGG1 in neurons subjected to metabolic/oxidative stress. The loss of mitochondrial OGG1 could be related to mitochondrial impairment, whereas the elevated OGG1 levels in nuclei of cells after oxidative insults and in aged cultured cells undergoing apoptosis indicates that increased oxidative nuclear DNA damage triggers the accumulation of OGG1 into the nucleus, a possibility consistent with previous studies showing translocation of OGG1 to the cell nucleus (Araneda et al, 2005). An increase in oxidized DNA bases could be due to either direct oxidation of DNA during oxidative stress or incorporation of oxidized nucleosides from the nucleotide pool during DNA repair and replication (Nakabeppu et al, 2004). DNA nick labeling revealed that DNA fragmentation, both SSB (nick labeling) and DSB (TUNEL) were present abundantly in rodent brains after MCAO (Liu et al, 1999 and the present study). Our data suggest that OGG1 has an important role in the repair of such lesions.
Our studies suggest that the accumulation of oxidative DNA damage in brain is a critical event in determining the fate of neurons after a stroke. In this context, DNA repair capacity, specifically the activity of the BER pathway, represents a primary response to such accumulation, and is fundamental to prevent cell death under these conditions. In the absence of an efficient BER pathway, these events may lead to increased DNA fragmentation and neuronal death. DNA-strand breaks can be directly introduced into the DNA by the attack of reactive species to the sugar-phosphate backbone, or may accumulate as a result of incomplete BER. An increase in mutation frequency in OGG1-deficient mice exposed to γ radiation has been previously demonstrated, and was associated with G:C to T:A transversions, likely from 8-oxoG mispairing during replication (Larsen et al, 2006). Accummulation of Fapy dA or Fapy dG is also mutagenic (Wiederholt et al, 2002). We observed a significant accumulation of oxidized purines in brains from OGG1-deficient mice compared with WT mice after stroke, indicating that mutagenic events may also have a role in the outcome of the stroke. It has been proposed that excision of adenine opposite 8-oxoG leads to the accumulation of SSBs, as they were suppressed by the knockdown of adenine DNA glycosylase encoded by MutY homolog (Oka et al, 2008). In the absence of OGG1, increased adenine incorporation opposite 8-oxoG during DNA replication may occur, resulting in A: 8-oxoG mispairs. MUTYH excises adenine opposite 8-oxoG through its adenine DNA glycosylase activity, and abasic sites could then be converted into SSBs by AP endonuclease (Oka et al, 2008). In addition, calcium release into the cytosol has been observed in stroke, which could activate Ca2+-dependent DNA endonucleases, resulting in excitotoxicity, DNA fragmentation, and apoptotic cell death.
We observed several alterations related to DNA damage and repair in cells in the contralateral (nonischemic) cerebral cortex of mice subjected to permanent MCAO. Levels of OGG1 were elevated in the contralateral cortex within 3 hours after a stroke and remained elevated at 24 hours; immunostaining results suggested that neurons exhibited a particularly robust increase in OGG1 levels in response to the stroke in the opposite cerebral hemisphere. We also found that levels of oxidatively modified DNA bases were increased in the contralateral cortex after a stroke, albeit to a lesser extent than in the ischemic cortex. Previous studies have provided evidence that neurons in the contralateral cortex are indeed subjected to at least a moderate amount of metabolic and oxidative stress after a focal ischemic stroke, but recover from this stress (Liu et al, 2009). The upregulation of OGG1 after a stroke in neurons in the contralateral hemisphere is likely an adaptive response that protects the neurons from being damaged and killed.
It had been thought that OGG1-deficient mice lack significant phenotypes, thus casting doubt on the oxidative damage theory of aging, which proposes that accumulation of oxidative DNA damage with aging leads to the aging phenotype. Our data clearly show that lack of OGG1, and thus lack of efficient BER, leads to an increased vulnerability to cell death after stroke. Although low BER capacity may be sufficient to maintain genomic stability under basal conditions, and increased input of oxidative lesions, such as after ischemia reperfusion or after exposure to certain toxins, requires an efficient and robust response of the BER pathway. Under these conditions, even small deficiencies in the repair capacity may render neurons vulnerable to dysfunction and death.
Acknowledgments
The authors acknowledge the expert technical assistance of Dr Miral Dizdaroglu from the DNA Measurements Group, National Institute of Standards and Technology, and Mohamed Mughal from the Laboratory of Neurosciences, National Institute on Aging.
The authors declare no conflict of interest.
Footnotes
This study was supported by the Intramural Research Program of the National Institute on Aging.
References
- Aksenova MV, Aksenov MY, Markesbery WR, Butterfield DA. Aging in a dish: age-dependent changes of neuronal survival, protein oxidation, and creatine kinase BB expression in long-term hippocampal cell culture. J Neurosci Res. 1999;58:308–317. [PubMed] [Google Scholar]
- Araneda S, Pelloux S, Radicella JP, Angulo J, Kitahama K, Gysling K, Forray MI. 8-oxoguanine DNA glycosylase, but not Kin17 protein, is translocated and differentially regulated by estrogens in rat brain cells. Neuroscience. 2005;136:135–146. doi: 10.1016/j.neuroscience.2005.06.080. [DOI] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Croteau DL, ap Rhys CM, Hudson EK, Dianov GL, Hansford RG, Bohr VA. An oxidative damage-specific endonuclease from rat liver mitochondria. J Biol Chem. 1997;272:27338–27344. doi: 10.1074/jbc.272.43.27338. [DOI] [PubMed] [Google Scholar]
- de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, Klungland A, Bohr VA. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine DNA glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1-defective mice. Cancer Res. 2001;61:5378–5381. [PubMed] [Google Scholar]
- Dizdaroglu M. Substrate specificities and excision kinetics of DNA glycosylases involved in base-excision repair of oxidative DNA damage. Mutat Res. 2003;531:109–126. doi: 10.1016/j.mrfmmm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Endres M, Biniszkiewicz D, Sobol RW, Harms C, Ahmadi M, Lipski A, Katchanov J, Mergenthaler P, Dirnagl U, Wilson SH, Meisel A, Jaenisch R. Increased postischemic brain injury in mice deficient in uracil-DNA glycosylase. J Clin Invest. 2004;113:1711–1721. doi: 10.1172/JCI20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ML, Van RH, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age. Proc Natl Acad Sci USA. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, de Souza-Pinto NC, Haraguchi K, Hogue BA, Jaruga P, Greenberg MM, Dizdaroglu M, Bohr VA. Repair of formamidopyrimidines in DNA involves different glycosylases: role of the OGG1, NTH1, and NEIL1 enzymes. J Biol Chem. 2005;280:40544–40551. doi: 10.1074/jbc.M508772200. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Jahani-Asl A, Cheung EC, Neuspiel M, MacLaurin JG, Fortin A, Park DS, McBride HM, Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem. 2007;282:23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- Jaruga P, Speina E, Gackowski D, Tudek B, Olinski R. Endogenous oxidative DNA base modifications analysed with repair enzymes and GC/MS technique. Nucleic Acids Res. 2000;28:E16. doi: 10.1093/nar/28.6.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A, Calvayrac G, Karahalil B, Bohr VA, Stevnsner T. Mammalian 8-oxoguanine DNA glycosylase 1 incises 8-oxoadenine opposite cytosine in nuclei and mitochondria, while a different glycosylase incises 8-oxoadenine opposite guanine in nuclei. J Biol Chem. 2003;278:19541–19548. doi: 10.1074/jbc.M301504200. [DOI] [PubMed] [Google Scholar]
- Kemeleva EA, Sinitsyna OI, Kolosova NG, Vasyunina EA, Zharkov DO, Conlon KA, Berrios M, Nevinsky GA. Immunofluorescent detection of 8-oxoguanine DNA lesions in liver cells from aging OXYS rats, a strain prone to overproduction of free radicals. Mutat Res. 2006;599:88–97. doi: 10.1016/j.mrfmmm.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott AB, Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann NY Acad Sci. 2008;1147:283–292. doi: 10.1196/annals.1427.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A, McNeill DR, Gleichmann M, Mattson MP, Wilson DM., III XRCC1 protects against the lethality of induced oxidative DNA damage in nondividing neural cells. Nucleic Acids Res. 2008;36:5111–5121. doi: 10.1093/nar/gkn480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Li W, Zhang F, Sun FY, Nagayama T, O'Horo C, Chen J. Inducible repair of oxidative DNA lesions in the rat brain after transient focal ischemia and reperfusion. J Cereb Blood Flow Metab. 2003;23:1324–1339. doi: 10.1097/01.WCB.0000091540.60196.F2. [DOI] [PubMed] [Google Scholar]
- Larsen E, Reite K, Nesse G, Gran C, Seeberg E, Klungland A. Repair and mutagenesis at oxidized DNA lesions in the developing brain of wild-type and Ogg1−/− mice. Oncogene. 2006;25:2425–2432. doi: 10.1038/sj.onc.1209284. [DOI] [PubMed] [Google Scholar]
- Li W, Luo Y, Zhang F, Signore AP, Gobbel GT, Simon RP, Chen J. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2006;26:181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de CR, Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res Mol Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y, Tsuchimoto D, Ichinoe A, Ohno M, Ide Y, Hirano S, Yoshimura D, Tominaga Y, Furuichi M, Sakumi K. Biological significance of the defense mechanisms against oxidative damage in nucleic acids caused by reactive oxygen species: from mitochondria to nuclei. Ann NY Acad Sci. 2004;1011:101–111. doi: 10.1007/978-3-662-41088-2_11. [DOI] [PubMed] [Google Scholar]
- Oka S, Ohno M, Tsuchimoto D, Sakumi K, Furuichi M, Nakabeppu Y. Two distinct pathways of cell death triggered by oxidative damage to nuclear and mitochondrial DNAs. EMBO J. 2008;27:421–432. doi: 10.1038/sj.emboj.7601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Xiong S, Li GM, Gu L, Mao G, Markesbery WR, Lovell MA. Altered 8-oxoguanine glycosylase in mild cognitive impairment and late-stage Alzheimer's disease brain. Free Radic Biol Med. 2008;45:813–819. doi: 10.1016/j.freeradbiomed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JA, Bourque BM, de Souza-Pinto NC, Bohr VA. No evidence of mitochondrial respiratory dysfunction in OGG1-null mice deficient in removal of 8-oxodeoxyguanine from mitochondrial DNA. Free Radic Biol Med. 2005;38:737–745. doi: 10.1016/j.freeradbiomed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Szczesny B, Hazra TK, Papaconstantinou J, Mitra S, Boldogh I. Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases. Proc Natl Acad Sci USA. 2003;100:10670–10675. doi: 10.1073/pnas.1932854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, You HJ, Doetsch PW. Phenotypic change caused by transcriptional bypass of uracil in nondividing cells. Science. 1999;284:159–162. doi: 10.1126/science.284.5411.159. [DOI] [PubMed] [Google Scholar]
- Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35:5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederholt CJ, Delaney MO, Greenberg MM. Interaction of DNA containing Fapy.dA or its C-nucleoside analogues with base excision repair enzymes. Implications for mutagenesis and enzyme inhibition. Biochemistry. 2002;41:15838–15844. doi: 10.1021/bi025903e. [DOI] [PubMed] [Google Scholar]
- Wilson DM, III, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]