Abstract
Tumor necrosis factor alpha (TNFα) and Fas receptor contribute to cell death and cognitive dysfunction after focal traumatic brain injury (TBI). We examined the role of TNFα/Fas in postinjury functional outcome independent of cell death in a novel closed head injury (CHI) model produced with weight drop and free rotational head movement in the anterior–posterior plane. The CHI produced no cerebral edema or blood–brain barrier damage at 24 to 48 hours, no detectable cell death, occasional axonal injury (24 hours), and no brain atrophy or hippocampal cell loss (day 60). Microglia and astrocytes were activated (48 to 72 hours). Tumor necrosis factor-α mRNA, Fas mRNA, and TNFα protein were increased in the brain at 3 to 6 hours after injury (P<0.001 versus sham injured). In wild-type (WT) mice, CHI produced hidden platform (P=0.009) and probe deficits (P=0.001) in the Morris water maze versus sham. Surprisingly, injured TNFα/Fas knockout (KO) mice performed worse in hidden platform trials (P=0.036) but better in probe trials than did WT mice (P=0.0001). Administration of recombinant TNFα to injured TNFα/Fas KO mice reduced probe trial performance to that of WT. Thus, TNFα/Fas influence cognitive deficits independent of cell death after CHI. Therapies targeting TNFα/Fas together may be inappropriate for patients with concussive TBI.
Keywords: cognition, concussion, inflammation, mice, traumatic brain injury, tumor necrosis factor
Introduction
Diffuse or concussive traumatic brain injury (TBI) caused by vehicular accidents, sports concussions, and explosive devices are associated with cognitive and psychiatric sequelae that may cause significant functional impairment through unknown mechanisms (Arciniegas et al, 1999). Inflammation has a significant role in secondary injury after human and experimental TBI, with beneficial and detrimental effects reported (Ziebell and Morganti-Kossmann, 2010). Tumor necrosis factor-α (TNFα) is a pleotropic cytokine, which acutely increases in the serum and cerebrospinal fluid of patients with head injury (Goodman et al, 1990; Ross et al, 1994). The Fas receptor is a 45-kDa transmembrane receptor protein in the TNF receptor (TNFR) superfamily that is also upregulated and involved in secondary damage after experimental and human TBI (Beer et al, 2000; Bermpohl et al, 2007; Grosjean et al, 2007). Both TNFα and Fas are increased in the brain after cerebral contusion (Qiu et al, 2002), and TNFα mRNA is increased in the brain after concussive TBI (Rooker et al, 2006). However, whether TNFα and Fas have similar roles in different subtypes of TBI (focal versus concussive) remains unknown.
Tumor necrosis factor-α signaling may be beneficial or detrimental after experimental TBI (Knoblach et al, 1999; Scherbel et al, 1999; Shohami et al, 1999). We have previously reported that genetic or genetic/pharmacological inhibition of TNFα and Fas together improves cognitive outcome after controlled cortical impact (CCI) in immature and adult mice, suggesting that TNFα and Fas together contribute to postinjury cognitive deficits after focal TBI (Bermpohl et al, 2007). In that study as well as in other studies, antagonism of TNFα and/or Fas was associated with improved motor and cognitive performance, as well as decreased acute cell death and brain tissue damage (Bermpohl et al, 2007; Knoblach et al, 1999; Scherbel et al, 1999; Shohami et al, 1999). As cell death is a prominent feature of TBI models in which TNFα and Fas have been studied, whether TNFα and Fas mitigate functional outcome after TBI independent of cell death is not known. To examine this question directly, we developed a closed head injury (CHI) model in mice that produces cognitive deficits in the absence of demonstrable acute cell death or chronic cell loss, and tested the hypothesis that TNFα and Fas are induced after CHI and contribute detrimentally to cognitive outcome.
Materials and methods
All experimental data were obtained by investigators blinded to study groups.
Animals
Adult male mice (a total of 280) were housed in 12-hour day–night cycles in a pathogen-free facility at the Massachusetts General Hospital in accordance with the NIH Guide for Care and Use of Laboratory Animals. Food and water was given ad libitum. Wild-type (WT) C57/BL6 mice (weighing 25 to 30 g, 2.5 to 4 months of age; Jackson Laboratories, Bar Harbor, ME, USA) were used, along with age-matched inbred TNFα knockout (TNFα KO) and TNFα/Fas double-KO mice (TNFα/Fas KO). The TNFα/Fas KO mice were generated on a C57/BL6 background from TNF−/− mice (stock no. 3008, Jackson Laboratories) and from Fas−/− mice (stock no. 3233, Jackson Laboratories) (Bermpohl et al, 2007). Mice were genotyped according to protocols published by Jackson Laboratories (Bermpohl et al, 2007).
Closed Head Injury
The trauma protocol was approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Mice were anesthetized with 4% isoflurane (Anaquest, Memphis, TN, USA) for 45 seconds in a 70% N2O–30% O2 Fluotec 3 vaporizer (Colonial Medical, Amherst, NH, USA). Mice were placed on a Kimwipe (Kimberly-Clark, Irving, TX, USA) grasped by the tail, and the head was positioned under a hollow tube (length 66 inches; diameter 10 mm). A metal bolt (53 g) was used to deliver the impact dorsally on the skull between the coronal and lambdoid sutures. On impact, the mouse head readily penetrated the thin Kimwipe and was free to rotate in the anterior–posterior plane. The control group consisted of age-matched sham-injured mice (anesthesia but no weight drop). Injured mice were recovered in room air in their cages. Loss of consciousness (LOC) times in seconds (s) was defined as latency to spontaneous ambulation after induction of anesthesia and/or CHI.
Physiologic Variables
To assess the physiologic changes as a result of the injury, a subset of WT animals (n=5) were anesthetized and the femoral artery cannulated with P10 tubing (intramedic polyethylene tubing P10, Becton-Dickenson, Franklin Lakes, NJ, USA). Arterial blood gases were drawn at baseline before CHI and then at 2, 4, and 10 minutes after injury. Blood pressure was monitored continuously for the duration of the experimental period (Power Lab, AD Instruments, Colorado Springs, MO, USA).
Assessment of Brain Edema
Brain edema was assessed by measuring brain water content using the (wet–dry)/wet brain weight method. Brains were removed at 24 or 48 hours after CHI (n=6 per time point) and bisected into left and right hemispheres. Each hemisphere was weighed (wet weight), then dried at 99°C for 72 hours, and dry weights were obtained. The percentage of brain water content was expressed as (wet–dry weight)/wet weight × 100%.
Assessment of the Blood–Brain Barrier Permeability
Evans blue (5 mL/kg of a 2% solution) was injected intravenously immediately before CHI. Mice (n=6 per group) were killed 24 hours after injury by transcardial perfusion with phosphate-buffered saline (PBS). Their brains were removed, bisected into left and right hemispheres, and immersed in 3 mL of N,N-dimethylformamide for 72 hours at room temperature. Evans blue in N,N- dimethylformamide was analyzed by spectrophotometry (612 nm). The amount of Evans blue (μg) per gram brain tissue was calculated from a standard curve and reported for each hemisphere.
Histochemical Detection of Acute Cellular Injury and Degeneration
For histologic studies, mice were anesthetized with 4% isoflurane and killed at 6, 24, 48, or 72 hours after CHI (n=4 to 6 per time point) or sham injury (n=4 to 5 per time point). The brain was removed intact, immediately frozen in nitrogen vapor, and stored at −80°C. Cryostat coronal brain sections (12 mm) cut at 200 to 250 μm intervals from the corpus callosum to the end of the hippocampus and from the anterior to posterior brainstem were mounted on poly--lysine-coated slides. Some frozen sections were stained with hematoxylin and eosin, mounted with Permount, and photographed using a Nikon Eclipse Ti-s fluorescence microscope (Tokyo, Japan) and NIS Elements BR 3.0 imaging software (Tokyo, Japan). Other sections were fixed in 100% ethanol at room temperature for 10 minutes and then labeled with 0.001% Fluoro Jade B (Chemicon, Billerica, MA, USA) or TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labeling) (Roche Diagnostics, Indianapolis, IN, USA) as per the manufacturer's instructions. Labeling was detected using excitation and emission filters of 490 and 520 nm, respectively. For propidium iodide (PI) labeling of cells with altered membrane permeability, mice were subjected to CHI or sham injury, and PI (Sigma, St Louis, MO, USA) was administered by intracerebroventricular injection (0.5 ng/mL in PBS, 4 μL) 1 hour before the sacrifice. Propidium iodide labeling in frozen brain sections was detected using excitation and emission wavelengths of 568 and 585 nm, respectively. For a positive control, cells injured by CCI were analyzed from the injured cortex (Bermpohl et al, 2007).
Assessment of Injured Cell Counts
For acute cellular injury and death studies, brain regions assessed for cell counts were all × 200 microscopic fields (1,100 × 1,100 μm2) in the cortex (n=12 fields per section, 6 sections per mouse, 5 mice per time point=360 fields per time point), the hippocampus (24 fields (in 5 sections) per mouse, 5 mice per time point=120 fields), the corpus callosum (28 fields (in 4 sections) per mouse, 5 mice per time point=140 fields), the thalamus, and the striatum (4 fields per section, 5 sections per mouse, 5 mice per time point=100 fields). Cell count data for each mouse were the average of the total brain fields counted.
Assessment of Amyloid Precursor Protein, Neutrophils, Microglia, and Astrocytes
For immunohistochemical detection of the amyloid precursor protein (APP), neutrophils, and astrocytes, frozen sections were air dried and fixed in 100% ethanol, washed in PBS (pH 7.4), and blocked in 3% normal goat serum/PBS for 30 minutes. Sections were incubated overnight at 4°C with rabbit anti-APP (1:300, Sigma) for APP, phycoerythrin-conjugated anti-mouse CD11b (1:200; BD-Biosciences, Franklin Lakes, NJ, USA) for neutrophils, or with Cy3-conjugated monoclonal anti-glial fibrillary acidic protein antibody (1:2,000; Sigma) for astrocytes. The next day, slides were analyzed using a Nikon Eclipse Ti-S fluorescence microscope (Micro Video Instruments, Avon, MA, USA) with 568/585 nm filters. For immunohistochemical detection of microglia, mice were transcardially perfused with 4% paraformaldehyde at 24 to 72 hours. The brain was postfixed for 24 hours in 4% paraformaldehyde and cryoprotected in 30% sucrose for 24 hours. Coronal sections were cut (16 to 20 μm) and mounted on poly--lysine-coated slides. Sections were washed in PBS, blocked in 3% normal goat serum in PBS for 1 hour, and incubated overnight at 4°C with rabbit anti-Iba1 antibody (1:200; Wako Pure Chemical Industries Ltd, Osaka, Japan). Slides were washed in PBS and incubated with the appropriate Cy3-conjugated secondary antibody (1:300; Jackson ImmunoResearch, West Grove, PA, USA) for 60 minutes, washed in PBS, and cover-slipped for analysis using excitation/emission spectra of 568/585 nm. Neutrophils were assessed in the hippocampus and in the cistern spaces around the hippocampus. Microglia, astrocytes, and APP-positive cells/axons were assessed in the cortex, hippocampus, thalamus and striatum, and corpus callosum. Microglia and astrocytes were assessed qualitatively. Amyloid precursor protein-positive axons were semi-quantitated as the number of positively stained brain sections per mouse per group in the cortex.
Assessment of Chronic Brain Tissue Loss
Mice were killed 60 days after CHI or sham injury (n=4 per group). Their brains were removed, frozen in nitrogen vapor, and brain sections were cut on a cryostat every 500 μm. Brain sections were stained with hematoxylin and photographed using a Nikon Ti-S microscope. Area measurements were performed using NIS Elements image analysis software. Volumetric analyses were performed by summing the areas of all brain sections counted and multiplying by 0.5. Volumetric data were expressed in mm3.
Assessment of Hippocampal Cell Counts
Hematoxylin-stained tissue sections were analyzed by light microscopy. Cell counts were obtained in each hemisphere from two brain sections (one from the anterior and one from the mid-hippocampus). For each hippocampal subregion (CA1, CA3, and dentate gyrus), all hematoxylin-stained cells were counted in a single × 200 field for a total of four × 200 fields for each hippocampal subregion per mouse. Cell counts were expressed as the average number of cells/200 × fields.
Wire-Grip Test
Vestibular-motor function was assessed between 1 and 7 days after injury using a wire-grip test (Bermpohl et al, 2007). In brief, mice were placed on a metal wire (45 cm) suspended between 2 upright poles 45 cm above a table. The animals were scored based on the manner in which they held onto the wire during a 60-second period. The wire-grip test was performed in triplicate and an averaged value calculated for each mouse on each day of testing.
Morris Water Maze
Two Morris water maze (MWM) paradigms were used to evaluate spatial learning and memory. In each, the apparatus consisted of a white pool 83 cm in diameter and 60 cm deep, filled with water to a depth of 29 cm. Highly visible cues were positioned on the walls of the tank and around the room. Water temperature was maintained at 25°C. A clear plexiglass goal platform 10 cm in diameter was positioned 0.5 cm below the surface of the water ∼15 cm from the southwest wall. In paradigm I, MWM testing was started 24 hours after injury. Each mouse was subjected to a series of two to three trials per day such that the testing paradigm was completed 4 days after injury. For each trial, mice were randomized to one of four starting locations (north, south, east, and west) and placed in the pool facing the wall. The maximum time allotted to find the submerged platform was 60 seconds. If the mouse failed to reach the platform within this time, it was placed on the platform by the experimenter and allowed to remain there for 10 seconds. To control for possible differences in visual acuity or sensorimotor function between groups, two sets of trials were performed using a visible platform (raised 0.5 cm above the water and marked with red tape). In paradigm II (Yager et al, 2008), TNFα/Fas KO and WT animals were subjected to a series of six visible platform trials, for which the platform was randomly changed for each trial. In this paradigm, only one starting location was used per trial. The maximum latency to the visible platform was 30 seconds. Paradigm II was started 24 hours after CHI and the six trials were completed on the same day. In both paradigms, performance in the MWM was quantified by latency (s) to the platform. For probe trials, mice were placed in the tank opposite the target quadrant and the time spent in the target quadrant was quantified (60 seconds maximum).
Administration of Recombinant Tumor Necrosis Factor-α
For TNFα reconstitution experiments, TNFα/Fas KO mice were anesthetized and administered 20 pg of recombinant mouse TNFα (R&D Systems, Minneapolis, MN, USA) or an equal volume of PBS (10 μL) to each cerebral ventricle immediately before CHI.
Quantitative Real-Time Reverse Transcriptase PCR
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA, USA) from the cortex and the hippocampus of CHI and sham-injured mice (n=8 per group at 3, 6, or 24 hours after injury). Complementary DNA was synthesized using the SuperScripts III First-Strand Synthesis System for reverse transcriptase-PCR (Invitrogen). Samples in triplicates and negative controls were run on an ABI Prism 7500 (Applied Biosystems, Foster City, CA, USA). Primer sequences for TNFα, Fas, TNFR1, and TNFR2 (Applied Biosystems catalog numbers Mm00443258-m1 (TNF), Mm00433237-m1 (Fas), Mm00441875-m1 (TNFR1), Mm00441889-m1 (TNFR2)) and Taqman FAM-labeled probes were mixed with AmpliTaq Fast Universal PCR Master Mix (Applied Biosystems). Gene products were normalized to endogenous 18S rRNA (HS-999999S1, Applied Biosystems).
Tumor Necrosis Factor-α Enzyme-Linked Immunosorbent Assay
Injured mice were killed at 6 or 24 hours (n=8 per time point) after CHI. Sham-injured mice were killed at 6 hours after anesthesia. The brains were removed, rapidly dissected on ice, and the entire cortex and hippocampus were isolated. The brain tissue was homogenized on ice in 500 μL of RIPA (radio immunoprecipitation assay) buffer (Boston BioProducts, Worcester, MA, USA). One tablet of protease inhibitor (Roche Diagnostics) was added to every 10 mL of RIPA buffer. Homogenates were centrifuged at 12,000 g × 15 minutes at 4°C, and supernatants were stored at −80°C. Samples were assayed for total protein using the Dc assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Protein samples (1 mg/mL) were plated in duplicate onto 96-well plates, and enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer's instructions (Quantikine, R&D Systems). Mouse recombinant TNFα provided in the ELISA kit was used as a positive control. Tumor necrosis factor-α was reported as picogram TNFα per milligram brain tissue.
Nuclear Factor-κB Transcription Factor Assay
A Nuclear Extraction Kit (Chemicon) was used to obtain nuclear extracts from brain tissue lysates (n=5 per time point). The Universal EZ-TFA Transcription Factor Colorimetric Assay (Millipore, Billerica, MA, USA) was used to detect nuclear factor (NF)κB p65 transcription factor DNA-binding activity in nuclear extracts according to the manufacturer's instructions. This assay combines the principle of the electrophoretic mobility shift assay with the 96-well-based ELISA. The capture probe is a double-stranded biotinylated oligonucleotide containing the consensus-binding sequence for NFκB. When mixed with the nuclear extract, NFκB binds to its consensus DNA sequence and is immobilized on the streptavidin-coated plate. The bound transcription factor is detected with a specific rabbit anti-mouse p65 primary antibody and a horseradish peroxidase-conjugated secondary antibody. Tumor necrosis factor-α-treated HeLa whole-cell extracts served as a positive control, the NFκB-specific competitor oligonucleotide served as a specificity control for DNA binding, and a transcription factor assay negative control was also included in the ELISA.
Statistical Analyses
All data are mean±s.e.m. Data were analyzed using Graphpad PRISM V (La Jolla, CA, USA). Wire-grip and MWM results were analyzed using two-factor repeated measures analysis of variance (ANOVA) (group × time). Loss of consciousness data, and volumetric and hippocampal cell count data were analyzed by t-test. Both mRNA and ELISA data and acute cell death data were analyzed by ANOVA and Dunnett's test (with sham injured as the control reference group). Probe trial data were analyzed by rank-sum test. Axon injury data were analyzed qualitatively. For all comparisons, P<0.05 was considered significant.
Results
After CHI, most of the mice had tonic-clonic seizure activity lasting ∼15 to 45 seconds, brief periods of gasping and shallow respirations before resuming a normal respiratory pattern during recovery in room air. The overall mortality for mice that were allowed to survive at least 1 week for behavioral testing was 20.1%, mainly occurring during the first few minutes of recovery from CHI. In acute experiments, mortality ranged from 10% to 20%. None of the mice exhibited skull fractures, cerebral contusions, or parenchymal brain hemorrhage; however, a small amount of intraventricular hemorrhage was observed in most of the mice examined. Loss of consciousness was significantly higher in CHI mice (420±20 seconds) than in sham-injured mice (36±1.8 seconds, anesthesia effect only; P<0.0001). Loss of consciousness in mice used for behavioral experiments (424±28 seconds) did not differ from LOC in histology experiments (384±32 seconds, P=0.26). Table 1 shows physiologic variables before and after CHI. Mean arterial blood pressure increased immediately after CHI (94±4.2 mm Hg) and returned to baseline (84.2±1.9 mm Hg; P=0.014) by 2 minutes after injury. Closed head injury also caused a transient decrease in PaO2 (P<0.0001); however, no mouse was hypoxic. The modest increase in PaCO2 that returned to baseline within 4 minutes of injury.
Table 1. Physiologic variables before and after closed head injury (CHI) in wild-type mice.
| Physiologic variable | Baseline (before CHI) | Two minutes after CHI | Four minutes after CHI | Ten minutes after CHI |
|---|---|---|---|---|
| pO2 (Torr) | 113±2 | 68±4* | 104±4 | 105±3 |
| pCO2 (Torr) | 25±2 | 37±1* | 33±2* | 37±1 |
| pH | 7.50±0.03 | 7.30±0.01 | 7.40±0.02 | 7.40±0.02 |
| MABP (mm Hg) | 84±2 | 82±1 | 84±3 | 85±4 |
Torr, a pressure of 1 Torr is approximately equal to 1 mm Hg; MABP, mean arterial blood pressure.
*P<0.001 versus baseline; n=5 per group.
Brain water content did not differ between sham (right hemisphere 77.8%±0.1%, left hemisphere 77.6%±0.2%) and CHI groups at 24 (right hemisphere 77.3%±0.2%, left hemisphere 77.2%±0.2%) or 48 hours (right hemisphere 77.3%±0.3%, left hemisphere 77.8%±0.3%). Blood–brain barrier leakage of Evans blue at 24 hours did not differ between sham-injured (right hemisphere 0.68±0.15 μg/g, left hemisphere 0.63±0.11 μg/g brain) and CHI (right hemisphere, 0.61±0.09 μg/g, left hemisphere 0.73±0.13 μg/g brain) groups.
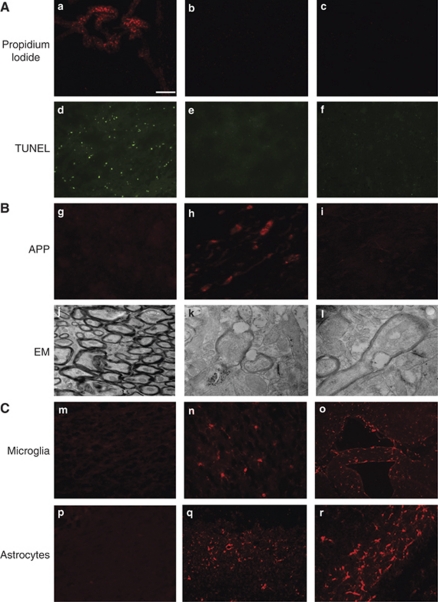
Figure 1A shows representative results of acute cell death analyses. No PI- or TUNEL-positive cells were detected in the cortex, CA1, CA3, dentate gyrus, or in the striatum/thalamus. In addition, no degenerative cells were identified by Fluoro Jade B or hematoxylin and eosin staining in any of the aforementioned regions (data not shown). Amyloid precursor protein histochemistry (Figure 1B, top panel) showed no examples of axonal injury in sham-injured (n=6) or CHI mice at 6 hours (n=4). Occasional axonal injury was noted in the cortex and in the periventricular white matter of injured mice at 24 hours (3 of 10 mice; 1, 4, and 6 brain sections per mouse with positive staining, respectively). No APP reactivity was observed in the cortex, hippocampus, corpus callosum, and thalamus in any injured mice at 48 and 72 hours (n=4 to 6 per time point). Axonal injury was detected in brainstem regions at 24 hours in 2 of 6 animals (3 to 4 sections per mouse with positive staining). No APP immunostaining was detected in brainstem regions between 48 and 72 hours (n=4 per group, data not shown). Electron microscopic analyses at 24 hours in 2 CHI mice confirmed the presence of occasional axon pathology (retraction bulbs, myelin damage, neurofilament, and microtubule disorganization) in cortical brain regions (Figure 1B, bottom panels).
Figure 1.
Histopathology of closed head injury (CHI). (A) Analysis of cellular injury and death after closed head injury (CHI). After CHI, no propidium iodide (PI)-positive cells were observed in the injured cortex (b) and the hippocampus (c), whereas PI was easily identified in choroid plexus cells, indicating adequate intracerebroventricular delivery of PI (a). Similarly, no TUNEL-positive cells were detected in the cortex (e) or the hippocampus (f), but were readily detected in the brain injured by CCI used as a positive staining control (d). Photomicrographs are representative pictures from CCI mice obtained at 6 hours and from CHI mice obtained at 48 hours after injury. Scale bar represents 100 μm in panels a to f. (B) Detection of axonal injury using anti-amyloid precursor protein (APP) immunohistochemistry and electron microscopy. Axonal damage was occasionally detected by APP staining in some injured mice at 24 hours (cortical region shown in panel h) but not in sham-injured (panel g, cortex) and most injured mice (panel i, the cortex of a representative injured mouse lacking APP staining). Scale bar represents 50 μm in panels g to i. Electron microscopic (EM) analysis of the corpus callosum of sham-injured mice did not reveal axon abnormalities (panel j), whereas analysis of cortical regions of CHI mice showed axolemmal blebbing (panels k and l) and axon bulb formation with disorganized microtubules (panel l). Magnification × 4,800 (panel j), × 13,000 (panel k), × 18,500 (panel l). (C) Detection of activated microglia and astrocytes after CHI. Sham-injured mice showed occasional resting microglia (panel m) and astrocytes (panel p). After CHI, mice had robust Iba1 (panels n and o) and GFAP staining (panels q and r) for microglia and astrocytes, respectively. Representative staining in the cortex (panels n and q) and white matter tracts (panels o and r) is shown. Scale bar represents 50 μm in panels n and r and 100 μm in panels m, o, p, and q. CCI, controlled cortical impact; GFAP, glial fibrillary acidic protein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Figure 1C shows results of analyses of cellular inflammation after CHI. Activated microglia were detected by Iba1 staining in the injured cortex, hippocampus, corpus callosum, periventricular white matter, and choroid plexus at 48 and 72 hours after injury. Reactive astrocytes were also observed at 48 to 72 hours after CHI mainly in the cortex, hippocampus, and corpus callosum. Neutrophils were detected in the cerebrospinal fluid spaces and in the choroid plexus of 3 of 9 animals at 24 hours and in 0 of 5 and 0 of 2 mice at 48 and 72 hours, respectively (data not shown). Little parenchymal neutrophil infiltration was observed at any of the time points examined (not shown). No evidence of microglial and astrocyte activation or neutrophil infiltration (not shown) was observed in the brains of sham-injured mice.
Volumetric analyses at 60 days after CHI showed no change in hemispheric volumes (injured left hemisphere 126.7+3.6 mm3; injured right hemisphere 124.1+3.2 mm3; sham-injured left hemisphere 122.7+4.1 mm3; sham-injured right hemisphere 126.0+3.0 mm3; P=0.49 (left injured versus left sham), P=0.69 (right injured versus right sham)). Hippocampal cell loss also did not differ between groups (injured CA1 725+32 cells/200 × field, sham CA1 623+38 cells/200 × field, P=0.08; injured CA3 845+35 cells/200 × field, sham CA3 816+19 cells/200 × field, P=0.49; injured dentate gyrus 1,206+56 cells/200 × field, sham dentate gyrus 1,318+62 cells/200 × field, P=0.23).
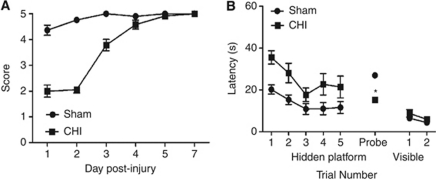
Figure 2A shows the results of wire-grip testing in CHI and sham-injured mice. Motor deficits induced by CHI resolved within 4 days (P<0.0001, group effect versus sham). Figure 2B shows the effect of CHI on MWM performance. The CHI induced significant increase in latency to the hidden platform (P=0.009, group effect) and decreased probe trial scores to chance levels (P<0.0001 versus sham). No difference between groups was observed in visible platform trials.
Figure 2.
Motor and Morris water maze performance in C57/Bl6 (wild type, WT) animals after closed head injury (CHI). (A) Compared with sham-injured mice, CHI mice had significant motor deficits (P<0.0001, group effect; n=12 per group), which returned to baseline within 4 days post-injury. (B) Compared with sham-injured mice, CHI mice had considerably worse hidden platform trial latencies (P=0.009, group effect; n=12 per group) and spent significantly less time in the target quadrant during the probe trial (*P<0.0001). No difference between groups was detected in visible platform trials.
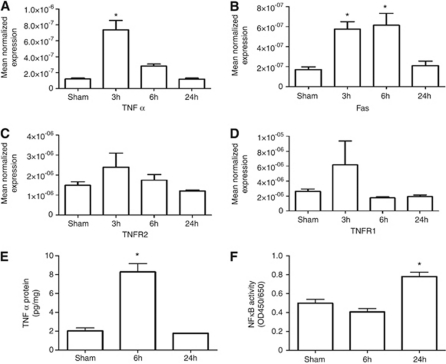
Figure 3 shows the results of quantitative reverse transcriptase-PCR analyses of TNFR-related molecules in the cortex and the hippocampus after CHI. Compared with sham injury, CHI induced a rapid increase in TNFα mRNA (P<0.0001 ANOVA) that peaked at 3 hours (six-fold increase versus sham, P<0.05) and returned to baseline by 24 hours. Enzyme-linked immunosorbent assay confirmed an increase in TNFα protein expression (P<0.001 ANOVA) that reached fourfold at 6 hours after CHI (8.2±0.9 pg/mg versus 2.0±0.3 pg/mg; P<0.05 versus sham). Both TNFα mRNA and protein levels returned to baseline at 24 hours after injury (TNFα protein, 1.80±0.03 pg/mg; P=NS versus sham injured, Dunnett's test). Fas mRNA expression also increased after CHI (P<0.0002 ANOVA). Fas mRNA was increased 3.5-fold versus sham at 3 and 6 hours (P<0.05 Dunnett's test for both time points) after CHI and returned to baseline by 24 hours. In contrast, no statistically significant change was observed in TNFR1 and TNFR2 mRNA compared with sham-injured mice. Assessment of NFκB activation showed that compared with sham-injured mice (0.4±0.03), CHI mice had increased NFκB p65 DNA-binding activity in the injured cortex/hippocampus at 24 hours (0.78±0.04; P<0.0001 ANOVA; P<0.05 for 24 hours, Dunnett's test; n=5 per group).
Figure 3.
TNFα/Fas signaling profiles and nuclear factor kappa B (NFκB)-binding activity after closed head injury (CHI). (A) TNFα and (B) Fas mRNA levels were significantly increased at 3 hours after CHI (*P<0.05 versus sham-injured; n=8 per group). The increase in mRNA levels of TNFR2 (C; P=0.095) and TNFR1 (D; P=0.059) were not statistically different from sham-injured mice. (E) TNFα protein level assessed by ELISA was significantly increased at 6 hours after CHI (*P<0.05 versus sham; n=8 per group). (F) A significant increase in NFκB-binding activity was detected at 24 hours after CHI (*P<0.05 versus sham; n=5 per group). ELISA, enzyme-linked immunosorbent assay; TNFα, tumor necrosis factor-α.
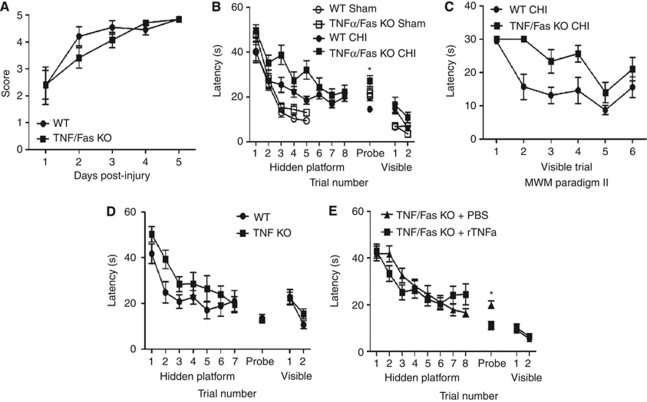
Given the involvement of TNFR and downstream signaling by NFκB, we tested the hypothesis that TNFα and Fas mediate functional deficits after CHI, using mice deficient in TNFα and the Fas receptor (TNFα/Fas KO). Naive (uninjured) TNFα/Fas KO mice do not differ with regard to WT in wire-grip test performance (Bermpohl et al, 2007). After CHI, TNFα/Fas KO and WT mice recovered their motor deficits similarly (Figure 4A). In MWM testing, initiated 24 hours after CHI or sham injury, both sham-injured TNFα/Fas KO and WT mice performed similarly in MWM testing (Figure 4B). Injured TNFα/Fas KO and WT animals showed significant improvement in hidden platform escape latency over time (P<0.0001 time effect, Figure 4B). Compared with injured WT, injured TNFα/Fas KO mice performed worse in hidden platform trials (P=0.036, group effect, Figure 4B) but significantly better (nearly two-fold improvement) in probe trials (P=0.0001, Figure 4B), with no differences between groups in visible platform trials (P>0.05, Figure 4B). In MWM paradigm II (visible platform trials only), sham-injured TNFα/Fas KO mice performed similarly to WT (data not shown), but injured TNFα/Fas KO mice performed worse than WT in visible platform trials (P=0.0015, group effect, Figure 4C), indicating that part of the observed learning deficits induced by CHI are procedural (nonhippocampal dependent, nonspatial deficits). Mice deficient in TNFα alone do not differ from WT in MWM performance at baseline (Bermpohl et al, 2007) and did not differ in hidden platform (P=0.09, group effect; n=12 per group) or in probe trial (P=0.73) performance after CHI (Figure 4D). Loss of consciousness did not differ between mice administered PBS and recombinant TNFα (1,092±144 seconds and 972±128 seconds, respectively, P=0.54). Finally, compared with PBS, administration of recombinant TNFα just before CHI in TNFα/Fas KO mice, significantly worsened probe trial latencies without affecting hidden platform performance (P=0.0025; n=13 per group Figure 4E). There was no difference between groups in hidden platform (P=0.83, group effect) or in visible platform (P=0.93, group effect) performance.
Figure 4.
Motor and Morris water maze (MWM) deficits (assessed at days 1 to 4 after CHI) in TNFα/Fas KO and WT mice after closed head injury (CHI). (A) After CHI, TNFα/Fas KO and WT animals had transient motor deficits versus sham-injured WT mice (P<0.01 compared with sham-injured mice in Figure 4). TNFα/Fas KO and WT mice did not differ in postinjury wire-grip performance after CHI (P=0.36; n=15 per group). (B) Sham-injured and injured TNFα/Fas KO and WT mice improved their MWM performance in hidden platform trials over time (P<0.0001 time effect for both groups). Sham-injured WT and TNFα/Fas KO animals (n=12 per group) did not differ in hidden platform, visible platform, or probe trial performance. Injured TNFα/Fas KO mice had worse hidden platform trial latencies compared with injured WT (P=0.036, group effect; n=15 per group); however, injured TNFα/Fas KO mice performed significantly better than injured WT in probe trials (*P=0.0001). (C) Injured TNFα/Fas KO mice preformed significantly worse than WT in visible platform trials in MWM paradigm II, suggesting a nonspatial, procedural deficit (P=0.0015, group effect; n=7 per group). (D) Compared with injured WT, injured TNFα KO mice had similar performance in hidden platform trials (P=0.09, group effect; n=12 per group) and probe trials (P=0.73). (E) TNFα/Fas KO mice were injected with recombinant TNFα or PBS and then subjected to CHI. TNF/Fas KO mice injected with TNFα had worse probe trial performance compared with TNFα/Fas KO mice administered PBS (*P=0.0025; n=13 per group). There was no difference between groups in hidden platform or visible platform trials (P>0.60 for hidden and visible platform trials). CHI, closed head injury; KO, knockout; PBS, phosphate-buffered saline; TNFα, tumor necrosis factor-α; WT, wild type.
Discussion
To our knowledge, this is the first report of a functional role for TNFα and Fas in a concussive TBI model. The CHI was produced by allowing the head to move freely after impact and yielded inertial injury but not contusion that may account for the remarkable lack of cell death. The CHI produced significant LOC and cognitive deficits in the absence of structural brain damage, blood–brain barrier damage, or edema, and was associated with early induction of TNFα and Fas mRNA and/or protein, NFκB-binding activity, and activation of microglia and astrocytes. Remarkably, acute or chronic cell death was not identified, and only occasional examples of axonal injury were detected after CHI. In CHI mice, genetic antagonism of TNFα and Fas together produced a dichotomous cognitive phenotype, with worse performance in hidden platform trials and improved probe trial performance in the MWM.
Taken together, the data suggest that TNFα/Fas signaling might influence postinjury cognitive function after concussive TBI fundamentally differently than in focal contusion models (Bermpohl et al, 2007). Alternatively, the timing of TNF/Fas inhibition may have an important role in the observed effects on cognitive function in this study. Studies in experimental TBI using three different models (namely fluid percussion, CCI, and a different CHI model) all conclude that early TNFα (within 4 to 6 hours) is detrimental (Knoblach et al, 1999; Scherbel et al, 1999; Shohami et al, 1999), whereas TNF activity at days to weeks is essential for functional recovery (Scherbel et al, 1999). In addition, administration of TNF antagonists before or up to 1 hour after injury, but not later, improves performance, suggesting that the timing of TNF antagonism could account for the observed effects on postinjury functional outcome. The compelling evidence from these studies suggests that enhanced early neuronal expression of TNFα after TBI may contribute to subsequent neurologic dysfunction independent of the mode of injury, and that TNF is a ‘double-edge sword' with early detrimental effects but later beneficial effects on functional recovery and regeneration (Knoblach et al, 1999; Scherbel et al, 1999; Oshima et al, 2009; Shohami et al, 1999).
We have previously reported that dual antagonism of TNFα/Fas resulted in improved hidden platform trial performance in adult and immature mice subjected to CCI (Bermpohl et al, 2007); however, probe trials were not assessed in that study. In the CCI model, TNFα/Fas gene KO was also associated with decreased numbers of acutely injured cells in the cortex and the hippocampus, and with decreased brain tissue loss during the chronic period after injury (Bermpohl et al, 2007). Thus, the beneficial effects of TNFα/Fas KO on postinjury cognitive function after CCI may have been attributed, at least in part, to inhibition of cell death in neuronal populations critical for learning and memory function.
In this study, using a model devoid of detectable cell death or loss, TNFα/Fas KO mice had worse hidden platform trial latencies but improved probe trial latencies after CHI. Mice deficient in TNFα alone were indistinguishable from WT, suggesting that TNFα and Fas signal redundantly as in CCI (Bermpohl et al, 2007), but that TNFα/Fas influence post-injury cognitive function (assessed by hidden and visible platform trials) differently in CHI compared with CCI. Reconstitution of TNFα in TNFα/Fas KO mice (by intracerebroventricular administration of recombinant mouse TNFα before injury) did not improve hidden platform performance but reversed the beneficial effects of TNFα/Fas KO on probe trial performance, offering further evidence for the specific involvement of TNFα and Fas together in the pathogenesis of cognitive deficits after concussive TBI. Previous studies in cerebral contusion models have shown a protective role for TNFα in the recovery of motor function and in postinjury axonal sprouting (Oshima et al, 2009; Scherbel et al, 1999; Shohami et al, 1999). To our knowledge, this study is the first to extend a protective effect of TNFα on the recovery of cognitive function in a concussive TBI model.
The reasons for a dichotomous role for TNFα/Fas in CHI (beneficial for hidden platform acquisition but detrimental for probe trials) are unknown. Hidden platform trials involve both spatial and nonspatial (i.e., procedural) learning mechanisms, visible platform trials involve nonspatial mechanisms, and probe trials assess (hippocampal-dependent) spatial memory (Gerlai, 2001). Why exogenous TNFα influenced probe trial scores but not hidden platform latency in injured TNF/Fas KO mice may be related to preferential binding of soluble TNFα to TNFR1 versus TNFR2 (McCoy and Tansey, 2008). We recently reported a key role for TNFR2/Fas but not for TNFR1/Fas in hidden platform performance after CCI, suggesting a key role for TNFR2 in this regard (Yang et al, 2010). As membrane-bound TNFα is required to activate TNFR2 (McCoy and Tansey, 2008), data from this study are consistent with a role for TNFR2 in hidden platform trials and for TNFR1 in probe trials after CHI. If true, hidden platform deficits in TNFα/Fas KO mice may not be amenable to manipulation by exogenous TNFα.
The lack of structural brain damage and overt cell death suggests that CHI models human concussive TBI (Shaw, 2002). The presence of significant LOC, tonic-clonic seizures, robust hidden platform, and probe trial deficits in the MWM, and up to 20% mortality in some experiments indicates a moderate level of concussive injury (Marmarou et al, 1994). In contrast, mild concussive TBI models in rats (Farkas et al, 2006; Lyeth et al, 1990; Singleton and Povlishock, 2004) and in mice (Stahel et al, 2000; Tang et al, 1997a, 1997b; Zohar et al, 2003) do not produce seizures or mortality. However, even the most mild concussive TBI models have delayed cell death in vulnerable brain regions such as the hippocampus, with few exceptions (Hicks et al, 1993; Kilbourne et al, 2009; Lyeth et al, 1990; Rooker et al, 2006; Tang et al, 1997b; Zohar et al, 2003) and most have evidence for axonal injury as well. Our CHI model is unique as it produces concussive TBI that lacks significant histopathological and biochemical indices of cellular or axonal injury and death, edema, blood–brain barrier damage, and gross changes in brain parenchyma. We are not aware of another concussive mouse TBI model that produces significant motor and cognitive deficits in the collective absence of these histopathological findings.
We assessed MWM deficits beginning 24 hours after injury and completed by day 4 post-injury to mimic human concussive TBI in which cognitive deficits occur immediately after injury and generally resolve within weeks. The exact temporal course of cognitive deficits in our CHI model requires further study; our unpublished data show no hidden platform but persistent probe deficits at 45 days after CHI. It is also likely that motor deficits do not significantly contribute to MWM deficits in CHI mice, because motor and cognitive deficits do not correlate well in experimental TBI models, and visible platform latencies (which control for differences in postinjury motor function) were not different between sham-injured and CHI animals.
We used in vivo PI labeling to identify injured cells with abnormal plasmalemma permeability that are detected for several days after focal TBI (Whalen et al, 2008). Positive labeling of choroid plexus cells after intracerebroventricular administration confirmed adequate brain delivery of PI to detect plasmalemma damage in this study. The absence of PI labeling at 6 to 72 hours after CHI differs from other reports of transient membrane permeability which occurs with or without degenerative changes in neurons injured by mild fluid percussion TBI (Farkas et al, 2006; Singleton and Povlishock, 2004). Data from this study suggest that plasmalemma permeability is not a feature of cellular injury in our CHI model.
The absence of degenerative changes detectable by Fluoro-Jade B, TUNEL, and hematoxylin and eosin staining, and the lack of chronic hippocampal cell loss, as well as hemispheric and ventricular volume loss strongly suggest that CHI does not induce significant cell death. The lack of histopathology in our CHI model suggests that the associated motor and cognitive deficits may be caused by axonal injury and/or to synaptic dysfunction rather than by the loss of critical neuronal subpopulations. Synaptic dysfunction (loss of long-term potentiation and enhanced long-term depression) occurs after experimental TBI (Albensi, 2001; Albensi et al, 2000; Biegon et al, 2004). Our findings, suggesting a role for TNFα/Fas in posttraumatic synaptic dysfunction, are consistent with known roles for TNFα and Fas in normal synaptic function (Albensi and Mattson, 2000; Corsini et al, 2009; Tancredi et al, 1992).
In brain contusion models, TNFα, Fas mRNA, and/or protein are induced in the injured brain, and inhibition of TNFα and/or Fas is beneficial to postinjury cell death and functional outcome (Bermpohl et al, 2007; Qiu et al, 2002; Scherbel et al, 1999; Shohami et al, 1996). In contrast, few studies have examined TNFα or Fas expression in concussive TBI models. In a weight-drop model of concussive TBI in rats, Rooker et al (2006) showed induction of TNFα mRNA in brain at 1 to 6 hours after injury, consistent with our data, and expression of interleukin-1β protein in activated microglia. Recent studies have also shown induction of insulin-like growth factor-1 signaling including extracellular signal-regulated kinase 1/2 phosphorylation, and inhibition of MWM deficits by administration of insulin-like growth factor-1 in a mouse weight-drop mild TBI model (Rubovitch et al, 2010).
In conclusion, our findings have potential implications for patients with TBI, as treatment strategies targeting TNFα/Fas may need to be stratified according to injury type (i.e., contusion versus concussion) and timing of TNF/Fas inhibition. It will also be important to examine the role of TNFα/Fas in experimental TBI models with mixed injury subtypes in which contusion and concussion mechanisms coexist, as they often do in human TBI.
The authors declare no conflict of interest.
Footnotes
This work was supported by grants from NICHD T32 HD040128-06 (WM), NINDS 5RO1NS047447 (MJW), and MGH Center for the Integration of Medicine and Technology (CIMIT) (MJW).
References
- Albensi BC. Models of brain injury and alterations in synaptic plasticity. J Neurosci Res. 2001;65:279–283. doi: 10.1002/jnr.1151. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Sullivan PG, Thompson MB, Scheff SW, Mattson MP. Cyclosporin ameliorates traumatic brain-injury-induced alterations of hippocampal synaptic plasticity. Exp Neurol. 2000;162:385–389. doi: 10.1006/exnr.1999.7338. [DOI] [PubMed] [Google Scholar]
- Arciniegas D, Adler L, Topkoff J, Cawthra E, Filley CM, Reite M. Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 1999;13:1–13. doi: 10.1080/026990599121827. [DOI] [PubMed] [Google Scholar]
- Beer R, Franz G, Schopf M, Reindl M, Zelger B, Schmutzhard E, Poewe W, Kampfl A. Expression of Fas and Fas ligand after experimental traumatic brain injury in the rat. J Cereb Blood Flow Metab. 2000;20:669–677. doi: 10.1097/00004647-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2007;27:1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci USA. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E, Koch P, Teodorczyk M, Kleber S, Klussmann S, Wiestler B, Brustle O, Mueller W, Gieffers C, Hill O, Thiemann M, Seedorf M, Gretz N, Sprengel R, Celikel T, Martin-Villalba A. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell. 2009;5:178–190. doi: 10.1016/j.stem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Farkas O, Lifshitz J, Povlishock JT. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury. J Neurosci. 2006;26:3130–3140. doi: 10.1523/JNEUROSCI.5119-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30:213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Grosjean MB, Lenzlinger PM, Stahel PF, Yatsiv I, Shohami E, Trentz O, Kossmann T, Morganti-Kossmann MC. Immunohistochemical characterization of Fas (CD95) and Fas ligand (FasL/CD95L) expression in the injured brain: relationship with neuronal cell death and inflammatory mediators. Histol Histopathol. 2007;22:235–250. doi: 10.14670/HH-22.235. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Smith DH, Lowenstein DH, Saint Marie R, McIntosh TK. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J Neurotrauma. 1993;10:405–414. doi: 10.1089/neu.1993.10.405. [DOI] [PubMed] [Google Scholar]
- Kilbourne M, Kuehn R, Tosun C, Caridi J, Keledjian K, Bochicchio G, Scalea T, Gerzanich V, Simard JM. Novel model of frontal impact closed head injury in the rat. J Neurotrauma. 2009;26:2233–2243. doi: 10.1089/neu.2009.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunol. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Lee S, Sato A, Oda S, Hirasawa H, Yamashita T. TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res. 2009;1290:102–110. doi: 10.1016/j.brainres.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Qiu J, Whalen MJ, Lowenstein P, Fiskum G, Fahy B, Darwish R, Aarabi B, Yuan J, Moskowitz MA. Upregulation of the Fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J Neurosci. 2002;22:3504–3511. doi: 10.1523/JNEUROSCI.22-09-03504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooker S, Jander S, Van Reempts J, Stoll G, Jorens PG, Borgers M, Verlooy J. Spatiotemporal pattern of neuroinflammation after impact-acceleration closed head injury in the rat. Mediators Inflamm. 2006;2006:90123. doi: 10.1155/MI/2006/90123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Halliday MI, Campbell GC, Byrnes DP, Rowlands BJ. The presence of tumor necrosis factor in CSF and plasma after severe head injury. Br J Neurosurg. 1994;8:419–425. doi: 10.3109/02688699408995109. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Edut S, Sarfstein R, Werner H, Pick CG. The intricate involvement of the insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol Dis. 2010;38:299–303. doi: 10.1016/j.nbd.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E, Marino MW, McIntosh TK. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci USA. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol. 2002;67:281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab. 1996;16:378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10:119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Povlishock JT. Identification and characterization of heterogeneous neuronal injury and death in regions of diffuse brain injury: evidence for multiple independent injury phenotypes. J Neurosci. 2004;24:3543–3553. doi: 10.1523/JNEUROSCI.5048-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel PF, Shohami E, Younis FM, Kariya K, Otto VI, Lenzlinger PM, Grosjean MB, Eugster HP, Trentz O, Kossmann T, Morganti-Kossmann MC. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J Cereb Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D'Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. 1992;146:176–178. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- Tang YP, Noda Y, Hasegawa T, Nabeshima T. A concussive-like brain injury model in mice (I): impairment in learning and memory. J Neurotrauma. 1997a;14:851–862. doi: 10.1089/neu.1997.14.851. [DOI] [PubMed] [Google Scholar]
- Tang YP, Noda Y, Hasegawa T, Nabeshima T. A concussive-like brain injury model in mice (II): selective neuronal loss in the cortex and hippocampus. J Neurotrauma. 1997b;14:863–873. doi: 10.1089/neu.1997.14.863. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Dalkara T, You Z, Qiu J, Bermpohl D, Mehta N, Suter B, Bhide PG, Lo EH, Ericsson M, Moskowitz MA. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:490–505. doi: 10.1038/sj.jcbfm.9600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager PH, You Z, Qin T, Kim HH, Takahashi K, Ezekowitz AB, Stahl GL, Carroll MC, Whalen MJ. Mannose binding lectin gene deficiency increases susceptibility to traumatic brain injury in mice. J Cereb Blood Flow Metab. 2008;28:1030–1039. doi: 10.1038/sj.jcbfm.9600605. [DOI] [PubMed] [Google Scholar]
- Yang J, You Z, Kim HH, Hwang SK, Khuman J, Guo S, Lo EH, Whalen M. Genetic analysis of the role of tumor necrosis factor receptors in functional outcome after traumatic brain injury in mice. J Neurotrauma. 2010;27:1037–1046. doi: 10.1089/neu.2009.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]