Abstract
Cerebrovascular reactivity to vasodilatory hypercapnic and vasoconstrictive hypocapnic challenges is known to be altered in several hemodynamic disorders, which is often attributable to changes in smooth muscle-mediated vascular compliance. Recently, attenuated reactivity to hypercapnia but enhanced reactivity to hypocapnia was observed in patients with chronic stroke. We hypothesize that the latter observation could be explained by a change in the basal vascular tone. In particular, reduced cerebral perfusion pressure, as is prevalent in these patients, may cause vasodilation through autoregulatory mechanisms, and this compensatory baseline condition may alter reactivity to vasoconstrictive hypocapnic challenges. To test this hypothesis, a predilated vascular condition was created in young, healthy subjects (n=11; age=23 to 36 years) using inhalation of 4% CO2. Using blood oxygenation level-dependent functional magnetic resonance imaging at 3 T, breath holding and cued deep breathing respiratory challenges were administered to assess hypercapnia and hypocapnia reactivity, respectively. During the predilated condition, vasoconstrictive reactivity to hypocapnia was significantly (21.1%, P=0.016) enhanced throughout the gray matter, whereas there was no significant change (6.4%, P=0.459) in hypercapnic vasodilatory reactivity. This suggests that baseline vasodilation may explain the enhanced hypocapnia reactivity observed in some stroke patients, and that hypocapnia challenges may help identify the level of vascular compliance in patients with reduced cerebral perfusion pressure.
Keywords: BOLD, cerebrovascular reactivity, CO2, hypercapnia, hypocapnia, respiration
Introduction
The human cerebrovasculature has evolved to have advanced regulatory mechanisms that enable vessels to dilate and constrict in response to changes in cerebral perfusion pressure (autoregulation), metabolic demand (neurovascular coupling), or the presence of vasoactive stimuli (reactivity). These parameters can be altered in pathologic states (Silvestrini et al, 2000; Aso et al, 2009; Bokkers et al, 2010), but precisely how age and disease affect the mechanisms underlying vascular responsiveness is still unclear.
One robust means of assessing cerebrovascular reactivity (CVR) is through the manipulation of arterial gas tensions. The potent vasodilatory nature of arterial carbon dioxide in humans was described by Kety and Schmidt (1948), and can be manipulated using inhalation of air with increased CO2 content to induce hypercapnia or using hyperventilation to induce hypocapnia (Cohen et al, 2002). Voluntary respiratory challenges such as breath holding or deep breathing can also cause transient hypercapnia and hypocapnia, and some of these methods could be relatively easy to implement in clinical environments (Bright et al, 2009). Vascular responses to these challenges can be characterized throughout the brain using noninvasive blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI), which reflects localized changes in blood volume and the concentration of deoxyhemoglobin caused by alterations in blood flow, blood volume, and/or tissue metabolism (Buxton et al, 2004; He and Yablonskiy, 2007).
In studies of normal aging, hypercapnia and hypocapnia cerebral blood flow (CBF) responses are both observed to be attenuated (Yamaguchi et al, 1979; Maeda et al, 1994; Ito et al, 2002), possibly because of arteriole rarefaction or mild hypertension, atherosclerosis, and the resulting impairment in vessel compliance that is frequently observed in older populations (Yamamoto et al, 1980; Hutchins et al, 1996). Attenuated hypercapnic CVR in patients has also been shown to offer diagnostic and prognostic clinical information in stroke populations (Silvestrini et al, 2000; Shiino et al, 2003; Aso et al, 2009; Goode et al, 2009). Similar clinical applications for hypocapnic CVR in stroke patients have not yet been as well examined.
A recent study by Zhao et al (2009) explored the effect of both hypercapnic and hypocapnic challenges in stable stroke patients, observed several years after the stroke. These patients exhibited significant deviation from normal CO2 reactivity relative to age-matched controls, and these deviations extended far beyond the region of ischemic tissue damage and even into the contralateral hemisphere. In particular, patients displayed an attenuated CBF response to a CO2 inhalation challenge, but an exaggerated CBF response to hyperventilation. In the context of the general observations of attenuated hypercapnic and hypocapnic reactivity in age and age-related disorders, it is likely that the enhancement of constrictive reactivity to hypocapnia observed by Zhao et al reflects specific consequences of stroke and pathologic hypoperfusion rather than effects of age and the related decline in compliance. However, an attenuated dilatory response to hypercapnia could reflect either consequences specific to stroke or effects of age and age-related vascular impairments that may be amplified in stroke patients of the study by Zhao et al.
It has been shown that patients with reduced perfusion pressure or stenosis of an internal carotid artery may show large areas of increased arterial cerebral blood volume through autoregulatory mechanisms to maintain sufficient blood supply to the downstream tissue (Derdeyn et al, 2002; Donahue et al, 2010). We hypothesize that widespread increases in basal blood volume may affect the vasodilatory or vasoconstrictive responses to changes in arterial gas tensions during reactivity challenges, and that this protective vasodilation may explain the widespread abnormal responses to hypocapnic CO2 modulations observed in chronic stroke patients.
In this study, we use a controlled experiment in young, healthy volunteers to explore how baseline dilation of the cerebrovasculature, which is often present in disease, can affect hypercapnic and hypocapnic CO2 reactivity independently of the effects of normal aging. To achieve this, a simulated pathologic condition, in which the cerebrovasculature is globally dilated, is created in healthy subjects using prolonged inhalation of 4% CO2 gas in air. During this altered baseline condition, and during the normal baseline condition, two types of respiratory challenges are executed to ascertain hypercapnia and hypocapnia reactivity independently.
Materials and methods
Data Acquisition
A total of 12 subjects (aged 23 to 36 years, 2 women) were scanned using a 3T Siemens TIM Trio scanner (Siemens, Erlangen, Germany) equipped with a transmit body coil and 12-channel receive head coil, using foam inserts to minimize head motion. A standard gradient-echo EPI (echo-planar imaging) sequence (repetition time/echo time=1,500/38 milliseconds, flip angle=73°, field of view=225 mm, 15 slices) was performed to obtain BOLD-weighted images with a spatial resolution of 3.5 × 3.5 × 4.0 mm3. Two BOLD data sets containing 476 volumes were collected, lasting ∼12 minutes each. A structural image was also obtained to enable registration of functional data and generation of anatomically derived masks (MPRAGE: repetition time/inversion time/echo time=1,778/900/4.4 milliseconds, 1.7 × 1.7 × 2.0 mm3, Mugler and Brookeman, 1990). Appropriate ethical approval for this study was obtained from the Oxfordshire Research Ethics Committee.
The BOLD data were preprocessed using FSL software tools for motion correction, slice timing correction, and brain extraction (Smith et al, 2004, http://www.fmrib.ox.ac.uk/fsl) before being high-pass filtered (315 seconds) and spatially smoothed using a Gaussian kernel (full-width half-maximum=5 mm). The functional data acquired from each scan were registered to the MPRAGE image (FLIRT, FSL, Jenkinson and Smith, 2001). The MPRAGE image was then transformed using nonlinear registration (Andersson et al, 2008) into the MNI (Montreal Neurological Institute) space (MNI152, nonlinearly derived, McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada). These transformations were concatenated and then inverted to enable the conversion of regions of interest (ROIs) defined in the MNI space into the functional space for every subject and scan.
Respiratory Protocol
Two distinct baseline conditions were established for each subject (in a randomized order) using inhalation of either normal air or a mixture of 4% CO2 in air. During each condition, three breath-hold (BH) challenges, and three cued deep breathing (CDB) challenges were executed (also in a randomized order), interleaved with 90 seconds of normal breathing.
A 15-second BH challenge, somewhat shorter than generally observed in the experimental literature (Kastrup et al, 1999) but comparable with clinical research studies (Donahue et al, 2009), was selected to ensure successful execution of the task during each baseline condition, and to reduce hyperventilation on resumption of breathing (and consequent head motion during the BOLD response). Subjects were instructed to cease breathing wherever they were in their natural breathing cycle on seeing the ‘hold' cue, eliminating any preparatory breaths that may delay the transient hypercapnic response.
Pilot studies revealed that the competing effects of deep exhalations and inhalation of 4% CO2 precluded transient hypocapnia normally associated with the CDB challenge. To achieve the hypocapnic response and detectable BOLD signal changes during both conditions, the CDB challenge was modified from its original form (Bright et al, 2009): a third deep breath was added to the original challenge (in both conditions), and fast switching of the gas delivered to the subject enabled the second and third inhalations of the challenge to be drawn from normal air during the 4% CO2 condition. A schematic of the paradigm, including the timing of gas switching, is illustrated in Figure 1. Cues were displayed as white letters on a black background and projected onto a screen in the scan room that was viewed through a mirror on the head coil.
Figure 1.
Experimental design. (A) Scanning protocol: after the stabilization of target gas delivery (either humidified air or 4% CO2 in air), a 12-minute BOLD-weighted gradient-echo EPI scan was performed. Six respiratory challenges were cued; three breath-hold (BH) and three cued deep breathing (CDB) challenges were administered in a randomized order, interleaved with 90 seconds of normal breathing. (B) Challenge cues: CDB and BH challenges were visually cued using textual instructions. During the 4% CO2 baseline condition, the CDB challenge required temporary cessation of CO2 inhalation. The period of gas switching is indicated with asterisks. (C) Schematic of two-source gas delivery system: this system was designed to allow instant switching of inspired gas content from outside the scan room. At the end of deep inhalation, the nearly empty reservoir bag could be refilled with a different gas while the subject exhaled (air versus CO2 mixture). BOLD, blood oxygenation level-dependent; EPI, echo-planar imaging.
A two-source gas delivery system (Figure 1C) was constructed to enable the fast switching of inhaled gas content required for the modified CDB challenge described above. Before the start of scanning, each length of tubing was flooded with its respective source gas, so that manual switching of the two valves in the control room caused near-instant switching of the gas delivered to a reservoir bag. Subjects wore a full coverage face mask (8920 Series, Hans Rudolph Inc., Kansas City, MO, USA) connected to the reservoir and room environment through one-way valves to ensure minimal rebreathing. The use and positioning of the filter and reservoir bag reduced breathing resistance and subject awareness of gas switching.
All subjects were brought to the scanning facility before the scan session to have the respiratory protocol explained. Outside the scanner, subjects practiced breathing with the mask and reservoir bag system and were gradually introduced to CO2 inhalation until reaching the 4% target. Several CDB and BH challenges were also performed with concurrent gas monitoring.
Physiologic Monitoring
Respiration was monitored using respiratory bellows, and the data were temporally aligned with scanner trigger output (BIOPAC MP150, Biopac, Goleta, CA, USA). Subjects were instructed to breathe in and out through their nose, and a nasal cannula worn beneath the face mask facilitated continuous monitoring of inspired and expired respiratory gas composition using a Normocapoxy (Datex Engstrom, Helsinki, Finland) gas monitor to simultaneously measure CO2 and O2. The bellows data were used to empirically calculate the lag in gas measurements caused by the length of sampling tube and strength of vacuum. End-tidal (ET) values, known to well represent the arterial CO2 and O2 content (Young et al, 1991), were extracted using code developed in IDL (ITT Visual Information Solutions, Boulder, CO, USA). The ET partial pressure of CO2 (PETCO2) trace was interpolated using splines to estimate PETCO2 at every scan volume.
Cerebrovascular Reactivity Analysis
A general linear model (GLM) was constructed to measure BOLD signal changes caused by the two types of respiratory challenges and normal fluctuations in PETCO2 during free breathing, and these results were transformed into hypercapnia, hypocapnia, and resting CVR maps. First, the interpolated scan-aligned PETCO2 trace was divided into three regressors representing CO2 changes associated with BH challenges, CDB challenges, or resting fluctuations. Three 40-second windows after the start of appropriate challenge cues were extracted to form BH and CDB regressors, and six 20-second windows preceding every challenge were extracted to form the resting fluctuations regressor. The mean resting PETCO2 value was measured and subtracted from each regressor, and all values outside the relevant windows of interest were set to zero. Example regressors are shown in Figure 2.
Figure 2.
Extraction of breath hold (BH), cued deep breathing (CDB), and resting fluctuation end-tidal (ET) PCO2 regressors from total scan PETCO2 data. ET values were extracted from the scan CO2 trace, and were temporally aligned and interpolated using splines to obtain PETCO2 values for every scan volume (top). The start times of challenge cues (dashed lines) were used to define three 40-second windows of interest, and the ET PCO2 data within these windows were extracted to create (A) BH or (B) CDB regressors. (C) Data within six 20-second windows preceding the start times of all challenges were extracted to form a regressor of ‘resting fluctuations.' All PETCO2 data were demeaned (mean value calculated in the ‘resting' periods), and all values outside the appropriate windows of interest were zeroed. The average BOLD time course for this scan is plotted (bottom) for reference. The resting fluctuation regressor is displayed at twice the scaling of the BH and CDB regressors to assist in visibility. BOLD, blood oxygenation level-dependent.
The three regressors of interest were convolved with a γ-variate function before incorporation into a general linear model (FILM, FSL, Woolrich et al, 2001) to determine BOLD signal changes explained by CO2 fluctuations. The regressor representing resting fluctuations was convolved with a γ-variate function with full-width at half-maximum=6 seconds and a mean lag of 6.3 seconds, as proposed previously (Wise et al, 2004).
The temporal delay in the BOLD response to breathing challenges versus normal breathing variations has been shown to differ significantly (Birn et al, 2008), and also exhibits significant regional heterogeneity across cortical and subcortical regions (Bright et al, 2009; Chang et al, 2008). To accurately explore regional differences in BH-CVR and CDB-CVR, it is necessary to incorporate this variation in hemodynamic delay times into regressor modeling.
The hemodynamic delay was assessed within eight ROIs. Regions of interest of the temporal, parietal, frontal, and occipital lobes, as well as those of the thalamus, putamen, and insula, were automatically defined using the MNI atlas (Mazziotta et al, 2001). An ROI of the visual cortex was constructed using the V1 and V2 regions of the Juelich atlas (Amunts et al, 2000; Eickhoff et al, 2007). A mask of gray matter voxels was created from each subject's MPRAGE image (FAST, FSL, Zhang et al, 2001). These ROIs were transformed into the subject- and condition-specific fMRI space.
The PETCO2 BH and CDB regressors were convolved with a γ-variate of width 7.0 seconds and mean lag estimated from the time to peak of the global mean BOLD time course. The average BOLD time course of each ROI was correlated with the BH and CDB regressors across a range of temporal shifts (−10 to +10 seconds in intervals of 0.5 seconds). The shift associated with maximum correlation was used to determine the optimal hemodynamic delay for that specific ROI and challenge. For every data set, GLM analysis was performed independently for each ROI, using appropriate hemodynamic delays for BH and CDB challenges to most accurately measure CVR in that region. Parameters derived from motion correction and the trace from respiratory bellows were included in the GLM as confounds.
Parameter estimate maps for CDB, BH, and resting fluctuation regressors were converted into reactivity maps in units of %BOLD/ΔPETCO2 (mm Hg). Although the BOLD response to CDB challenges is negative, it is associated with a negative change in PETCO2, resulting in a positive CVR value. This terminology, although not uniformly adopted in the literature, is implemented to ensure consistency even in the presence of paradoxical blood flow reductions during hypercapnia that have been observed in certain pathologies (Mandell et al, 2008) and would indicate true negative CVR.
The median CVR values associated with BH, CDB, and resting fluctuations across all voxels within the gray matter were calculated. As there may be different EPI distortion artifacts between the two functional scans, the aligned segmentation-based gray matter mask may not accurately register with the functional space for both conditions. For comparison, an additional ‘gray matter' mask was created using a combined BH and CDB CVR threshold of 0.05 %BOLD/ΔPETCO2 (mm Hg), which removed the majority of white matter and CSF voxels. For each ROI, CVR maps obtained using the appropriate optimized BH and CDB hemodynamic delay timings were used to calculate the median BH and CDB CVR values within the transformed ROI mask.
Results
Although all subjects successfully completed the respiratory protocol, one subject data set was removed from further analysis because of an interruption of the EPI sequence caused by MRI system errors. End-tidal PCO2 statistics for the 11 remaining subjects are described in Table 1, and PETO2 data are presented in Table 2.
Table 1. End-tidal PCO2 statistics for normal air and 4% CO2 baseline conditions (in units of mm Hg).
| Subject |
Normal air condition |
4% CO2 condition |
||||
|---|---|---|---|---|---|---|
| Baseline | CDB | BH | Baseline | CDB | BH | |
| PETCO2 (mm Hg) | ΔPETCO2 (mm Hg) | ΔPETCO2 (mm Hg) | PETCO2 (mm Hg) | ΔPETCO2 (mm Hg) | ΔPETCO2 (mm Hg) | |
| 1 | 42.7±0.7 | −3.3±2.9 | 3.9±3.0 | 48.5±0.4 | −7.2±0.5 | 3.5±2.7 |
| 2 | 37.1±2.3 | −7.2±1.0 | 6.3±0.4 | 48.3±0.5 | −9.2±0.2 | 3.9±2.9 |
| 3 | 47.5±0.6 | −9.4±2.7 | 2.1±3.9 | 54.3±0.5 | −9.9±1.2 | 5.9±2.3 |
| 5 | 43.8±1.2 | −11.9±1.8 | 4.7±1.2 | 46.9±0.4 | −12.4±2.9 | 4.8±1.7 |
| 6 | 44.9±0.4 | −5.6±0.6 | 4.4±2.8 | 51.1±0.5 | −8.8±0.9 | 3.0±1.9 |
| 7 | 39.5±0.5 | −6.8±0.3 | 7.3±0.2 | 48.4±0.4 | −7.3±1.4 | 6.6±0.8 |
| 8 | 44.1±0.8 | −7.8±1.4 | 5.2±2.5 | 47.5±0.5 | −8.2±0.2 | 4.0±0.3 |
| 9 | 43.5±0.8 | −8.1±0.8 | 6.8±0.5 | 49.5±0.9 | −9.2±0.6 | 4.5±1.7 |
| 10 | 40.7±3.2 | −4.5±1.7 | 7.6±2.1 | 49.0±0.9 | −8.4±0.5 | 7.5±0.8 |
| 11 | 39.8±1.7 | −4.6±2.6 | 3.1±2.7 | 47.2±0.5 | −8.5±1.3 | 5.1±0.4 |
| 12 | 42.1±0.6 | −3.5±1.8 | 7.1±1.3 | 45.2±0.6 | −6.5±0.2 | 6.9±0.8 |
| Mean±s.d. | 42.3±2.9 | −6.6±2.6 | 4.9±2.3 | 48.7±2.4† | −8.7±1.6† | 4.6±2.0 |
BH, breath hold; CDB, cued deep breathing; PET, end-tidal partial pressure.
†Significant difference across two baseline conditions, paired Student's t-test P<0.005.
Table 2. End-tidal PO2 statistics for normal air and 4% CO2 baseline conditions (in units of mm Hg).
| Subject |
Normal air condition |
4% CO2 condition |
||||
|---|---|---|---|---|---|---|
| Baseline | CDB | BH | Baseline | CDB | BH | |
| PETO2 (mm Hg) | ΔPETO2 (mm Hg) | ΔPETO2 (mm Hg) | PETO2 (mm Hg) | ΔPETO2 (mm Hg) | ΔPETO2 (mm Hg) | |
| 1 | 114.6±1.7 | 6.1±5.5 | −13.1±2.9 | 129.4±1.7 | 4.9±1.3 | −10.6±3.1 |
| 2 | 122.8±4.6 | 12.6±2.6 | −15.4±0.2 | 127.1±1.5 | 8.2±1.0 | −9.5±4.9 |
| 3 | 108.6±4.3 | 16.6±7.5 | −10.3±8.1 | 124.4±1.9 | 7.5±1.2 | −18.2±6.9 |
| 5 | 101.8±6.9 | 24.7±2.3 | −16.9±1.5 | 127.2±4.3 | 14.1±4.0 | −14.3±2.6 |
| 6 | 112.1±2.2 | 14.7±2.7 | −17.3±3.7 | 129.3±2.4 | 6.1±1.3 | −13.7±6.1 |
| 7 | 117.8±1.8 | 12.1±1.1 | −19.8±0.4 | 130.5±2.6 | 4.5±1.4 | −16.4±4.6 |
| 8 | 106.2±6.4 | 17.9±6.2 | −18.1±5.2 | 118.8±2.1 | 11.6±0.4 | −15.4±1.6 |
| 9 | 113.3±4.0 | 15.1±4.6 | −19.3±2.5 | 131.9±1.5 | 3.7±0.9 | −17.7±5.9 |
| 10 | 118.0±9.8 | 8.8±5.0 | −27.4±7.9 | 123.8±1.9 | 8.7±1.1 | −29.9±3.3 |
| 11 | 120.9±2.4 | 9.8±1.2 | −10.7±0.9 | 123.3±1.1 | 4.6±1.5 | −14.0±0.7 |
| 12 | 113.7±1.7 | 7.2±4.5 | −18.3±4.9 | 126.3±1.5 | 7.2±2.8 | −16.6±4.5 |
| Mean±s.d. | 113.6±6.3 | 13.2±5.4 | −15.5±6.7 | 126.5±3.8† | 7.4±3.2† | −14.7±6.9 |
BH, breath hold; CDB, cued deep breathing; PET, end-tidal partial pressure.
†Significant difference across two baseline conditions, paired Student's t-test P<0.005.
During the normal air condition, we observed a mean PETCO2 of 42.3±2.9 mm Hg and a mean PETO2 of 113.6±6.3 mm Hg across subjects, and we measured significantly increased values of 48.7±2.4 mm Hg PETCO2 and 126.5±3.8 mm Hg PETO2 during the 4% CO2 condition (P<0.005). The BH challenge had comparable gas tension responses in both conditions, whereas the CDB challenge caused significantly larger ΔPETCO2 and significantly smaller ΔPETO2 during the 4% CO2 condition (see tables).
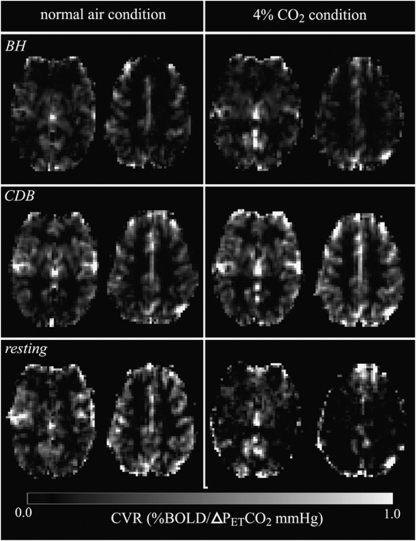
Example CVR maps derived from BH, CDB, and resting fluctuation PETCO2 regressors during each condition for a representative single subject are displayed in Figure 3 in units of %BOLD signal change per ΔPETCO2 (mm Hg). Both BH-CVR and CDB-CVR maps show good qualitative agreement, and both challenge maps reveal structural features in the brain, such as large vessels, basal ganglia, and gray and white matter contrast. The CVR maps derived from resting fluctuations appear noisier, although they occasionally reveal the same structure as respiratory challenge CVR maps.
Figure 3.
Example of slices from CVR maps for a single subject during normal air (left) and 4% CO2 (right) conditions, and derived using BH (top), CDB (middle), or resting fluctuation (bottom) PETCO2 regressors. BH, breath hold; CDB, cued deep breathing; CVR; cerebrovascular reactivity; PET, end-tidal partial pressure.
The hemodynamic delay times for BH and CDB challenges exhibited some significant variability in the eight ROIs examined in our analysis. Delay times for BH and CDB challenges during the two baseline conditions, relative to the delay optimized for the gray matter time course, are shown in Figure 4. In most regions, the BH and CDB delay times are similar, with the occipital lobe and the visual cortex being notable exceptions. In these regions, the CDB response timing is significantly delayed relative to the whole brain response, whereas the BH response is not significantly different and even showing a trend towards earlier responses. The gray matter hemodynamic delay parameter was not significantly different for either challenge between the two conditions. The BH and CDB challenge delay times were comparable during the normal air condition, but significantly different during the 4% CO2 condition (the CDB delay is 2.27 seconds longer than BH delay, P=0.026, paired Student's t-test).
Figure 4.
Optimal hemodynamic delays for BH and CDB regressors relative to the hemodynamic delay calculated for the gray matter time course, measured in eight regions of interest (ROIs). The mean and s.d. of 11 subjects are plotted for each challenge and each baseline condition, and significant relationships were determined using paired Student's t-tests (*P<0.05, *P<0.01, ***P<0.005, corrected for multiple comparisons). The occipital lobe and visual cortex ROIs indicate a significant difference in the BH and CDB response dynamics during both conditions. BH, breath hold; CDB, cued deep breathing.
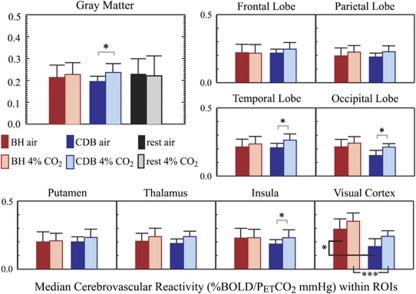
To quantify the relationship between these different metrics of reactivity, the median CVR values within several ROIs were calculated and compared across the population using paired Student's t-tests. The results are displayed graphically in Figure 5. Within gray matter voxels, there was no significant difference between BH-CVR measured in the normal air and 4% CO2 conditions (6.4%, P=0.459), whereas CDB-CVR increased significantly (21.1%, P=0.016) at elevated CO2. The choice of gray matter mask (derived from segmentation of the structural image or loose CVR thresholding) did affect the absolute values of median CVR, but did not affect the significant relationships between the two conditions. The enhanced CDB-CVR trend is apparent in many of the smaller ROIs analyzed, although not always reaching statistical significance after correction for multiple comparisons. A notable variation is seen in the visual cortex, where we observe significantly larger BH-CVR relative to CDB-CVR during both conditions.
Figure 5.
Region of interest (ROI) analysis of cerebrovascular reactivity (CVR) derived from breath-hold (BH), cued deep breathing (CDB), and resting CO2 changes in units of %BOLD signal change per ΔPETCO2 mm Hg. Regional variability in the hemodynamic delay between hypercapnic and hypocapnic alterations and the BOLD response has been incorporated into the GLM and ROI analyses, as discussed in the ‘Materials and methods' section. Significant enhancement of CDB-CVR during the 4% CO2 condition is measured in whole-brain gray matter and several smaller ROIs. Voxels within the visual cortex reveal significant deviation of BH-CVR and CDB-CVR during both baseline conditions. (In total gray matter, P=0.016. In smaller ROIs, significance indicated using *P<0.05; **P<0.01; ***P<0.005, paired Student's t-test, corrected for multiple comparisons.) BOLD, blood oxygenation level-dependent; PET, end-tidal partial pressure.
Discussion
We observed modified, challenge-dependent BOLD reactivity patterns in healthy subjects during an altered baseline state in which the cerebrovasculature was globally dilated. We have shown that predilation of healthy vessels is associated with a moderately enhanced response to a hypocapnia respiratory challenge, possibly suggesting that enhanced hypocapnic CVR in patients, such as those presented in the study by Zhao et al, may be caused by widespread protective vasodilation. However, we do not observe any significant attenuation in BH-CVR during the predilated condition. It is possible that attenuated hypercapnia CVR observed by Zhao et al (2009) is related to widespread impairment of dilatory vascular responsiveness in an elderly and pathologic population and not directly reflective of an interaction of reactivity with protective dilation. Therefore, it may not have been present in our study of much younger volunteers.
As there are complicated interactions of multiple chemical factors that are implicated in vasoconstriction and vasodilation, the current study is unable to discern the specific mechanisms behind enhanced vasoconstrictive hypocapnia reactivity during increased basal dilation. It is possible that basal dilation affects stretch-activated ion channels in smooth muscle cells, which could alter the resting concentrations of ions in the intracellular and extracellular space and perhaps the sensitivity of cells to pH changes. As the respiratory challenges used in our experiments cause bolus-like changes in PaCO2 and do not reach a maximum steady-state value, enhanced CDB-CVR could reflect either an amplified constriction response or a faster constriction response.
Physiologic Response to Respiratory Challenges
It is important to address physiologic differences in the CDB challenge during the two baseline conditions to assess whether they could be the cause of the observed CVR enhancement. In particular, Tables 1 and 2 indicate that the PETCO2 response to the CDB challenge is significantly greater and that the PETO2 response to the CDB challenge is significantly smaller during the 4% CO2 condition than during the normal air condition. As the magnitude of the CO2 effect is directly taken into account in our measurement of CVR (we normalize the %BOLD signal change by challenge-induced ΔPETCO2), it is unlikely that this could explain the amplified CDB-CVR observation. It is possible that hypocapnia challenges like CDB act nonlinearly on the mechanisms behind BOLD contrast, and that this normalization is not appropriate. However, the range of PETCO2 values studied throughout the entire experiment lie well within the range of PETCO2 in which CBF responds linearly (Figure 6 showing linear response range supported by the positron emission tomography, transcranial Doppler sonography, and arterial spin labeling literature (Rhodes et al, 1981; Poeppel et al, 2007; Pollock et al, 2009). Better understanding of the dynamic blood volume response to CDB is required to determine whether our BOLD metric for CVR is appropriate.
Figure 6.
Summary of group end-tidal PCO2 statistics. Mean values (and s.d.) across 11 subjects are plotted for resting PETCO2 and challenge-induced PETCO2 responses during the normal air and 4% CO2 conditions. ‘a' indicates the range of ‘normal' PETCO2 values for humans (Levitzky, 2003). ‘b' indicates the range of PETCO2 values in which the CBF response is observed to be linear (Rhodes et al, 1981; Poeppel et al, 2007; Pollock et al, 2009). CBF, cerebral blood flow; PET, end-tidal partial pressure.
Another possible contributor to the enhanced CDB reactivity response in the predilated condition is the decreased PETO2 response. During the 4% CO2 condition, subjects tended to hyperventilate slightly, which is reflected in the increased resting PETO2 levels despite inhalation of a slightly hypoxic gas mixture. Transient hypocapnia and hyperoxia induced by the CDB challenge are determined by the relative depth and rate of the challenge breathing versus the resting breathing, and therefore a different resting respiratory rate will alter the physiologic responses to the challenge. Conversely, hypercapnia achieved during BH challenges is predominantly determined by baseline tissue metabolism producing carbon dioxide, and is therefore not significantly affected by the mild hyperventilation during the 4% CO2 baseline condition. The temporary gas switching during the 4% CO2 condition may also have a small role in exaggerating this effect.
The mean difference in ΔPETO2 caused by CDB challenges during normal air versus 4% CO2 conditions was 5.9±4.1 mm Hg, which was highly significant (P<0.005). Oxygen has competing mechanisms affecting BOLD contrast: increased arterial oxygen content will increase relative levels of oxygenated hemoglobin in the vasculature and thus increase the BOLD signal, while simultaneously acting as a vasoconstricting agent that decreases the BOLD signal during iso-metabolic conditions (Bulte et al, 2007). We do not expect oxygen effects of this scale to cause any significant decreases in flow (Bulte et al, 2007), and we calculate that 5.9 mm Hg of additional arterial oxygen would contribute at most a 0.025% increase in the BOLD signal (using the %BOLD–%PaO2 relationship empirically obtained by Chiarelli et al, 2007). Our measured CVR enhancement in the gray matter of 0.041 %BOLD/ΔPETCO2 (mm Hg), when associated with a mean CDB ΔPETCO2 of 6.6 mm Hg (Table 1), reflects an ∼0.27% BOLD signal difference, which is an order of magnitude beyond what could be explained solely by oxygen effects.
The response to the BH challenge implemented in this study does not differ significantly across the two baseline conditions. This is most likely owing to the independence of BH-induced gas responses and the resting breathing rate, as described earlier. We also selected a short 15-second BH challenge to make the task tolerable, in particular while the subject is already hypercapnic during the 4% CO2 condition. This duration of BH should preclude the sensation of air hunger and diaphragm twitching that occurs with longer challenges, and studies have shown that 15-second BHs during elevated PETCO2 (7 to 8 mm Hg above normal levels) are not associated with increases in neuronal activity beyond what is observed during normocapnia conditions (McKay, 2002). Our choice of a short BH challenge should minimize confounding neuronal effects associated with air hunger or breathlessness between baseline conditions.
Further modifications to the BH challenge may better help standardize the task across trials, and the merits of methods such as paced breathing, controlled breathing depth, and beginning the hold immediately after exhalation are discussed in the literature (Thomason and Glover, 2008; Scouten and Schwarzbauer, 2008). Owing to subject variability in respiratory response to CO2 inhalation (in which we observe a trend of increased breathing rate but variable breathing depth), we opted to allow subjects to free breathe at a pace and depth that reflected their natural ventilatory response to hypercapnia conditions.
Regional Variability in Hypercapnic and Hypocapnic Cerebrovascular Reactivity Relationships
Another finding of our study is the presence of regional variability in the agreement of CDB- and BH-derived CVR measurements, even in the normal air condition. Within the visual cortex (automatically delineated using the V1 and V2 ROIs defined in the Juelich atlas), BH-CVR was significantly larger than CDB-CVR during both conditions as indicated in Figure 5. In previous CDB experiments, we observed regional heterogeneity in the BOLD response timing to the challenge, including a relatively slower response in the posterior cortices (Bright et al, 2009). In this study, we observe a significant difference in the BH and CDB dynamics in this region, which was not present in the original methodology. The hemodynamic delay measured in this study is calculated using the average BOLD time course of all voxels in an ROI, which will give more weight to the timing of voxels with large BOLD signal changes rather than small response amplitudes. In the previous study, every voxel timing was assessed equally, regardless of the magnitude of the BOLD signal change. This suggests that the visual cortex contains voxels with a range of CVR values that may be partially correlated with response timing.
It is unclear what mechanisms are driving the large BH-CVR (and relatively small CDB-CVR response) in the visual cortex. The cues for all breathing challenges in this experiment were presented visually, and thus there is an unavoidable concurrent level of neuronal stimulation in the visual cortex. It is conceivable that the dilatory effects of neuronal stimulation and the dilatory response to breath holding act in an additive manner, whereas the constrictive CDB challenge competes with the concurrent neuronally derived vasodilation. For example, increased local demand for oxygen causes the release of adenosine, potassium, and hydrogen ions into the extracellular space, the latter of which may interfere with the customary relationship between PETCO2 and pH (Kontos et al, 1977).
The visual cortex is also supplied by two arterial pathways: the posterior and middle cerebral arteries. This unique vascular arrangement may alter the blood flow and BOLD response to local dilation and constriction relative to other cortical areas. Our analysis of optimal hemodynamic delay times also indicates that the visual cortex exhibits significantly different response dynamics to hypercapnic and hypocapnic modulations, which may alter our assessment of BH- and CDB-CVR using transient challenges.
Limitations of the Current Study
The BOLD contrast measured in our experiment reflects a complicated interaction of CBF, CBV, and the cerebral metabolic rate of oxygen consumption (CMRO2) (Buxton et al, 2004; He and Yablonskiy, 2007). It is frequently assumed that the effect of hypercapnia or hypocapnia on CMRO2 is negligible, despite controversy in the literature. A recent study by Zappe et al (2008) examined the effect of 6% CO2 inhalation on neuronal activity (and thus local CMRO2) in anesthetized monkeys using BOLD fMRI and electrophysiology. They observed significant depression of neuronal activity during mild hypercapnia, which is associated with an increased BOLD signal as local demands for oxygen were reduced. Taking these findings into account, the use of 4% CO2 inhalation to create our simulated ‘pathologic' basal vascular condition may also be reducing CMRO2, and this may detrimentally affect the accuracy of the CVR measurement.
There are also well-documented neuronal networks that are known to be associated with respiration or respiratory control (McKay et al, 2003; Pattinson et al, 2009), which are likely activated during inhalation of 4% CO2 and the resulting ventilatory response. These networks, which include regions of the supplementary motor cortex, superior precentral gyrus, subcortical gray matter, and brainstem, may also be activated by the voluntary respiratory challenges used in this study.
Therefore, our experimental protocol may alter CMRO2, both positively and negatively, and locally and globally. Further studies that explore alternative means of achieving global vasodilation or initiating vascular responses without simultaneously activating respiratory, motor, and visual networks would be necessary to better understand this issue.
This study focuses on vascular reactivity to transient CO2 challenges, which may reflect different mechanisms than steady-state CO2 modulations like those used by Zhao et al (2009). It would be valuable to ascertain how vascular responsiveness to short and prolonged stimuli are differentially affected by impaired compliance and basal vascular tone, because these tools may offer more complementary diagnostic information in elderly and diseased patients.
In addition, although we achieve excellent characterization of the challenge response using BOLD fMRI, it does not allow for straightforward quantification of blood flow and blood volume. It would be advantageous to understand the dynamic CBF and CBV responses to transient BH and CDB challenges, to best understand the BOLD measurements discussed in this study. Arterial spin labeling techniques, as used in the study by Zhao et al (2009), offer quantitative measurement of CBF and would provide specific physiologic insight into the vascular properties affected in our experiments. However, the respiratory challenges cause large, transient BOLD signal changes that confound the pairwise image subtraction used in arterial spin labeling analysis methods. The 4% CO2 condition also affects arterial transit times, making it difficult to quantitatively compare CBF between the two conditions.
At this time, the scope of our experiments does not allow for quantitative CBF and CBV measurements at the temporal resolution required to accurately characterize challenge response dynamics. Further improvements in arterial spin labeling and other MRI techniques may address these issues in the future.
Clinical Relevance
Kavec et al (2004) reported an elevated mean CBV of 3.7 mL/100 mL in patients with ⩾70% stenosis of a carotid artery versus 3.5 mL/100 mL in healthy controls using dynamic susceptibility contrast MRI. Using positron emission tomography, cortical CBV has been measured to be significantly increased in healthy volunteers during inhalation of 7% CO2 (3.7 mL/100 mL relative to 3.3 mL/100 mL during normal conditions, Ito et al, 2003). In our study, we use 4% CO2 inhalation to cause global vasodilation; therefore, we estimate that our simulated predilated condition is a reasonable approximation of the CBV changes observed in patients. Total CBV may not be the best indicator of volume changes associated with CO2 inhalation or autoregulatory dilation, which primarily involve the arteriolar resistance vessels, and emerging methods that isolate precapillary blood volume have been used to measure autoregulatory dilation of these vessels in stenosis patients (Donahue et al, 2010). However, it is difficult to directly compare these results with the existing hypercapnia literature to assess whether the precapillary volume changes in our study are comparable with clinical observations.
It is important to consider the different mechanisms driving the dilation in our 4% CO2 condition relative to the autoregulatory vasodilation prevalent in patients with early stages of hemodynamic impairment. Carrera et al (2009) showed no correlation between baseline cerebral autoregulation (pressure–flow relationship) and CVR (CO2–flow relationship), suggesting that these effects are based, at least in part, on distinct physiologic mechanisms. Therefore, the simulated pathologic condition created in this study is innately different than autoregulatory-driven vasodilated conditions. It is not clear whether the different mechanisms causing the decreased vascular tone would lead to different effects on hypercapnic and hypocapnic reactivity. Certainly in our simulated pathologic condition, CBF is globally increased beyond normal levels, whereas autoregulatory vasodilation would attempt to maintain stable CBF. Further characterization of this discrepancy is required to understand possible consequences on BOLD reactivity measurements.
The enhanced response to hypocapnia is observed in our simulated pathologic dilated condition and in chronic stroke patients, but not in most studies of elderly patients with hypertension, atherosclerosis, and other mild pathologies that are commonly associated with impaired vessel compliance and attenuated reactivity. Hypocapnia challenges may assist in the differentiation of compliant and noncompliant vasculature in populations that exhibit widespread alterations in vascular tone. This knowledge could aid clinicians in understanding the extent of vascular damage versus the extent of vascular stress associated with ischemic lesions.
Our results also support the use of simple CDB challenges to measure hypocapnic CVR, which can hopefully enable broader application of CVR measurements in clinical settings. For example, in studying the relationship between subarachnoid hemorrhage and delayed cerebral ischemia, Pennings et al (2004) observed enhanced hypocapnic reactivity in patients shortly after subarachnoid hemorrhage. This study required invasive imaging techniques to assess local hypocapnic CVR, and the transient hypocapnic challenge explored in this study may be of interest to the hemorrhagic stroke research community for probing the same parameters using noninvasive MRI methods. Another study using transcranial Doppler sonography to assess CVR showed that attenuated hypercapnic CVR was predictive of delayed cerebrovascular ischemia after subarachnoid hemorrhage (Carrera et al, 2010), indicating that BH and CDB challenges may be complementary techniques in these patients.
In summary, we have used a controlled experiment in healthy volunteers to determine the effect of baseline dilation of the cerebrovasculature on hypercapnic and hypocapnic CO2 reactivity independently of the effects of normal aging or impaired vascular compliance. We have shown that widespread protective vasodilation may manifest as enhanced reactivity to hypocapnia, which suggests that simple deep breathing challenges could offer clinical insights into the different mechanisms behind certain diseases and normal aging. We have also observed surprising deviations from normal vasodilatory and vasoconstrictive reactivity patterns in localized regions of the brain. Further work is necessary to characterize the metabolic effects of CO2 inhalation, respiratory challenges, and neuronal stimulation on our measurement of CVR.
Acknowledgments
The authors thank Stephen Knight for his assistance with the experiments.
The authors declare no conflict of interest.
Footnotes
This research was supported (in part) by the Intramural Research Program of the NIH, NINDS, as well as the EPSRC, UK MRC, Oxford NIHR Biomedical Research Centre, and Dunhill Medical Trust.
References
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable. NeuroImage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Andersson JA, Smith S, Jenkinson M.2008FNIRT—FMRIB's Non-linear Image Registration Tool [abstract]In: 14th Annual Meeting of the Organization for Human Brain Mapping; 15 June – 18 June 2008; Melbourne, Australia. Abstract 496
- Aso K, Ogasawara K, Sasaki M, Kobayashi M, Suga Y, Chida K, Otawara Y, Ogawa A. Preoperative cerebrovascular reactivity to acetazolamide measured by brain perfusion SPECT predicts development of cerebral ischemic lesions caused by microemboli during carotid endarterectomy. Eur J Nucl Med Mol Imaging. 2009;36:294–301. doi: 10.1007/s00259-008-0886-y. [DOI] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokkers RP, van Osch MJ, van der Worp HB, de Borst GJ, Mali WP, Hendrikse J. Symptomatic carotid artery stenosis: impairment of cerebral autoregulation measured at the brain tissue level with arterial spin-labeling MR imaging. Radiology. 2010;256:201–208. doi: 10.1148/radiol.10091262. [DOI] [PubMed] [Google Scholar]
- Bright MG, Bulte DB, Jezzard P, Duyn JH. Characterization of regional heterogeneity in cerebrovascular reactivity dynamics using novel hypocapnia task and BOLD fMRI. NeuroImage. 2009;48:166–175. doi: 10.1016/j.neuroimage.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte DP, Chiarelli PA, Wise RG, Jezzard P. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab. 2007;27:69–75. doi: 10.1038/sj.jcbfm.9600319. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. NeuroImage. 2004;23:220–233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Carrera E, Kurtz P, Badjatia N, Fernandez L, Claassen J, Lee K, Schmidt JM, Connolly ES, Marshall RS, Mayer SA. Cerebrovascular carbon dioxide reactivity and delayed cerebral ischemia after subarachnoid hemorrhage. Arch Neurol. 2010;67:434–439. doi: 10.1001/archneurol.2010.43. [DOI] [PubMed] [Google Scholar]
- Carrera E, Lee LK, Giannopoulos S, Marshall RS. Cerebrovascular reactivity and cerebral autoregulation in normal subjects. J Neurol Sci. 2009;285:191–194. doi: 10.1016/j.jns.2009.06.041. [DOI] [PubMed] [Google Scholar]
- Chang C, Thomason ME, Glover GH. Mapping and correction of vascular hemodynamic latency in the BOLD signal. NeuroImage. 2008;43:90–102. doi: 10.1016/j.neuroimage.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Wise RG, Gallichan D, Jezzard P. A calibration method for quantitative BOLD fMRI based on hyperoxia. NeuroImage. 2007;37:808–820. doi: 10.1016/j.neuroimage.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Derdeyn CP, Videen TO, Yundt KD, Fritsch SF, Carpenter DA, Grubb RL, Powers WJ. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodyamic impairment revisited. Brain. 2002;125:595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Sideso E, MacIntosh BJ, Kennedy J, Handa A, Jezzard P. Absolute arterial cerebral blood volume quantification using inflow vascular-space-occupancy with dynamic subtraction magnetic resonance imaging. J Cereb Blood Flow Metab. 2010;30:1329–1342. doi: 10.1038/jcbfm.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, van Laar PJ, van Zijl PCM, Stevens RD, Hendrikse J. Vascular space occupancy (VASO) cerebral blood volume-weighted MRI identifies hemodynamic impairment in patients with carotid artery disease. J Magn Reson Imaging. 2009;29:718–724. doi: 10.1002/jmri.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisted. NeuroImage. 2007;36:511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Goode SD, Krishan S, Alexakis C, Mahajan R, Auer DP. Precision of cerebrovascular reactivity assessment with use of different quantification methods for hypercapnia functional MR imaging. Am J Neuroradiol. 2009;30:972–977. doi: 10.3174/ajnr.A1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med. 2007;57:115–126. doi: 10.1002/mrm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins PM, Lynch CD, Cooney PT, Curseen KA. The microcirculation in experimental hypertension and aging. Cardiovasc Res. 1996;32:772–780. [PubMed] [Google Scholar]
- Ito H, Kanno I, Ibaraki M, Hatazawa J. Effect of aging on cerebral vascular response to PaCO2 changes in humans as measured by positron emission tomography. J Cereb Blood Flow Metab. 2002;22:997–1003. doi: 10.1097/00004647-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanno I, Ibaraki M, Hatazawa J, Miura S. Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2003;23:665–670. doi: 10.1097/01.WCB.0000067721.64998.F5. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Li TQ, Glover GH, Moseley ME. Cerebral blood flow-related signal changes during breath-holding. Am J Neuroradiol. 1999;20:1233–1238. [PMC free article] [PubMed] [Google Scholar]
- Kavec M, Usenius JP, Tuunanen PI, Rissanen A, Kauppinen RA. Assessment of cerebral hemodynamics and oxygen extraction using dynamic susceptibility contrast and spin echo blood oxygenation level-dependent magnetic resonance imaging: applications to carotid stenosis patients. NeuroImage. 2004;22:258–267. doi: 10.1016/j.neuroimage.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos HA, Raper AJ, Patterson JL. Analysis of vasoreactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke. 1977;8:358–360. doi: 10.1161/01.str.8.3.358. [DOI] [PubMed] [Google Scholar]
- Levitzky MG.(ed). (2003Pulmonary Physiology New York, NY: McGraw-Hill [Google Scholar]
- Maeda H, Matsumoto M, Handa N, Hougaku H, Ogawa S, Itoh T, Tsukamoto Y, Kamada T. Reactivity of cerebral blood flow to carbon dioxide in hypertensive patients: evaluation by the transcranial Doppler method. J Hypertens. 1994;12:191–197. [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, Mikulis DJ. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparision with arterial spin labeling MRI. Stroke. 2008;39:2021–8. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC.2002The neural basis for the control of breathing in humans determined using functional MRIPhD thesis, University of London
- McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol. 2003;95:1170–1178. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP-RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, Mayhew SD, Rogers R, Tracey I, Wise RG. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. NeuroImage. 2009;44:295–305. doi: 10.1016/j.neuroimage.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Pennings FA, Bouma GJ, Ince C. Direct observation of the human cerebral microcirculation during aneurysm surgery reveals increased arteriolar contractility. Stroke. 2004;35:1284–1288. doi: 10.1161/01.STR.0000126039.91400.cb. [DOI] [PubMed] [Google Scholar]
- Poeppel TD, Terborg C, Hautzel H, Herzog H, Witte OW, Mueller HW, Krause BJ. Cerebral haemodynamics during hypo- and hypercapnia; determination with simultaneous 15O-butanol-PET and transcranial Doppler sonography. Nuklearmedizin. 2007;46:93–100. [PubMed] [Google Scholar]
- Pollock JM, Deibler AR, Whitlow CT, Tan H, Kraft RA, Burdette JH, Maldjian JA. Hypercapnia-induced hyperperfusion: an underrecognized clinical entity. Am J Neuroradiol. 2009;30:378–385. doi: 10.3174/ajnr.A1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CG, Lenzi GL, Frackowiak RSJ, Jones T, Pozzilli C. Measurement of CBF and CMRO2 using the continuous inhalation of C15O2 and 15O2; Experimental validation using CO2 reactivity in the anaesthetised dog. J Neurol Sci. 1981;50:381–389. doi: 10.1016/0022-510x(81)90150-7. [DOI] [PubMed] [Google Scholar]
- Scouten A, Schwarzbauer C. Paced respiration with end-expiration technique offers superior BOLD signal repeatability for breath-hold studies. NeuroImage. 2008;43:250–257. doi: 10.1016/j.neuroimage.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Shiino A, Morita Y, Tsuji A, Maeda K, Ito R, Furukawa A, Matsuda M, Inubushi T. Estimation of cerebral perfusion reserve by blood oxygenation level-dependent imaging: comparison with single-photon emission computed tomography. J Cereb Blood Flow Metab. 2003;23:121–135. doi: 10.1097/01.WCB.0000037546.46809.CA. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Caltagirone C. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–2127. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, DeLuca M, Drobnjak I, Flitney D, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Glover GH. Controlled inspiration depth reduced variance in breath-holding-induced BOLD signal. NeuroImage. 2008;39:206–214. doi: 10.1016/j.neuroimage.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. NeuroImage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Meyer JS, Sakai F, Yamamoto M. Normal human aging and cerebral vasoconstrictive responses to hypocapnia. J Neurol Sci. 1979;44:87–94. doi: 10.1016/0022-510x(79)90226-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Meyer JS, Sakai F, Yamaguchi F. Aging and cerebral vasodilator responses to hypercarbia. Arch Neurol. 1980;37:489–496. doi: 10.1001/archneur.1980.00500570037005. [DOI] [PubMed] [Google Scholar]
- Young WL, Prohovnik I, Ornstein E, Ostapkovich N, Matteo RS. Cerebral blood flow reactivity to changes in carbon dioxide calculated using end-tidal versus arterial tensions. J Cereb Blood Flow Metab. 1991;11:1031–1035. doi: 10.1038/jcbfm.1991.171. [DOI] [PubMed] [Google Scholar]
- Zappe AC, Uludag K, Oeltermann A, Ugurbil K, Logothetis NK. The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb Cortex. 2008;18:2666–2673. doi: 10.1093/cercor/bhn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhao P, Alsop DC, Abduljalil A, Selim M, Lipsitz L, Novak P, Caplan L, Hu K, Novak V. Vasoreactivity and peri-infarct hyperintensities in stroke. Neurology. 2009;72:643–649. doi: 10.1212/01.wnl.0000342473.65373.80. [DOI] [PMC free article] [PubMed] [Google Scholar]