Abstract
Aim:
Analyzing safety aspects of a drug from individual studies can lead to difficult-to-interpret results. The aim of this paper is therefore to assess the general safety and tolerability, including incidences of the most common adverse events (AEs), of vildagliptin based on a large pooled database of Phase II and III clinical trials.
Methods:
Safety data were pooled from 38 studies of ≥12 to ≥104 weeks’ duration. AE profiles of vildagliptin (50 mg bid; N = 6116) were evaluated relative to a pool of comparators (placebo and active comparators; N = 6210). Absolute incidence rates were calculated for all AEs, serious AEs (SAEs), discontinuations due to AEs, and deaths.
Results:
Overall AEs, SAEs, discontinuations due to AEs, and deaths were all reported with a similar frequency in patients receiving vildagliptin (69.1%, 8.9%, 5.7%, and 0.4%, respectively) and patients receiving comparators (69.0%, 9.0%, 6.4%, and 0.4%, respectively), whereas drug-related AEs were seen with a lower frequency in vildagliptin-treated patients (15.7% vs 21.7% with comparators). The incidences of the most commonly reported specific AEs were also similar between vildagliptin and comparators, except for increased incidences of hypoglycemia, tremor, and hyperhidrosis in the comparator group related to the use of sulfonylureas.
Conclusions:
The present pooled analysis shows that vildagliptin was overall well tolerated in clinical trials of up to >2 years in duration. The data further emphasize the value of a pooled analysis from a large safety database versus assessing safety and tolerability from individual studies.
Keywords: type 2 diabetes, dipeptidyl peptidase-4, edema, safety, vildagliptin
Introduction
Vildagliptin is an orally effective dipeptidyl peptidase-4 (DPP-4) inhibitor that has been studied in a large clinical program as monotherapy and combination therapy.1 It binds covalently to the catalytic site of DPP-4, eliciting prolonged enzyme inhibition. This raises intact glucagon-like peptide-1(GLP-1) levels both after meal ingestion and in the fasting state. By increasing concentrations of active GLP-1, vildagliptin improves β- and α-cell sensitivity to glucose.2 This results in glucose-sensitive modulation of insulin and glucagon secretion, improving both fasting and postprandial glycemia, with a low risk for hypoglycemia and no weight gain.
Areas of potential safety concern related to type 2 diabetes (T2DM) itself (ie, cardiovascular and hepatic safety), as well as potential safety concerns specific to DPP-4 inhibitors (ie, immune system, skin, and pancreatitis), have been analyzed previously for vildagliptin based on a large pooled database, with no increased risks identified versus comparators.3,4 However, other safety aspects, such as general safety and tolerability, including incidences of most common specific adverse events (AEs), have so far been reviewed in the literature from individual studies only.1,5,6 Although this is often the only possible approach early in the development of a new drug, such data from single and often relatively small studies are less reliable than analyses from larger datasets. Furthermore, specific design features, study duration, sample size, and, in particular, the comparator chosen for individual studies can influence the AE reporting rates in a specific study, which needs to be weighed against the overall experience in a clinical trial program. For example, one issue that arose from an individual study with vildagliptin was related to edema. In contrast to other studies, Bolli et al,7 in a trial comparing vildagliptin and pioglitazone as add-on therapy with metformin, reported peripheral edema as the most common AE for vildagliptin with an incidence even somewhat higher than for the thiazolidinedione (TZD) itself.
Based on these considerations, it was of interest to assess the general safety and tolerability of vildagliptin, as well as the specific risk of edema-related AEs with vildagliptin treatment, using the previously described large pooled database of vildagliptin Phase II and III clinical studies.4 We report here the results of these new pooled safety analyses.
Methods
Populations
The safety analyses are based on the previously reported pool of 38 Phase II and Phase III studies that used vildagliptin as monotherapy or in combination with metformin, TZDs, sulfonylureas (SUs), or insulin for ≥12 weeks up to ≥104 weeks.4
For the analysis of overall AEs, AEs by system organ class (SOC) or preferred term (PT), serious AEs (SAEs), discontinuations due to AEs, and deaths, as well as edema-related AEs, the “all studies (excluding open-label) safety population”, which excludes open-label studies in order to minimize reporting bias, was used. Supplementary Table 1 briefly describes each of the studies included in this pooled dataset.
Peto odds ratios (ORs) were additionally calculated for edema-related AEs, as a single study had previously reported a higher incidence with vildagliptin.7 Calculation of ORs requires a comparator; thus, calculation of ORs and the resulting Forest plot for edema-related AEs used data pooled from all controlled studies excluding open-label trials. This population is termed “all controlled studies (excluding open-label) safety population” (see Supplementary Table 1 for details).
In addition to being analyzed in the all studies (excluding open-label) safety population, confirmed hypoglycemic episodes (as defined in this article) were also assessed in monotherapy (monotherapy [excluding open-label] safety population), which was deemed more appropriate considering that the risk of hypoglycemia is influenced by antidiabetic background therapy. Confirmed hypoglycemia is thus not reflected under the most common AEs in the all studies (excluding open-label) safety population in Table 1. The monotherapy (excluding open-label) safety population includes 21 studies (see Supplementary Table 1 for details on the contributing studies).
Table 1.
Adverse event (AE) summary and most common AEs (all studies [excluding open-label] safety population)
| n (%) |
Vildagliptin 50 mg bid |
Comparatorsa |
|---|---|---|
| N = 6116 | N = 6210 | |
| Mean exposure (weeks) | 62.4 | 54.7 |
| AEs | 4225 (69.1) | 4228 (69.0) |
| Drug-related AEs | 961 (15.7) | 1349 (21.7) |
| Serious AEs | 545 (8.9) | 557 (9.0) |
| Discontinuations due to AEsb | 347 (5.7) | 400 (6.4) |
| Deaths | 24 (0.4) | 23 (0.4) |
| Most common AEs (occurring in ≥3% of patients in either group): | ||
| Nasopharyngitis | 577 (9.4) | 528 (8.5) |
| Headache | 431 (7.0) | 371 (6.0) |
| Dizziness | 390 (6.4) | 460 (7.4) |
| Back pain | 356 (5.8) | 321 (5.2) |
| Diarrhea | 345 (5.6) | 418 (6.7) |
| Upper respiratory tract infection | 317 (5.2) | 254 (4.1) |
| Bronchitis | 297 (4.9) | 278 (4.5) |
| Hypertension | 297 (4.9) | 315 (5.1) |
| Influenza | 290 (4.7) | 282 (4.5) |
| Arthralgia | 289 (4.7) | 236 (3.8) |
| Nausea | 247 (4.0) | 268 (4.3) |
| Pain in extremity | 217 (3.5) | 238 (3.8) |
| Fatigue | 210 (3.4) | 253 (4.1) |
| Cough | 206 (3.4) | 210 (3.4) |
| Urinary tract infection | 204 (3.3) | 185 (3.0) |
| Asthenia | 198 (3.2) | 306 (4.9) |
| Tremor | 184 (3.0) | 471 (7.6) |
| Edema peripheral | 180 (2.9) | 219 (3.5) |
| Hyperhidrosis | 169 (2.8) | 422 (6.8) |
Notes:
Comparators = placebo plus active comparators;
Only AEs that caused the study drug to be permanently discontinued are included.
Assessments
All AEs were recorded and assessed by the investigator as to the severity and possible relationship to the study medication. This included laboratory abnormalities if considered an AE by the investigator. All laboratory assessments were performed by central laboratories (for details, see Ligueros-Saylan et al4).
Confirmed hypoglycemia was defined as symptoms suggestive of low blood glucose confirmed by self-monitored blood glucose measurement <3.1 mmol/L plasma glucose equivalent.
Standardization of terms
AEs were encoded in all studies using the Medical Dictionary for Regulatory Activities (MedDRA, Version 12.1) system. This is a medically validated terminology database developed by the International Conference on Harmonization. Within the MedDRA, AEs are grouped by SOC, eg, “cardiac disorders” or “gastrointestinal disorders”. Within an SOC, specific AEs are identified by PT. The PTs included in the analysis of edema-related AEs are allergic edema, generalized edema, local swelling, localized edema, edema, peripheral edema, pitting edema, skin edema, skin swelling, and swelling.
Data analysis
For AEs, SAEs, discontinuations due to AEs, and deaths, incidences were calculated as number of patients with an event divided by the number of patients in the treatment group. For edema-related AEs (as defined previously), exposure-adjusted incidences were additionally calculated as number of patients having events per 100 subject-year exposure (SYE), defined as 100×(number of patients with an event divided by the total exposure time in years).
To further compare edema-related AEs between vildagliptin and comparators, for each trial, Peto OR and the corresponding 95% confidence interval (CI) were calculated. The pooled estimate was obtained using a fixed-effect model and presented in a forest plot. An OR below unity is indicative of a treatment effect favoring vildagliptin. Correction for continuity using the inverse of the opposite arm size was used when zero events occurred.8 This correction causes less bias than the standard continuity correction of 0.5 when the sizes of the treatment arms are unbalanced.9
Safety data of vildagliptin 50 mg bid (the highest approved and most commonly used dosage of the drug) are reported along with pooled safety data of all comparators (active or placebo) from the safety populations.
Ethics and good clinical practice
All study participants provided written informed consent. All protocols were approved by the independent ethics committee/institutional review board at each study site or country. All studies were conducted using good clinical practice and in accordance with the Declaration of Helsinki.
Results
Exposure and demography
As detailed in Ligueros-Saylan et al,4 in the all studies (excluding open-label) safety population, 6116 patients received vildagliptin 50 mg bid (representing 7313.6 SYE) and 6210 patients received any comparator (representing 6512.7 SYE). The comparators group included placebo (23.7%), SUs (41.7%), metformin (18.8%), TZDs (12.3%), and acarbose (3.5%).
The mean duration of exposure was 62.4 weeks with vildagliptin 50 mg bid and 54.7 weeks with comparators (Table 1). This allows for direct comparisons between the two groups and provides a conservative estimate, as the slightly longer exposure with vildagliptin tends to favor the comparator group.
The demographic and baseline characteristics of patients in the all studies (excluding open-label) safety population have also been described previously.4 In brief, the population studied was representative of a broad spectrum of T2DM patients, with a mean age, body mass index, glycosylated hemoglobin A, fasting plasma glucose, and duration of T2DM of approximately 56 years, 31.4 kg/m2, 8.1%, 9.8 mmol/L, and >4 years, respectively, and with nearly one-third of patients having some degree of renal insufficiency (glomerular filtration rate [Modification of Diet in Renal Disease] ≤80 mL/min per 1.73 m2).
Overall safety and tolerability
Tables 1 and 2 report AE profiles for vildagliptin 50 mg bid and comparators in the all studies (excluding open-label) safety population.
Table 2.
Adverse events (AEs) by system organ class (SOC) (all studies [excluding open-label] safety population)
| n (%) | Vildagliptin 50 mg bid | Comparatorsa |
|---|---|---|
| N = 6116 | N = 6210 | |
| Mean exposure (weeks) | 62.4 | 54.7 |
| AEs by SOC: | ||
| Blood and lymphatic system disorders | 125 (2.0) | 114 (1.8) |
| Cardiac disorders | 375 (6.1) | 375 (6.0) |
| Congenital, familial, and genetic disorders | 12 (0.2) | 13 (0.2) |
| Ear and labyrinth disorders | 189 (3.1) | 221 (3.6) |
| Endocrine disorders | 40 (0.7) | 32 (0.5) |
| Eye disorders | 368 (6.0) | 356 (5.7) |
| Gastrointestinal disorders | 1440 (23.5) | 1393 (22.4) |
| General disorders/administration site conditions | 884 (14.5) | 1069 (17.2) |
| Hepatobiliary disorders | 102 (1.7) | 99 (1.6) |
| Immune system disorders | 63 (1.0) | 63 (1.0) |
| Infections and infestations | 2162 (35.3) | 2014 (32.4) |
| Injury, poisoning, and procedural complications | 595 (9.7) | 522 (8.4) |
| Investigations | 368 (6.0) | 427 (6.9) |
| Metabolism and nutrition disorders | 476 (7.8) | 706 (11.4) |
| Musculoskeletal and connective tissue disorders | 1374 (22.5) | 1313 (21.1) |
| Benign, malignant, and unspecified neoplasms | 149 (2.4) | 144 (2.3) |
| Nervous system disorders | 1320 (21.6) | 1474 (23.7) |
| Pregnancy, puerperium and perinatal conditions | 2 (0.0) | 8 (0.1) |
| Psychiatric disorders | 480 (7.8) | 474 (7.6) |
| Renal and urinary disorders | 291 (4.8) | 255 (4.1) |
| Reproductive system and breast disorders | 241 (3.9) | 220 (3.5) |
| Respiratory, thoracic and mediastinal disorders | 601 (9.8) | 570 (9.2) |
| Skin and subcutaneous tissue disorders | 769 (12.6) | 893 (14.4) |
| Social circumstances | 12 (0.2) | 3 (0.0) |
| Surgical and medical procedures | 18 (0.3) | 11 (0.2) |
| Vascular disorders | 475 (7.8) | 484 (7.8) |
Note:
Comparators = placebo plus active comparators.
Overall AEs, SAEs, discontinuations due to AEs, and deaths were all reported with a similar frequency in patients receiving vildagliptin (69.1%, 8.9%, 5.7%, and 0.4%, respectively) and patients receiving comparators (69.0%, 9.0%, 6.4%, and 0.4%, respectively), and drug-related AEs were seen with a lower frequency in vildagliptin-treated patients (15.7% vs 21.7% with comparators) (Table 1).
When the reported AEs were analyzed by SOC (Table 2), the incidences with vildagliptin and comparators were also similar overall. The four SOCs with the highest incidence of AEs were infections and infestations (35.3% for vildagliptin vs 32.4% for comparators), gastrointestinal disorders (23.5% vs 22.4%), musculoskeletal and connective tissue disorders (22.5% vs 21.1%), and nervous system disorders (21.6% vs 23.7%). Of note, there were no imbalances between vildagliptin and comparators in the overall reporting rates under the cardiac (6.1% with vildagliptin vs 6.0% with comparators), hepatobiliary (1.7% vs 1.6%), skin (12.6% vs 14.4%), and vascular (7.8% in both groups) SOCs. The most notable difference was observed in the metabolism/nutrition SOC, with incidences of 7.8% for vildagliptin and 11.4% for comparators, which were mainly due to hypoglycemia.
Overall, there were no appreciable trends in SAEs reported, and the majority of SAEs were scattered across many different SOCs. The primary SOC with the highest incidence of SAEs was cardiac disorders, with no imbalance between vildagliptin (1.7%) and comparators (1.9%). The only other SOCs with an incidence of SAEs ≥ 1% were infections and infestations (1.5% with vildagliptin 50 mg bid vs 1.4% with comparators); benign, malignant, and unspecified neoplasms (including cysts and polyps) (1.2% vs 1.1%); nervous system disorders (1.1% vs 1.0%); and gastrointestinal disorders (1.0% vs 0.9%).
There were no meaningful imbalances across the treatment groups in the incidence of AEs leading to discontinuation in any SOC. The SOC with the highest incidence of AEs leading to discontinuation was gastrointestinal disorders (1.2% with vildagliptin vs 1.4% with comparators).
A summary of the most commonly (≥3% in either group) reported specific AEs for vildagliptin 50 mg bid and comparators is also provided in Table 1. All of the individual AEs were reported with a low frequency of <10%. The most common AEs across treatment groups were nasopharyngitis (9.4% with vildagliptin vs 8.5% with comparators), dizziness (6.4% vs 7.4%), headache (7.0% vs 6.0%), and diarrhea (5.6% vs 6.7%). The incidences of the most commonly reported AEs were overall similar between vildagliptin and comparators. The most notable differences were lower incidences with vildagliptin of tremor (3.0% vs 7.6%) and hyperhidrosis (2.8% vs 6.8%). Furthermore, confirmed hypoglycemia was reported less frequently with vildagliptin (1.7%) than with comparators (5.8%). Hypoglycemia was additionally assessed in a pooled monotherapy population, which was deemed more appropriate than the assessment in the overall pooled data-set, considering that the risk of hypoglycemia is influenced by antidiabetic background therapy. In the pooled monotherapy safety population, confirmed hypoglycemic events were reported in 0.5% of patients treated with vildagliptin versus 0.3% treated with placebo and 0.6% treated with all comparators (the comparator group consisted of 38.3% metformin, 20.7% placebo, 17.9% SU, 7.2% acarbose, and 15.9% TZD).
Edema
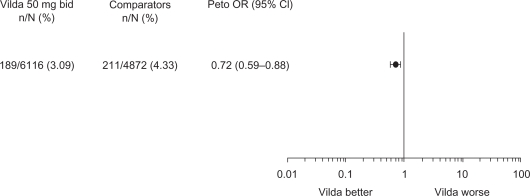
As depicted in Figure 1, there was no evidence of an increased risk of edema-related AEs with vildagliptin 50mg bid relative to comparators. The Peto OR for vildagliptin 50 mg bid was 0.72 (95% CI 0.59–0.88), indicating a statistically significant risk reduction versus the comparator group.
Figure 1.
Incidences and Peto odds ratio (OR) for edema-related adverse events with vildagliptin 50 mg bid versus comparators (placebo and active comparators) in the all controlled studies (excluding open-label) safety population.
Abbreviation: Vilda, vildagliptin
The overall incidence of any edema-related AE was low in both treatment groups (Table 3). The unadjusted and SYE-adjusted incidences of any edema-related AEs were lower for vildagliptin than for comparators (Table 1). The most commonly reported edema-related AE was peripheral edema, and for this AE the incidence was also lower with vildagliptin (2.9%, 2.46 events per 100 SYE) than with comparators (3.5%, 3.36 events per 100 SYE). For all other specific edema-related AEs, the SYE-adjusted incidences with vildagliptin were the same as or lower than with comparators.
Table 3.
Edema-related adverse events (AEs) (all studies [excluding open-label] safety population)
| Vildagliptin 50 mg bid | Comparatorsa | |
|---|---|---|
| N = 6116 | N = 6210 | |
| n (%) | ||
| Subject-year exposure adjusted | ||
| Any edema-related AE | 198 (3.2) | 242 (3.9) |
| 2.71 | 3.72 | |
| Generalized edema | 2 (0.0) | 2 (0.0) |
| 0.03 | 0.03 | |
| Local swelling | 1 (0.0) | 1 (0.0) |
| 0.01 | 0.02 | |
| Edema | 10 (0.2) | 9 (0.1) |
| 0.14 | 0.14 | |
| Peripheral edema | 180 (2.9) | 219 (3.5) |
| 2.46 | 3.36 | |
| Pitting edema | 9 (0.1) | 12 (0.2) |
| 0.12 | 0.18 | |
| Skin swelling | 0 (0.0) | 1 (0.0) |
| 0.00 | 0.02 | |
Note:
Comparators = placebo plus active comparators.
Discussion
The present paper has evaluated in a large pooled database safety aspects of the DPP-4 inhibitor vildagliptin that were previously assessed only from individual study results. Although the latter approach represents generally a good approach to judging the efficacy of a drug, it has considerable limitations when assessing safety and tolerability and can lead to difficult-to-interpret or even misleading results. On the one hand, sample sizes of individual trials are often too small to reliably assess whether any imbalances observed in individual AEs reflect a true excess over the comparator treatment studied or rather a chance finding. On the other hand, the study duration, the safety surveillance measures, and, in particular, the comparator chosen for individual studies can influence the AE reporting rates in a specific study. Another complication arises if the results of an individual study are extrapolated as being representative for the overall safety of a drug, as happens in the literature, especially if pooled data are not available.
For vildagliptin, a higher incidence of peripheral edema, for example, was observed in a study that compared the drug with the TZD pioglitazone, for which edema is a known side effect.7 In contrast, the new pooled analysis presented here did not confirm an increased incidence of edema-related events with vildagliptin but rather showed a statistically significant risk reduction versus the comparator group (OR = 0.72). Peripheral edema specifically occurred at an incidence rate of 2.46 events per 100 SYE with vildagliptin 50 mg bid versus 3.36 events per 100 SYE with comparators. Of note, only 12% of patients in the comparator group were treated with TZDs. Another study reported an imbalance with vildagliptin versus comparator treatment for the AE of hypertension.10 In contrast, hypertension was well balanced when analyzed in the large pooled dataset (4.9% with vildagliptin vs 5.1% with comparators). These examples clearly highlight the value and importance of pooled safety analyses.
The safety of vildagliptin versus all comparators was previously assessed with regard to organs, systems, or tissues of particular interest in T2DM and areas of potential concern with DPP-4 inhibitors.4 The meta-analyses indicated that vildagliptin was not associated with an increased risk of hepatic events or hepatic enzyme elevations indicative of drug-induced liver injury, pancreatitis, skin-related toxicity, or infections. In line with these results, the data presented here did not show any imbalances between vildagliptin and comparators for AEs in the SOCs of hepatobiliary disorders, skin and subcutaneous tissue disorders and infection and infestations.
The present pooled analysis further shows a general safety profile of vildagliptin 50 mg bid in clinical trials of up to >2 years in duration that was very similar to that of comparators regarding the overall incidences of AEs, SAEs, discontinuations due to AEs, and deaths. This also holds true when AEs were analyzed by SOCs or the most common AEs were evaluated. The only notable differences were for confirmed hypoglycemia and the likely hypoglycemia-related AEs of tremor and hyperhidrosis, for which lower incidences were observed with vildagliptin than with comparator treatment, mainly due to the use of SUs as a comparator in several studies (representing >40% of the comparator group). Because hypoglycemia incidences are largely influenced by antidiabetic background therapy, it is important to review hypoglycemia rates for specific treatment regimens. The overall safety population used for the present safety analyses consists of a broad range of studies with different treatment regimens, including add-on to insulin; thus, the frequency of confirmed hypoglycemia was also assessed in a pooled monotherapy safety population. Of the patients treated with vildagliptin monotherapy, 0.5% reported confirmed hypoglycemic episodes, which is very similar to the rate found with placebo (0.3%).
Taken together, the present pooled analysis provides a more comprehensive and reliable assessment of the general safety and tolerability of vildagliptin than can be obtained by extracting safety data from individual studies only.
Supplementary material
Table S1.
Vildagliptin studies contributing to safety analyses
| Study no. | Study description | Phase/population | Randomized patients* | Treatment duration** | Publication*** |
|---|---|---|---|---|---|
| Monotherapy | |||||
| 1 | Placebo-controlled dose-ranging study in drug-naïve T2DM patients (HbA1c 6.8%–10%) | II/a,b,c | 279 | 12 weeks | 1 |
| 2 | Uncontrolled 40-week extension to Study 1 | II/a,c | 141 | 52 weeks | Not available |
| 3 | Placebo-controlled low-dose efficacy/safety study in drug-naïve T2DM patients (HbA1c 6.8%–11%) | II/a,b,c | 100 | 12 weeks | 2 |
| 4 | Placebo-controlled dose-ranging study (efficacy/safety) in drug-naïve T2DM patients (HbA1c 7.5%–10%) | III/a,b,c | 632 | 24 weeks | 3 |
| 5 | Uncontrolled 28-week extension to Study 4 | III/a,c | 440 | 52 weeks | NCT00138541 |
| 6 | Placebo-controlled long-term efficacy/safety study in drug-naïve T2DM patients with mild hyperglycemia (HbA1c 6.2%–7.5%) | III/a,b,c | 306 | 52 weeks | 4,5 |
| 7 | Placebo-controlled 52-week extension to Study 6 | III/a,b,c | 131 | 104 weeks | 6 |
| 8 | Active-controlled (metformin) long-term efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 780 | 52 weeks | 7 |
| 9 | Active-controlled (metformin) 52-week extension to Study 8 | III/a,b,c | 463 | 104 weeks | 8 |
| 10 | Active-controlled (gliclazide) long-term efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 1092 | 104 weeks | 9 |
| 11 | Active-controlled (acarbose) efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 661 | 24 weeks | 10 |
| 12 | Active-controlled (rosiglitazone) efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 786 | 24 weeks | 11 |
| 13 | Active-controlled (rosiglitazone) 80-week extension to Study 12 | III/a,b,c | 598 | 104 weeks | 12 |
| 14 | Active-controlled (pioglitazone) dose regimen comparison study in drug-naïve T2DM patients (HbA1c 9%–11%) | III/a,b,c | 273 | 12 weeks | NCT00101673 |
| 15 | Placebo-controlled efficacy/safety study in patients with IGT | III/a,b,c | 179 | 12 weeks | 13 |
| 16 | Placebo-controlled mechanistic study (β-cell function) in drug-naïve T2DM patients with mild hyperglycemia (HbA1c ≤ 7.5%) | III/a,b,c | 89 | 52 weeks | NCT00260156 |
| 17 | Placebo-controlled dose-ranging study (efficacy/safety) in drug-naïve T2DM patients (HbA1c 7.5%–10%) | III/a,b,c | 354 | 24 weeks | 14 |
| 18 | Active-controlled (metformin) efficacy/safety study in drug-naïve elderly (≥65 years) T2DM patients (HbA1c 7%–9%) | III/a,b,c | 335 | 24 weeks | 15 |
| Combination therapy with metformin | |||||
| 19 | Placebo-controlled dose-selection study in patients inadequately controlled by metformin (HbA1c 7.0%–9.5%) | II/a,b | 132 | 12 weeks | 16 |
| 20 | Placebo-controlled 40-week extension to Study 19 | II/a,b | 71 | 52 weeks | 16 |
| 21 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) | III/a,b | 544 | 24 weeks | 17 |
| 22 | Uncontrolled 28-week extension to Study 21 | III/a | 417 | 52 weeks | NCT00138515 |
| 23 | Active-controlled (glimepiride) long-term efficacy/safety study in T2DM patients treated with metformin (HbA1c > 6.5%–8.5%) | III/a,b | 3118 | ≥104 weeks | 18,19 |
| 24 | Active-controlled (gliclazide) long-term efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) | III/a,b | 1007 | 52 weeks | 20 |
| 25 | Active-controlled (pioglitazone) long-term efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) | III/a,b | 576 | 52 weeks | 21,22 |
| 26 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) to compare a.m. vs p.m. dosing regimens | III/a,b | 370 | 24 weeks | 23 |
| 27 | Efficacy/safety study in T2DM patients treated with metformin (HbA1c 6.5%–9%) to compare vildagliptin as add-on to metformin vs uptitration of metformin | III/a,b,c**** | 914 | 24 weeks | 24 |
| 28 | Efficacy/safety study of initial fixed combination therapy of vildagliptin and metformin in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c**** | 1179 | 24 weeks | 25 |
| Combination therapy with TZD | |||||
| 29 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled by TZD (HbA1c 7.5%–11%) | III/a,b | 463 | 24 weeks | 26 |
| 30 | Uncontrolled 28-week extension to Study 29 | III/a | 312 | 52 weeks | NCT00138554 |
| 31 | Initial combination (vildagliptin/pioglitazone) efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c**** | 607 | 24 weeks | 27 |
| Combination therapy with SU | |||||
| 32 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled by SU (HbA1c 7.5%–11%) | III/a,b | 515 | 24 weeks | 28 |
| 33 | Uncontrolled 28-week extension to Study 32 | III/a | 332 | 52 weeks | NCT00138580 |
| Combination therapy with insulin | |||||
| 34 | Placebo-controlled efficacy/safety study in T2DM patients treated with insulin (HbA1c 7.5%–11%) | III/a,b | 296 | 24 weeks | 29 |
| 35 | Uncontrolled 28-week extension to Study 34 | III/a | 200 | 52 weeks | 30 |
Notes:
For extension studies: patients who entered extension;
For extension studies: duration of core + extension study.
ClinicalTrials.gov identifier number is provided if data are not yet published;
Monotherapy arms only. Population a = all studies (excluding open-label) safety population. Population b = all controlled studies (excluding open-label) safety population; Population c = monotherapy (excluding open-label) safety population.
Abbreviations: HbA1c, glycosylated hemoglobin A; IGT, impaired glucose tolerance; SU, sulfonylurea; T2DM, type 2 diabetes; TZD, thiazolidinedione.
References
- 1.Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7:692–698. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 2.Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38:423–428. doi: 10.1055/s-2006-944546. [DOI] [PubMed] [Google Scholar]
- 3.Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res. 2007;39:218–223. doi: 10.1055/s-2007-970422. [DOI] [PubMed] [Google Scholar]
- 4.Scherbaum WA, Schweizer A, Mari A, et al. Efficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycemia. Diabetes Obes Metab. 2008;10:675–682. doi: 10.1111/j.1463-1326.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 5.Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed {beta}-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab. 2008;93:103–109. doi: 10.1210/jc.2007-1639. [DOI] [PubMed] [Google Scholar]
- 6.Scherbaum WA, Schweizer A, Mari A, et al. Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab. 2008;10:1114–1124. doi: 10.1111/j.1463-1326.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 7.Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA1c over one year in drug-naïve patients with type 2 diabetes. Diabet Med. 2007;24:955–961. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 8.Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naive patients with type 2 diabetes: comparison with metformin. Horm Metab Res. 2008;40:892–895. doi: 10.1055/s-0028-1082334. [DOI] [PubMed] [Google Scholar]
- 9.Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naive patients with type 2 diabetes. Horm Metab Res. 2009;41:905–909. doi: 10.1055/s-0029-1234042. [DOI] [PubMed] [Google Scholar]
- 10.Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008;25:435–441. doi: 10.1111/j.1464-5491.2008.02391.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenstock J, Baron MA, Dejager S, et al. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care. 2007;30:217–223. doi: 10.2337/dc06-1815. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J, Niggli M, Maldonado-Lutomirsky M. Long-term 2-year safety and efficacy of vildagliptin compared with rosiglitazone in drug-naive patients with type 2 diabetes mellitus. Diab Obes Metab. 2009;11:571–578. doi: 10.1111/j.1463-1326.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock J, Foley JE, Rendell M, et al. Effects of the DPP-4 inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose tolerance. Diabetes Care. 2008;31:30–35. doi: 10.2337/dc07-1616. [DOI] [PubMed] [Google Scholar]
- 14.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–138. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Schweizer A, Dejager S, Bosi E. Comparison of vildagliptin and metformin monotherapy in elderly patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Obes Metab. 2009;11:804–812. doi: 10.1111/j.1463-1326.2009.01051.x. [DOI] [PubMed] [Google Scholar]
- 16.Ahrén B, Gomis R, Standl E, et al. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–2880. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- 17.Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–893. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 18.Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptin vs glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157–166. doi: 10.1111/j.1463-1326.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Dejager S, Ahren B, et al. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglyceamic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab. 2010;12(9):780–789. doi: 10.1111/j.1463-1326.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- 20.Filozof C, Gautier JF. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diab Med. 27(3):318–326. doi: 10.1111/j.1464-5491.2010.02938.x. 201; [DOI] [PubMed] [Google Scholar]
- 21.Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008;10:82–90. doi: 10.1111/j.1463-1326.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 22.Bolli G, Dotta F, Colin L, et al. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab. 2009;11:589–595. doi: 10.1111/j.1463-1326.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 23.Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res. 2009;41:368–373. doi: 10.1055/s-0028-1104604. [DOI] [PubMed] [Google Scholar]
- 24.Filozof C, Schwartz S, Foley JE. Effect of metformin as add-on therapy to a low dose metformin. World J Diabetes. 2010;1:19–26. doi: 10.4239/wjd.v1.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11:506–515. doi: 10.1111/j.1463-1326.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- 26.Garber AJ, Schweizer A, Baron MA, et al. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–174. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstock J, Baron MA, Camisasca RP, et al. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared to component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:175–185. doi: 10.1111/j.1463-1326.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 28.Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–1056. doi: 10.1111/j.1463-1326.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca V, Schweizer A, Albrecht D, et al. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca V, Baron M, Shao Q, Dejager S. Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus. Horm Metab Res. 2008;40:427–430. doi: 10.1055/s-2008-1058090. [DOI] [PubMed] [Google Scholar]
Acknowledgments
The authors acknowledge the patients, investigators, and staff at participating sites for all the studies. This work was funded by Novartis Pharmaceuticals Corporation.
Footnotes
Declaration
All authors are employees of Novartis.
References
- 1.Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs. 2010;70(16):2089–2112. doi: 10.2165/11206370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Ahrén B, Schweizer A, Dejager S, et al. Vildagliptin improves islet glucose sensing in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1236–1243. doi: 10.1210/jc.2008-2152. [DOI] [PubMed] [Google Scholar]
- 3.Schweizer A, Dejager S, Foley JE, et al. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large phase III type 2 diabetes population. Diabetes Obes Metab. 2010;12:485–494. doi: 10.1111/j.1463-1326.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 4.Ligueros-Saylan M, Foley JE, Schweizer A, et al. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials. Diabetes Obes Metab. 2010;12:495–509. doi: 10.1111/j.1463-1326.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- 5.Gerich J. DPP-4 inhibitors: what may be the clinical differentiators? Diabetes Res Clin Pract. 2010;90:131–140. doi: 10.1016/j.diabres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2010;30(5):463–484. doi: 10.1592/phco.30.5.463. [DOI] [PubMed] [Google Scholar]
- 7.Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008;10:82–90. doi: 10.1111/j.1463-1326.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox DR. The continuity correction. Biochmetrika. 1970;57:217–219. [Google Scholar]
- 9.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 10.Ahrén B, Gomes R, Standl E, et al. Twelve and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;12:2874–2880. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Vildagliptin studies contributing to safety analyses
| Study no. | Study description | Phase/population | Randomized patients* | Treatment duration** | Publication*** |
|---|---|---|---|---|---|
| Monotherapy | |||||
| 1 | Placebo-controlled dose-ranging study in drug-naïve T2DM patients (HbA1c 6.8%–10%) | II/a,b,c | 279 | 12 weeks | 1 |
| 2 | Uncontrolled 40-week extension to Study 1 | II/a,c | 141 | 52 weeks | Not available |
| 3 | Placebo-controlled low-dose efficacy/safety study in drug-naïve T2DM patients (HbA1c 6.8%–11%) | II/a,b,c | 100 | 12 weeks | 2 |
| 4 | Placebo-controlled dose-ranging study (efficacy/safety) in drug-naïve T2DM patients (HbA1c 7.5%–10%) | III/a,b,c | 632 | 24 weeks | 3 |
| 5 | Uncontrolled 28-week extension to Study 4 | III/a,c | 440 | 52 weeks | NCT00138541 |
| 6 | Placebo-controlled long-term efficacy/safety study in drug-naïve T2DM patients with mild hyperglycemia (HbA1c 6.2%–7.5%) | III/a,b,c | 306 | 52 weeks | 4,5 |
| 7 | Placebo-controlled 52-week extension to Study 6 | III/a,b,c | 131 | 104 weeks | 6 |
| 8 | Active-controlled (metformin) long-term efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 780 | 52 weeks | 7 |
| 9 | Active-controlled (metformin) 52-week extension to Study 8 | III/a,b,c | 463 | 104 weeks | 8 |
| 10 | Active-controlled (gliclazide) long-term efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 1092 | 104 weeks | 9 |
| 11 | Active-controlled (acarbose) efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 661 | 24 weeks | 10 |
| 12 | Active-controlled (rosiglitazone) efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c | 786 | 24 weeks | 11 |
| 13 | Active-controlled (rosiglitazone) 80-week extension to Study 12 | III/a,b,c | 598 | 104 weeks | 12 |
| 14 | Active-controlled (pioglitazone) dose regimen comparison study in drug-naïve T2DM patients (HbA1c 9%–11%) | III/a,b,c | 273 | 12 weeks | NCT00101673 |
| 15 | Placebo-controlled efficacy/safety study in patients with IGT | III/a,b,c | 179 | 12 weeks | 13 |
| 16 | Placebo-controlled mechanistic study (β-cell function) in drug-naïve T2DM patients with mild hyperglycemia (HbA1c ≤ 7.5%) | III/a,b,c | 89 | 52 weeks | NCT00260156 |
| 17 | Placebo-controlled dose-ranging study (efficacy/safety) in drug-naïve T2DM patients (HbA1c 7.5%–10%) | III/a,b,c | 354 | 24 weeks | 14 |
| 18 | Active-controlled (metformin) efficacy/safety study in drug-naïve elderly (≥65 years) T2DM patients (HbA1c 7%–9%) | III/a,b,c | 335 | 24 weeks | 15 |
| Combination therapy with metformin | |||||
| 19 | Placebo-controlled dose-selection study in patients inadequately controlled by metformin (HbA1c 7.0%–9.5%) | II/a,b | 132 | 12 weeks | 16 |
| 20 | Placebo-controlled 40-week extension to Study 19 | II/a,b | 71 | 52 weeks | 16 |
| 21 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) | III/a,b | 544 | 24 weeks | 17 |
| 22 | Uncontrolled 28-week extension to Study 21 | III/a | 417 | 52 weeks | NCT00138515 |
| 23 | Active-controlled (glimepiride) long-term efficacy/safety study in T2DM patients treated with metformin (HbA1c > 6.5%–8.5%) | III/a,b | 3118 | ≥104 weeks | 18,19 |
| 24 | Active-controlled (gliclazide) long-term efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) | III/a,b | 1007 | 52 weeks | 20 |
| 25 | Active-controlled (pioglitazone) long-term efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) | III/a,b | 576 | 52 weeks | 21,22 |
| 26 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled with metformin (HbA1c 7.5%–11%) to compare a.m. vs p.m. dosing regimens | III/a,b | 370 | 24 weeks | 23 |
| 27 | Efficacy/safety study in T2DM patients treated with metformin (HbA1c 6.5%–9%) to compare vildagliptin as add-on to metformin vs uptitration of metformin | III/a,b,c**** | 914 | 24 weeks | 24 |
| 28 | Efficacy/safety study of initial fixed combination therapy of vildagliptin and metformin in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c**** | 1179 | 24 weeks | 25 |
| Combination therapy with TZD | |||||
| 29 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled by TZD (HbA1c 7.5%–11%) | III/a,b | 463 | 24 weeks | 26 |
| 30 | Uncontrolled 28-week extension to Study 29 | III/a | 312 | 52 weeks | NCT00138554 |
| 31 | Initial combination (vildagliptin/pioglitazone) efficacy/safety study in drug-naïve T2DM patients (HbA1c 7.5%–11%) | III/a,b,c**** | 607 | 24 weeks | 27 |
| Combination therapy with SU | |||||
| 32 | Placebo-controlled efficacy/safety study in T2DM patients inadequately controlled by SU (HbA1c 7.5%–11%) | III/a,b | 515 | 24 weeks | 28 |
| 33 | Uncontrolled 28-week extension to Study 32 | III/a | 332 | 52 weeks | NCT00138580 |
| Combination therapy with insulin | |||||
| 34 | Placebo-controlled efficacy/safety study in T2DM patients treated with insulin (HbA1c 7.5%–11%) | III/a,b | 296 | 24 weeks | 29 |
| 35 | Uncontrolled 28-week extension to Study 34 | III/a | 200 | 52 weeks | 30 |
Notes:
For extension studies: patients who entered extension;
For extension studies: duration of core + extension study.
ClinicalTrials.gov identifier number is provided if data are not yet published;
Monotherapy arms only. Population a = all studies (excluding open-label) safety population. Population b = all controlled studies (excluding open-label) safety population; Population c = monotherapy (excluding open-label) safety population.
Abbreviations: HbA1c, glycosylated hemoglobin A; IGT, impaired glucose tolerance; SU, sulfonylurea; T2DM, type 2 diabetes; TZD, thiazolidinedione.