Abstract
Reduced infarct volume in TLR2-knockout mice compared with C57Bl/6 wild-type mice has recently been shown in experimental stroke and confirmed in this study. We now also show a significant decrease of CD11b-positive cell counts and decreased neuronal death in the ischemic hemispheres of TLR2-deficient mice compared with C57Bl/6wt mice 2 days after transient focal cerebral ischemia. To examine the potential benefit of intravascular TLR2 inhibition, C57Bl/6wt mice were treated intraarterially with TLR2-blocking anti-TLR2 antibody (clone T2.5) after 45 minutes of cerebral ischemia and compared with control antibody (isotype) treated wild-type mice. Whereas T2.5-treated mice had no reduction in infarct volumes at 48 hours after reperfusion, they did have decreased numbers of CD11b-positive inflammatory cells and decreased neuronal death compared with isotype-treated control mice. Comparison of the isotype antibody treatment to control (saline) treatment showed no effects on infarct volumes or neuronal survival. However, mice treated with the control isotype antibody had increased numbers of CD11b-positive inflammatory cells compared with saline-treated animals. Thus, antibody treatment itself (i.e., control isotype antibody, but potentially of any antibody) may have adverse effects and limit therapeutic benefit of anti-TLR2-antibody therapy. We conclude that TLR2 mediates leukocyte and microglial infiltration and neuronal death, which can be attenuated by TLR2 inhibition. The TLR2 inhibition in vivo improves neuronal survival and may represent a future stroke therapy.
Keywords: cerebral ischemia, MCAO, neuroprotection, stroke, TLR2, toll-like receptor
Introduction
Ischemic brain injury after focal cerebral ischemia results from a complex pattern of pathophysiological events including exitotoxicity, periinfarct depolarizations, inflammation, and apoptosis (Dirnagl et al, 1999; Kariko et al, 2004; Hossmann, 2006; McColl et al, 2009). The contribution of the inflammatory mechanisms to the postischemic neuronal damage is well known (Dirnagl, 1999; Hossmann, 2006; Trendelenburg, 2008). Toll-like receptors (TLRs) were recently shown to have an important role for the postischemic inflammatory response after transient focal cerebral ischemia (Babcock et al, 2006; Cao et al, 2007; Caso et al, 2008; Ziegler et al, 2007).
The TLRs represent a family of pattern-recognition transmembrane receptors, which recognize a large number of conserved structural motifs, called pathogen-associated molecular patterns. Although TLRs have a crucial role in the innate immune response to invading pathogens and infection, recent findings also suggest an important role in neurodegenerative disorders: TLRs are thought to be activated by endogenous ‘danger signals' released from injured or necrotic cells and contribute to the initiation of the inflammatory response as well as apoptotic cell death (Asea et al, 2002; Kariko et al, 2004; Aliprantis et al, 2000; Park et al, 2004; O'Neill et al, 2009; Kielian, 2006; Matzinger, 2002; Trendelenburg, 2008; Yao et al, 2009).
It was shown that activation of TLR signaling mediates ischemic preconditioning (Pradillo et al, 2009; Marsh et al, 2009; Stevens and Stenzel-Poore, 2006; Stevens et al, 2008; Kariko et al, 2004). Moreover, TLR2 and TLR4 were recently shown to contribute to ischemic brain damage in mice, potentially by activating proapoptotic pathways and the release of proinflammatory cytokines (Caso et al, 2008; Cao et al, 2007; Marsh et al, 2009; Kilic et al, 2008; Kariko et al, 2004; McColl et al, 2009; Tang et al, 2007; Ziegler et al, 2007). Furthermore, an association of TLR4 gene polymorphisms with ischemic stroke was found in human patients (Lin et al, 2005). The TLR2 is mainly expressed in microglia, but also in selected neurons (Babcock et al, 2006; Ziegler et al, 2007; Tang et al, 2007; Lalancette-Hébert et al, 2009). The TLR2 is activated by endogenous ‘danger signals,' such as HMGB1, apolipoprotein(apo)CIII, or heat shock proteins HSP70 and GP96 (Asea et al, 2002; Vabulas et al, 2002; Kawakami et al, 2008; Curtin et al, 2009) and was shown to contribute to neuroinflammation and neuronal death (Tang et al, 2007; Hoffmann et al, 2007).
There is growing interest in pharmacological modulation of TLR signaling due to the TLR-mediated effects in stroke and various other diseases. However, whereas specific TLR4 inhibitors were already tested in human patients, there is only limited information with regard to the use of TLR2 inhibitors in vivo (O'Neill et al, 2009): administration of anti-TLR2 antibody in vivo was shown to attenuate the proatherogenic effect of apoCIII-rich very low-density lipoprotein (Kawakami et al, 2008; O'Neill et al, 2009) and was shown to reduce myocardial ischemia/reperfusion injury (Arslan et al, 2010). 4,4′-Diisothio-cyanostilbene-2,2′-disulfonic acid protects against neuronal injury in vitro by blocking TLR2 activation (Yao et al, 2009). The monoclonal anti-TLR2 antibody T2.5 was shown to antagonize TLR2-specific activation of mouse and human macrophages both in vitro and in vivo (Zhang et al, 2006). Meng et al (2004, 2005) showed that administration of T2.5 in vivo prevents TLR2-driven lethal shock-like syndrome. The same antibody was used to block TLR2-mediated activation of monocytes by Pneumocystis murina in vitro (Zhang et al, 2006). Recently, it was shown that intravascular applied monoclonal antibodies permeate rodent brain after induction of focal cerebral ischemia (Chekhonin et al, 2004). However, there is no information about therapeutical use of TLR2 inhibitors in cerebral ischemia in vivo up to now.
This study was designed to address the question whether the application of anti-TLR2 antibody after transient focal ischemia in vivo protects against ischemic brain injury measured by infarct volume, inflammatory cell accumulation, or neuronal death.
Materials and methods
Animals
Adult 10–12 weeks old male TLR2−/− mice (Takeuchi et al, 1999) were generously provided by Dr S Akira (Department of Host Defense, Osaka University, Osaka, Japan). The TLR2−/− mice had been backcrossed to the original C57Bl/6J background for >10 generations. Thus, male C57Bl/6J mice (Charles River, Sulzfeld, Germany) were used as wild-type (control) mice, as well as for TLR2-inhibition studies. During surgery and ischemia, body temperature was measured in all animals and maintained between 37.0°C and 37.5°C, with a heating pad. All animal handling and surgery were performed in accordance with the Guidelines for the Use of Animals in Neuroscience Research (Society for Neuroscience) and approved by local and state authorities (LAGeSo No. G0382/05). Mice were housed under diurnal lighting conditions and allowed access to food and water ad libitum. All experiments were performed in a randomized manner: surgery, neurologic grading, and determination of infarct volume were performed by investigators masked to the groups.
Induction of Focal Cerebral Ischemia
Middle cerebral artery occlusion (MCAO) was induced by inserting a silicone-coated filament (Xantopren M Mucosa and Activator NF Optosil Xantopren, Heraeus Kulzer, Wehrheim, Germany) via the internal carotid artery as described by Hara et al (1996). For sham control, the operation was performed without inserting the filament. Mice were anesthetized with 2.5% isofluran for induction of surgery and maintained 1.5% isofluran in 70% N2O and 30% O2 during surgery via a face mask. Anesthesia did not exceed 10 minutes. The animals were reanesthetized for 1 minute after induction of MCAO (occlusion time: 60 minutes in the experiments with the TLR2-knockout mice, 45 minutes in the experiments with the in vivo application of TLR2 inhibitor), and the filament was removed to permit reperfusion, if not stated otherwise. Animals were killed 48 hours after the start of reperfusion for determination of infarct volumes, neuronal count, and leukocyte accumulation. Body weight of C57Bl/6 mice before surgery was 25.5±1.3 g compared with 25.4±1.5 g in TLR2−/− mice. After 48 hours of reperfusion, wild-type mice weighed 20.9±1.2 g compared with 21.5±1.5 g in TLR2−/− mice.
In Vivo Application of T2.5 and TLR2 Isotype Control Antibody
C57Bl6 wild-type mice were treated with TLR2-blocking antibody T2.5 or isotype control antibody, respectively, after 45 minutes MCAO. In all, 45 minutes MCAO was chosen to compensate for increased infarct sizes observed in the in vivo-treated mice when compared with the mice used in the TLR2−/− versus TLR2+/+ experiments, which were performed with 60 minutes occlusion time. Antibody was applied intraarterially instead of intraperitoneally (Meng et al, 2004) to reduce the necessary antibody amount per animal: after induction of cerebral ischemia and removal of the filament, 0.05 μg of antibody solution was applied via flexible tube directly into the internal carotid artery. During surgery, ischemia, and drug application, body temperature was measured and maintained between 37.0°C and 37.5°C by using a heating pad. In all, 11- to 12 week-old-male mice were used with a body weight of 23.9±0.8 g (T2.5 anti-TLR2 antibody-treated group), respectively 24.1±0.5 g (isotype control antibody-treated group). For examination of potential toxicity of antibody application into the cerebral arteries, 11- to 12-week-old male C57Bl/6J wild-type mice treated with 60 μL (equals 0.05 μg) isotype control antibody were compared with mice treated with 60 μL 1 × phosphate-buffered saline (PBS), injected as described above.
Assessment of Infarct Volume
At 48 hours after the induction of ischemia, mice were deeply anesthetized and killed. The brains were removed rapidly from the skull and snap frozen in 2-methylbutane on dry ice. Brains were sectioned (12 μm) on a microtome, dried, and stained with hematoxylin (Merck, Darmstadt, Germany). The sections were digitized, the area of infarction (‘direct infarct volume') was quantified on a PC using Sigma Scan Pro Software (Jandel Scientific, San Rafael, CA, USA; Sigma, St Louis, MO, USA) and infarct volumes were calculated. A correction for edema was applied by calculating the ‘indirect' infarct volume as the volume of the contralateral hemisphere minus the noninfarcted volume of the ipsilateral hemisphere. The difference between ‘direct' and ‘indirect' infarct volumes represents brain swelling.
Statistical Analysis
For comparison of infarct volumes, inflammatory cell count, and neuronal survival between the treated groups analysis of variance (P<0.005), followed by Tukey honestly significant difference (HSD) test was used, and for pairwise comparisons one-tailed Mann–Whitney U-test, if not stated otherwise. P values below 0.05 were considered statistically significant. Power calculation was performed using simple interactive statistical analysis (SISA)-Binominal (Uitenbroek, 1997). On the basis of the known variance of previous experiments, the MCAO experiments were powered (α=0.05; β=0.8) to detect effect sizes d (Cohen, 1988) of at least 1, i.e., of 1 s.d.
Immunohistochemistry
Staining was performed on 12 μm coronal cryosections at interaural positions 6.6, 5.3, 3.9, 1.9, and 0 mm from mice after 48 hours of reperfusion. The cryosections were thawmounted onto glass slides. Sections were used to determine stroke volume (see above). Slides were air dried for 30 minutes and fixed for 5 minutes in −20°C methanol and acetone (1:1). For counting leukocyte and activated microglia, sections were blocked using a solution containing 3% normal goat serum and 0.3% Triton X-100 (Sigma) in PBS for 60 minutes. The slides were incubated over night at 4°C with rat anti-CD11b antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at a dilution of 1:200. Neuron staining was performed according to the manufacture's instructions using M.O.M. Vector Kit (Vector, Burlingame, CA, USA) as well as mouse anti-NeuN (Chemicon, Temecula, CA, USA) at a dilution of 1:200. For detection of primary antibody Cy3-conjugated goat anti-mouse and goat anti-rat were used for secondary antibody (Molecular Probes, Leiden, The Netherlands). Omission of the primary antibody was used to ensure specificity of the secondary antibodies.
Cell Counting
A measure of 12 μm coronal cryosections were immunostained with anti-NeuN and anti-Cd11b antibodies. Labeled cells were counted in the whole affected ischemic hemisphere at interaural position 3.9 using a microscope and Stereo Investigator 7 Software (MicroBrightField Bioscience, San Diego, CA, USA) to reduce bias caused by different assignments of specific brain structures or different infarct localizations despite loosing regional information in contrast to cell number measurements in specific areas (Collins et al, 2010; Harhausen et al, 2010; Ziegler et al, 2009). The neuronal survival was determined by the ratio, which was calculated by the number of NeuN-positive cells in the ischemic hemisphere divided by the number of NeuN-positive cells in the nonischemic/contralateral hemisphere.
In Vitro Assays
Murine macrophage RAW 264.7 cells were grown in high-glucose DMEM (Biochrom KG, Berlin, Germany) supplemented with 10% FCS (Biochrom KG) and seeded at a density of 25,000 cells/cm2. After 2 days, cells were preincubated with 50 μg/mL anti-TLR2 antibody T2.5 (eBioscience Inc., San Diego, CA, USA) for 30 minutes and then stimulated with 0.1 μg/mL pam3Cys-SKKK (EMC Microcollections GmbH, Tübingen, Germany). Six hours after stimulation, 90 μL of supernatants were harvested for tumor necrosis factor bioassay and after 24 hours, the nitrite concentration was measured by Griess reaction. Tumor necrosis factor-α Bioassay and Griess reaction was performed as described previously (Freyer et al, 1999).
Results
TLR2 Deficiency Reduces Direct Infarct Volume, Neuroinflammation, and Preserves Neuronal Survival in the Ischemic Hemisphere
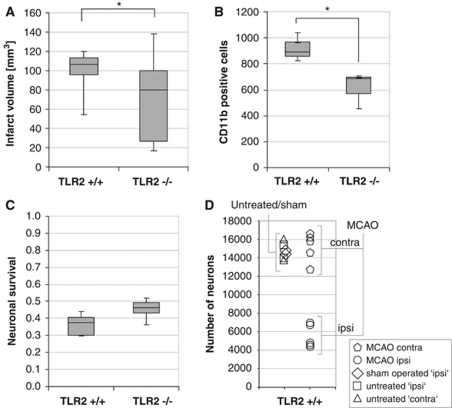
As it is known that TLR2-deficient mice had reduced infarct volumes at 48 hours of reperfusion compared with wild-type mice (Ziegler et al, 2007), we now tested whether TLR2-deficient mice also have reduced inflammatory cell accumulation, as measured by the CD11b-positive cell counts (CD11b stains inflammatory cells such as macrophages/monocytes and activated microglia) in the infarcted brain at 48 hours reperfusion after 1 hour MCAO compared with wild-type control mice. As shown in Figure 1A and reported before (Ziegler et al, 2007), TLR2-deficient mice had significant reduced (direct) infarct volume compared with wild-type mice. Moreover, there were less CD11b-positive cells in the ischemic hemisphere in TLR2-deficient mice (mean value±s.d. 614±140) compared with wild-type control mice (mean±s.d. 918±112; P<0.05) (Figure 1B).
Figure 1.
The TLR2 deficiency is associated with attenuated infarct volume, inflammatory cell accumulation, and with improved neuronal survival. (A) Infarct volume (‘direct infarct volume') is reduced in TLR2-deficient mice (TLR2−/−) at 48 hours of reperfusion after 1 hour middle cerebral artery occlusion (MCAO) compared with wild-type mice (TLR2+/+) (nTLR2−/−=12; nTLR2+/+=10; P<0.05). (B) CD11b-positive cells were counted in the ipsilateral (ischemic) brain hemisphere (at interaural position 3.9) 48 hours of reperfusion after 1 hour MCAO in C57Bl/6 wild-type (TLR2+/+) and TLR2-deficient mice (TLR2−/−) (nTLR2−/−=3; nTLR2+/+=3; P<0.05). (C) Survival ratio of neurons was calculated as the number of NeuN-positive cell count in the ischemic hemisphere (at interaural position 3.9) divided by the number of NeuN-positive cells in the nonischemic (contralateral) hemisphere in C57Bl/6 wild-type (TLR2+/+) and TLR2-deficient mice (TLR2−/−) (nTLR2−/−=6; nTLR2+/+=5; P<0.05). Cell counts are visualized as box-and-whisker plots. Statistical analysis was performed using the Mann–Whitney U-test. In all box plots, the top of the box represents the 75th percentile, the bottom of the box represents the 25th percentile, and the line in the middle represents the 50th percentile. The whiskers (the lines that extend out the top and bottom of the box) represent the highest and lowest values that are not outliers or extreme values. (D) Number of NeuN-positive cells (neurons) in the whole hemisphere (at interaural position 3.9) of untreated, sham-operated (mice that underwent anesthesia and surgery, but no MCAO), and MCAO-treated wild-type (TLR2+/+) mice (48 hours of reperfusion after 1 hour MCAO) and comparison of the left (ipsi) and right (contra) hemisphere (nuntreated ipsi=2, nuntreated contra=2, nsham ipsi=2, nMCAO ipsi=5, nMCAO contra=5). *P<0.05.
In parallel to the decreased cell accumulation of CD11b-positive cells, there was an attenuated loss of neuronal cells in the ischemic hemispheres of TLR2-deficient mice compared with that of wild-type mice. It was measured as the relative survival of neurons (neuronal survival ratio: 0.46±0.06 versus 0.36±0.06; given as mean±s.d.; P<0.05;) at 48 hours of reperfusion and induction of MCAO for 1 hour (Figures 1C and 1D).
TLR2 Antibody Blocks TLR2-Mediated Signaling In Vitro
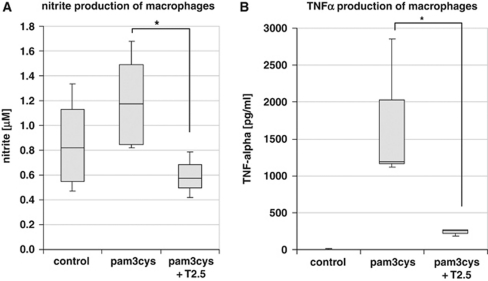
To evaluate whether the monoclonal anti-TLR2 antibody clone T2.5 (Meng et al, 2004) blocks TLR2 activation in vitro, murine macrophage RAW 264.7 cells were stimulated with the TLR2-agonist pam3Cys-SKKK (0.1 μg/mL) with or without previous incubation with 50 μg/mL anti-TLR2 antibody (T2.5). The primary reaction product of NO, nitrite, was measured 24 hours after stimulation. Nitrite concentration in the supernatant of TLR2.5-preincubated and pam3-Cys-SKKK-stimulated cells dropped to values of control cells (no preincubation with TLR2.5 or stimulation with pam3-Cys-SKKK), whereas supernatant of not T2.5 preincubated but pam3-Cys-SKKK-stimulated cells displayed high values of nitrite concentration. Thus, T2.5 efficiently blocks TLR2-mediated NO release in vitro (Figure 2A). Next, tumor necrosis factor-α concentration was measured after stimulation with the TLR2-agonist pam3-Cys-SKKK and with (and without, respectively) previous incubation with the anti-TLR2 antibody T2.5: tumor necrosis factor-α concentration was significantly (Δ=83.4%±6.7% P<0.001) attenuated in cells incubated with the anti-TLR2 antibody (T2.5) after stimulation with pam3-Cys-SKKK compared with untreated cells (Figure 2B). Thus, T2.5 anti-TLR2 antibody efficiently inhibits TLR2-mediated proinflammatory signaling in vitro.
Figure 2.
The TLR2-mediated inflammatory response can be blocked by anti-TLR2 antibody in vitro. The primary reaction product of NO, nitrite, was measured in the supernatant at 24 hours after stimulation of RAW 247.7 murine macrophage cells with the TLR2-agonist pam3-Cys-SKKK (0.1 μg/mL) with (or without) previous incubation with the anti-TLR2 antibody T2.5 (50 μg/mL) (A). Nitrite concentration after TLR2 stimulation in the supernatant of cells, which were preincubated with the blocking antibody (T2.5) drops to values obtained in the control experiment (without TLR2 stimulation), thus demonstrating that T2.5 efficiently blocks TLR2-mediated NO release (ncontrol=4; npam3cys=5; npam3cys+T2.5=3; P<0.05). (B) Tumor necrosis factor (TNF)-α concentration was measured after stimulation with the TLR2-agonist pam3-Cys-SKKK with (and without, respectively) previous incubation with the anti-TLR2 antibody T2.5. The TNF-α concentration was significantly (P<0.001) attenuated in cells incubated with the anti-TLR2 antibody (T2.5) after stimulation with pam3-Cys-SKKK compared with untreated cells (ncontrol=4; npam3cys=5; npam3cys+T2.5=3; P<0.05). *P<0.05.
TLR2-Blocking Antibody and Influence on the Infarct Volume
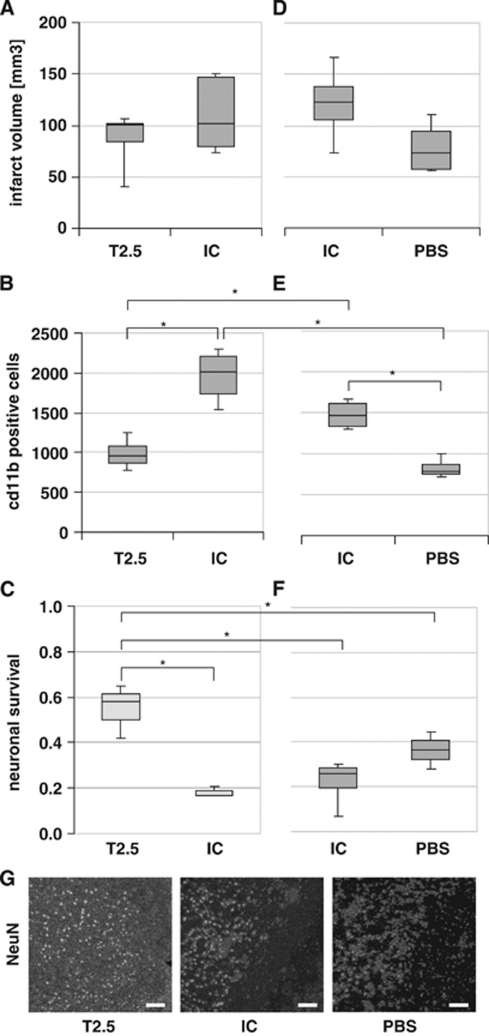
To evaluate a potential therapeutical effect of TLR2 inhibition, 0.05 μg TLR2-blocking antibody T2.5 was applied via flexible tube into the internal carotid artery of C57Bl/6 wild-type mice directly at the begin of reperfusion after induction of cerebral ischemia. Infarct volumes of wild-type mice treated with anti-TLR2 antibody were compared with those of wild-type mice treated with the same amount of isotype control antibody, which does not block TLR2 signaling. As shown in Figure 3A, there is no significant reduction of infarct volume in T2.5-treated mice compared with isotype-treated mice (mean±s.d. 88±25 mm3 versus 109±34 mm3). For type II error considerations, see Materials and methods (Statistics).
Figure 3.
(A–C) TLR2 inhibition reduces inflammatory response and increases neuronal survival. (A) TLR2 inhibition and its effect on the infarct volume. Direct infarct volume in male C57Bl/6 wild-type mice at 48 hours of reperfusion after 45 minutes middle cerebral artery occlusion (MCAO) treated with 0.05 μg T2.5 anti-TLR2 antibody (T2.5) and 0.05 μg isotype control (IC) antibody, respectively. Values are visualized as box-and-whisker plots (nT2.5=6; nIC=8; P>0.05). (B) TLR2 inhibition and its effect on the inflammatory response. CD11b-positive cells in the postischemic hemisphere at interaural position 3.9 mm at 48 hours after induction of MCAO for 45 minutes in male C57Bl/6 wild-type mice (P<0.001; nT2.5=6; nIC=8). (C) TLR2 inhibition and its effect on neuronal survival. NeuN-positive cells in the postischemic hemisphere at interaural position 3.9 at 48 hours after induction of MCAO for 45 minutes (P<0.05; nT2.5=3; nIC=3). ‘Neuronal survival' is shown, a ratio that was calculated as the number of NeuN-positive cells in the ischemic hemisphere divided by the number of NeuN-positive cells in the nonischemic (contralateral) hemisphere. (D–F) Effect of isotype control antibody on the postischemic brain injury. (D) Application of isotype antibody and its effect on the direct infarct volume. Direct infarct volumes in male C57Bl/6 wild-type mice are shown at 48 hours reperfusion after 45 minutes MCAO treated intraarterially with 0.05 μg isotype control antibody (IC), and phosphate-buffered saline (PBS), respectively. Cell counts are visualized as box-and-whisker plots (P>0.05, nIC=4; nPBS=4). (E) Antibody application and its effect on the inflammatory response. CD11b-positive cells in the postischemic hemisphere at interaural position 3.9 mm at 48 hours after induction of MCAO for 45 minutes in male C57Bl/6 wild-type mice (P<0.05, nIC=4; nPBS=4). (F) Antibody application and its effect on neuronal survival. ‘Neuronal survival' ratio was calculated as the number of NeuN-positive cells in the ischemic hemisphere divided by the number of NeuN-positive cells in the nonischemic (contralateral) hemisphere (P>0.05, nIC=4; nPBS=4). Statistical analysis between the different treatment groups of the two separate experiments (T2.5 versus IC, and IC versus PBS) was performed using analysis of variance (P<0.005), followed by pairwise comparisons using the Mann–Whitney U-test. (G) Representative images of NeuN-specific cell stain, located at the infarct border zone in the ischemic hemisphere at interaural position 3.9 at 48 hours of reperfusion after induction of MCAO for 45 minutes in male C57Bl/6 wild-type mice after intraarterial injection of T2.5 anti-TLR2 antibody (T2.5), isotype control (IC) antibody, or PBS, respectively. Scale bar=100 μm. *P<0.05.
TLR2 Inhibition Reduces Postischemic Inflammatory Response In Vivo
Further, we tested whether TLR2-blocking antibody applied in vivo reduces the postischemic response: CD11b-positive cells were counted at 48 hours reperfusion after 45 minutes MCAO in C57Bl/6 wild-type mice treated with either 0.05 μg TLR2-blocking antibody T2.5, or with the same amount of isotype control antibody. As shown in Figure 3B, there is a significant reduction of CD11b-positive cells in T2.5-treated mice compared with isotype-treated mice (mean±s.d. 986±177 versus 1,970±288; P=0.001). Thus, TLR2 inhibition in vivo inhibits the accumulation of inflammatory CD11b-positive cells.
TLR2 Inhibition In Vivo Protects Against Postischemic Neuronal Death
To test whether inhibition of TLR2 signaling by intraaterial application of TLR2-blocking antibody T2.5 after induction of MCAO preserves neuronal survival in the ischemic brain hemisphere, NeuN-positive cells were counted at 48 hours of reperfusion after MCAO for 45 minutes in C57Bl/6 wild-type mice. The animals were treated with either 0.05 μg TLR2-blocking antibody T2.5, or with the same amount of isotype control antibody. There were significantly more NeuN-positive cells in the ischemic hemisphere of T2.5-treated mice compared with the ischemic hemisphere of isotype-treated mice (mean±s.d. 8,677±2,340 versus 2,831±554; P<0.05) (data not shown). As shown in Figure 3C, there was a significant improvement of the ‘neuronal survival' (calculated as the number of NeuN-positive cells in the ischemic hemisphere divided by the number of NeuN-positive cells in the nonischemic/contralateral hemisphere) in the T2.5-treated mice compared with the isotype control antibody-treated group, which shows that TLR2 inhibition in vivo highly efficiently reduces postischemic neuronal death (Figures 3C and 3G).
Isotype Control Antibody Application and Its Influence on Postischemic Outcome in Wild-Type Mice
To test whether antibody application by itself influences postischemic tissue damage, infarct volumes at 48 hours of reperfusion after induction of MCAO for 45 minutes were compared between wild-type mice treated with isotype control antibody and mice treated with only PBS. Treatment with 0.05 μg isotype control antibody did not lead to a significant increase of infarct volumes (mean±s.d. 121±39 mm3 versus 79±27 mm3; P>0.05) at 48 hours of reperfusion after induction of cerebral ischemia compared with the sole application of PBS (Figure 3D). CD11b staining was performed to test whether the antibody application influences postischemic inflammation. Interestingly, there was a significant increase (mean±s.d. 1,478±182 versus 825±121; n=8, P=0.02) with regard to the number of CD11b-positive cells (myelomonocytic cells/macrophages and activated mircoglia) in wild-type mice at 48 hours after MCAO treated with 0.05 μg isotype control antibody compared with PBS-treated animals (Figure 3E). Moreover, there was a tendency without significance toward reduced numbers of NeuN-positive cells (mean±s.d. 3,342±1,612 versus 5,451±1,075) at 48 hours after induction of MCAO in isotype control antibody-treated mice compared with PBS-treated mice, which also corresponds to a nonsignificant change of the neuronal survival ratio (calculated as the number of NeuN-positive cells in the ischemic hemisphere divided by the number of NeuN-positive cells in the nonischemic/contralateral hemisphere) (Figures 3F and 3G).
Analysis of Brain Swelling (Edema) After Treatment
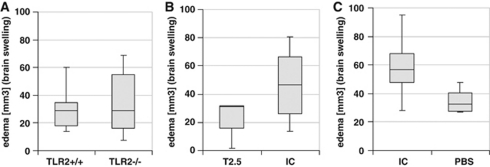
Brain swelling (which was calculated as the difference between ‘indirect' and ‘direct' infarct volumes) was compared at 48 hours after induction of MCAO in TLR-deficient, wild-type mice, as well as in T2.5-, isotype-, and PBS-treated wild-type mice (Figures 4A–4C). There was no significant difference observed between each group when using Mann–Whitney U-test for statistical analysis.
Figure 4.
(A–C) Brain swelling after middle cerebral artery occlusion (MCAO) and toll-like receptor (TLR) inhibition. The sections were digitized, the area of infarction (‘direct infarct volume') was quantified on a PC using Sigma Scan Pro Software (Jandel Scientific, San Rafael, CA, USA) and infarct volumes were calculated. ‘Indirect' infarct volume was calculated as the volume of the contralateral hemisphere minus the noninfarcted volume of the ipsilateral hemisphere and edema (brain swelling) was calculated as the difference between ‘direct' and ‘indirect' infarct volumes. (A) Brain swelling in TLR2-deficient and wild-type mice at 48 hours reperfusion after induction of MCAO. (B, C) Brain swelling in male C57Bl/6 wild-type mice at 48 hours of reperfusion after 45 minutes MCAO treated with 0.05 μg T2.5 anti-TLR2 antibody (T2.5), 0.05 μg isotype control (IC) antibody, and phosphate-buffered saline (PBS), respectively. Statistical analyses were performed using the Mann–Whitney U-test.
Discussion
Our data show a reduced postischemic neuronal death in vivo by TLR2 inhibition in a standard experimental stroke model. We applied TLR2-blocking antibody after MCAO to mimic a potential therapeutical use and because of speculated postischemic breakdown of the blood–brain barrier (Belayev et al, 1996), which should improve the ability of the antibodies to reach their target cells (most probably macrophages/monocytes and microglia), as shown in a rat MCAO model (Chekhonin et al, 2004). The relative large variability in the stroke volumes obtained in knockout mice compared with wild-type mice (Figure 1A) suits similar findings in other knockout mice (Harhausen et al, 2010; Trendelenburg et al, 2002; Ziegler et al, 2009).
Although there was only a tendency to reduced infarct volumes and brain swelling in mice treated with TLR2-blocking antibody, neuronal death as well as postischemic accumulation of inflammatory cells were significantly attenuated in T2.5-treated mice at 48 hours of reperfusion, which represents a time point at which infarct volumes were thought to be already consolidated and which was used for the analysis of TLR2-deficient mice in stroke (Ziegler et al, 2007). Our current findings are in good agreement with the recent data, which show a protection of TLR2-knockout mice against focal cerebral ischemia (Ziegler et al, 2007; Tang et al, 2007; Marsh et al, 2009). Moreover, the protective effect (as measured by the preservation of neurons as well as by the reduction of inflammatory cell accumulation in the ischemic hemisphere) observed by the application of the T2.5 antibody reaches a degree, which is comparable to that observed when TLR2-deficient mice were compared with wild-type mice (Figure 1).
However, it remains to be noted, that the isotype treatment itself has a detrimental effect compared with a pure PBS vehicle control, despite a significant benefit of the T2.5 treatment compared with control animals. Thus, the point of the right control (isotype versus vehicle) needs to be considered carefully. Whereas the application of the isotype was chosen in our study to detect TLR2-specific effects, the results of the experiments with PBS itself have demonstrated that an additional vehicle control is recommended in future studies using antibody treatment to control for unspecific or opposite effects of the antibodies.
Interestingly, a very recent study using systemic application of another TLR2-blocking antibody (OP301), which was applied 5 minutes before reperfusion in an animal model of myocard ischemia, revealed a similar protection against ischemic heart injury (Arslan et al, 2010). However, great therapeutical enthusiasm about stroke treatment with blocking antibodies seems to be premature with regard to the effects observed with the isotype control antibody, which show that isotype control antibody leads to an increased inflammatory response compared with sham-treated (PBS-treated) animals (Figures 3D–3G).
Unfortunately, recent clinical studies regarding antiinflammatory stroke therapies using blocking antibodies have been discouraging so far. For example, the Enlimomab (anti-intracellular adhesion molecule-1 (ICAM-1)) antibody Acute Stroke Trail was halted following enrollment of 625 patients, because of its impairing effect on patient outcome after treatment compared with placebo-treated patients (Furuya et al, 2001). Whether this lack of therapeutical benefit may be caused by adverse effects of the IgG by itself requires further examination. Moreover, it would be interesting to see, whether a double TLR inhibition by the use of anti-TLR2 and anti-TLR4 antibodies, as used recently in a sepsis model by the group of Spiller et al (2008) would even improve the observed neuroprotective effect of single TLR2 inhibition. It also needs to be clarified, whether systemic, and not local parenchymal TLR2 inhibition is responsible for the neuroprotective effect observed, as it was shown recently in myocardial ischemia/reperfusion injury (Arslan et al, 2010).
Taken together, our data confirm high potential of TLR inhibition in stroke therapy. As several pharmacological TLR inhibitors are currently under development (O'Neill et al, 2009; Marsh et al, 2009; McColl et al, 2009), further studies using other TLR inhibitors in experimental stroke models are eagerly awaited.
The authors declare no conflict of interest.
Footnotes
This work was supported by a grant of the German Research Society (DFG No TR742/1-1, 1-2), by the Center for Stroke Research (founded by the Federal Government for Education and Research), and the European Union (contract no QLG3-CT-2000-00934; ARISE and EUROSTROKE).
References
- Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B, Owens T. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;49:12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Cao C, Yang Q, Lv F, Jie C, Fu H, Wang J. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Comm. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and the worsening of experimental stroke. Stroke. 2008;39:1314–1320. doi: 10.1161/STROKEAHA.107.498212. [DOI] [PubMed] [Google Scholar]
- Chekhonin VP, Lebedev SV, Ryabukhin IA, Petrov SV, Gurina OI, Dmitrieva TB, Volkov AI, Kashparov IA, Skoblov YS. Selective accumulation of monoclonal antibodies against neurospecific enolase in brain tissue of rats with middle cerebral artery occlusion. Bull Exp Biol Med. 2004;138:343–347. doi: 10.1007/s10517-005-0037-4. [DOI] [PubMed] [Google Scholar]
- Cohen J.1988Statistical power analysis for the behavioral sciences2nd ed.Mahwah, NJ: Lawrence Erlbaum Associates [Google Scholar]
- Collins CE, Young NA, Flaherty DK, Airey DC, Kaas JH. A rapid and reliable method of counting neurons and other cells in brain tissue: a comparison of flow cytometry and manual counting methods. Front Neuroanat. 2010;4:5. doi: 10.3389/neuro.05.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Freyer D, Manz R, Ziegenhorn A, Weih M, Angstwurm K, Döcke WD, Meisel A, Schumann RR, Schönfelder G, Dirnagl U, Weber JR. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J Immunol. 1999;163:4308–4314. [PubMed] [Google Scholar]
- Furuya K, Takeda H, Azhar S, McCarron RM, Chen Y, Ruetzler CA, Wolcott KM, DeGraba TJ, Rothlein R, Hugli TE, del Zoppo GJ, Hallenbeck JM. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32:2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz M. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide sythease after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Harhausen D, Prinz V, Ziegler G, Gertz K, Endres M, Lehrach H, Gasque P, Botto M, Stahel PF, Dirnagl U, Nietfeld W, Trendelenburg G. CD93/AA4.1: a novel regulator of inflammation in murine focal cerebral ischemia. J Immunol. 2010;184:6407–6417. doi: 10.4049/jimmunol.0902342. [DOI] [PubMed] [Google Scholar]
- Hoffmann O, Braun JS, Becker D, Halle A, Freyer D, Dagand E, Lehnardt S, Weber JR. Toll-like receptor 2 mediates neuroinflammation and neuronal damage. J Immunol. 2007;178:6476–6481. doi: 10.4049/jimmunol.178.10.6476. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26:1055–1081. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling—a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Osaka M, Aikawa M, Uematsu S, Akira S, Libby P, Shimokado K, Sacks FM, Yoshida M. Toll-like 2 receptor mediates apolipoprotein CIII-induced monocyte activation. Circ Res. 2008;103:1402–1409. doi: 10.1161/CIRCRESAHA.108.178426. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti C L, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Phaneuf D, Soucy G, Weng YC, Kriz J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain. 2009;132:940–954. doi: 10.1093/brain/awn345. [DOI] [PubMed] [Google Scholar]
- Lin YC, Chang YM, Yu JM, Yen JH, Chang JG, Hu CJ. Toll-like receptor 4 gene C119A but not Asp299Gly polymorphism is associated with ischemic stroke among ethnic Chinese in Taiwan. Atherosclerosis. 2005;180:305–309. doi: 10.1016/j.atherosclerosis.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest. 2004;113:1473–1481. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Grabiec A, Rutz M, Metzger J, Luppa PB, Wagner H, Bauer S, Kirschning CJ. Murine TLR2 expression analysis and systemic antagonism by usage of specific monoclonal antibodies. Immunol Lett. 2005;98:200–207. doi: 10.1016/j.imlet.2004.11.015. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Fernández-López D, García-Yébenes I, Sobrado M, Hurtado O, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem. 2009;109:287–294. doi: 10.1111/j.1471-4159.2009.05972.x. [DOI] [PubMed] [Google Scholar]
- Spiller S, Elson G, Ferstl R, Dreher S, Mueller T, Freudenberg M, Daubeuf B, Wagner H, Kirschning CJ. TLR4-induced IFN-gamma production increases TLR2 sensitivity and drives gram-negative sepsis in mice. J Exp Med. 2008;205:1747–54. doi: 10.1084/jem.20071990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Stenzel-Poore MP. Toll-like receptors and tolerance to ischaemic injury in the brain. Biochem Soc Trans. 2006;34:1352–1355. doi: 10.1042/BST0341352. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gramnegative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Tang S, Arumugam T, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus X, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg G. Acute neurodegeneration and the inflammasome: central processor for danger signals and the inflammatory response. J Cereb Blood Flow Metab. 2008;28:867–881. doi: 10.1038/sj.jcbfm.9600609. [DOI] [PubMed] [Google Scholar]
- Trendelenburg G, Prass K, Priller J, Kapinya K, Polley A, Muselmann C, Ruscher K, Kannbley U, Schmitt AO, Castell S, Wiegand F, Meisel A, Rosenthal A, Dirnagl U. Serial analysis of gene expression identifies metallothionein-II as major neuroprotective gene in mouse focal cerebral ischemia. J Neurosci. 2002;22:5879–5888. doi: 10.1523/JNEUROSCI.22-14-05879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitenbroek DG.1997SISA-binomial(http://www.quantitativeskills.com/sisa/calculations/power.htm)
- Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, da Costa C, Rammensee HG, Wagner H, Schild H. The ER-resident heat shock protein Gp96 activates dendritic cells via the TLR2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- Yao H, Felfly H, Wang J, Zhou D, Haddad GG. DIDS protects against neuronal injury by blocking toll-like receptor 2 activated-mechanisms. J Neurochem. 2009;108:835–846. doi: 10.1111/j.1471-4159.2008.05838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang SH, Lasbury ME, Tschang D, Liao CP, Durant PJ, Lee CH. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect Immun. 2006;74:1857–1864. doi: 10.1128/IAI.74.3.1857-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Prinz V, Albrecht MW, Harhausen D, Khojasteh U, Nacken W, Endres M, Dirnagl U, Nietfeld W, Trendelenburg G. Mrp-8 and -14 mediate CNS injury in focal cerebral ischemia. Biochim Biophys Acta. 2009;1792:1198–1204. doi: 10.1016/j.bbadis.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ziegler G, Harhausen D, Schepers C, Hoffmann O, Röhr C, Prinz V, König J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]