Abstract
Background:
Patients with systemic lupus erythematosus (SLE) are 5–8 times more likely to develop coronary heart disease than the general population. The aim of this study was to find out the prevalence of the small dense low-density lipoprotein (LDL) cholesterol particle in patients with SLE.
Methods:
We recruited 50 consecutive patients with SLE who had no evidence of hypertension or renal failure. Fifty age- and gender-matched healthy controls were also recruited. We measured serum lipid levels and LDL particle diameters by gradient gel electrophoresis in both patients and controls.
Results:
Patients with SLE had significant dyslipidemia, characterized by elevated plasma triglycerides, LDL cholesterol, Apoprotein B, triglyceride:high-density (HDL) lipoprotein cholesterol ratio, and decreased plasma concentrations of HDL cholesterol. The LDL particle size in SLE (24.8 ± 1.23 nm) was significantly (P < 0.01) smaller than that in controls (26.1 ± 1.31 nm). The prevalence of the LDL phenotype B (the atherogenic phenotype) was 52% in SLE but only 20% in healthy controls.
Conclusion:
We conclude that the high prevalence of small dense LDL in SLE may contribute to the high incidence of coronary heart disease seen in this disorder.
Keywords: low-density lipoprotein, particle size, atherosclerosis, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the activation of T and polyclonal B lymphocytes, the production of autoantibodies, and the formation of immune complexes causing tissue and organ damage.1,2 Although its exact etiology is unknown, its clinical consequences can be devastating. For example, in prospective cohorts where incidence cases have been recorded, the prevalence of coronary heart disease in SLE has been 6%–10%3–5 and the estimated incidence of new coronary heart disease events is approximately 1.2%–1.5% per annum. Manzi et al1 utilized data from the Pittsburgh lupus cohort and compared them with data from women followed in the Framingham Offspring study. Overall, women with SLE had a 5–6-fold increased risk of coronary heart disease. Moreover, women with SLE aged 35–44 years were 52 times more likely to develop coronary heart disease. Employing data from the California Discharge Database, Ward5 found that women with SLE aged 18–44 years were more likely than aged-matched controls to have myocardial infarction, congestive heart failure, or stroke. Extrapolating from the population prevalence of SLE, Ward estimated that, overall, myocardial infarction, heart failure, and stroke were 8.5, 13.2, and 10.1 times more likely in women with SLE. In a case-control study from the UK General Practice Research Database, Fischer et al6 also reported that patients with SLE were at higher risk of developing coronary heart disease.
The pathogenesis of coronary heart disease in SLE is likely to be multifactorial, and may be related to vasculitis, corticosteroid use, renal disease, hypertension, hyperlipidemia, or thrombosis associated with antiphospholipid antibodies. Patri et al2 reported that patients with SLE were more likely to have a sedentary lifestyle, obesity, and hypercholesterolemia, while Borha et al7,8 found that, compared with controls, patients with SLE had lower high-density lipoprotein (HDL) cholesterol as well as higher very low-density lipoprotein (VLDL) cholesterol, triglycerides, and lipoprotein (a) concentrations. Increased carotid intima thickness, increased plasma concentrations of circulating oxidized LDL and homocysteine, as well as endothelial defects, have all been incriminated in the premature coronary heart disease seen in patients with SLE.9–11
Human plasma low-density lipoproteins (LDL) are a heterogenous collection of particles which vary in buoyant density, size, and lipid and protein composition.12–14 Small-sized LDL cholesterol has been associated with coronary heart disease in some case-control studies,15–18 although other studies have not found this association.19,20
There are only two reports in the literature on LDL particle size in SLE. Using nuclear magnetic resonance to measure LDL particle sizes in patients with SLE, both Chung et al21 and Hua et al22 did not find any differences in small dense LDL particle size between patients with SLE and controls. Therefore, we decided to use a different method to measure LDL particle size in 50 patients with SLE and 50 healthy controls. We measured LDL particle diameters by gradient gel electrophoresis (Quantimatrix Lipoprint™ LDL System, Quantimetrix Corporation, Redondo Beach, CA) in both patients and controls.
Methods and materials
Subjects
Fifty patients (all women) meeting the 1997 revised criteria of the American College of Rheumatology for SLE were recruited from the rheumatology clinic at the Al-Amiri Teaching Hospital in Kuwait over a period of one year from December 2005–December 2006. Inclusion criteria were disease onset at age <18 years and current age >22 years. Exclusion criteria were pregnancy, use of lipid-lowering drugs, current smoking, proteinuria, a plasma creatinine concentration >120 μmol/L, abnormal liver function, and a fasting plasma glucose >6.1 mmol/L. At initial presentation, the disease activity of each patient was assessed by the SLE Disease Activity Index. The patients were then treated with glucocorticoid and/or immunosuppressive agents until they were stable. Fifty age-matched healthy controls (again all women) were also recruited. The controls were matched with the SLE patients for body mass index (BMI). Informed consent was obtained from each participant before the study. The protocol for the study was approved by the local ethics committee.
Collection of blood samples
Following an overnight 12-hour fast (9 pm–9 am), 10 mL of blood was collected in citrated containers from each patient (before and after treatment) and control, and plasma was separated and kept frozen at −80°C until ready for analysis. The following tests were done on plasma samples from patients (before and after treatment) and controls.
Laboratory analysis
Erythrocyte sedimentation rate was measured using a modified Westergren method. Anti-double-stranded DNA antibody levels were measured using a commercial enzyme-linked immunoassay kit (Inova Diagnostics, San Diego, CA). The Beckman LX20 automated analytical system (Beckman-Coulter, Brea, CA) was used to measure glucose by the hexokinase method, total cholesterol and HDL cholesterol by the cholesterol esterase method, and triglycerides by the lipase method. Apolipoprotein B (ApoB) was measured by a nephelometric method on a Beckman IMMAGE automated analytical system (Beckman-Coulter). LDL cholesterol was calculated from total cholesterol, HDL-cholesterol, and triglyceride values using the formula, LDL cholesterol = total cholesterol-HDL-triglycerides/2.2.23
LDL subclass analysis
A modified tube gel electrophoresis technique, the Lipoprint System (Quantimetrix Corporation)24 was used. A serum sample (25 μL) was applied to the “ready to use” polyacrylamide gel tube along with 200 μL of a loading gel solution containing a lipophilic dye. The sample loading gel mixture was photopolymerized for 30 minutes prior to electrophoresis at a constant of 3 mA/tube for one hour. This system resolved up to 12 serum lipoprotein fractions as follows: VLDL (1), mid-band (3), LDL (7), and HDL (1). HDL migrated the fastest (retention factor [Rf] = 1), while VLDL migrated the slowest to the top of gel (Rf = 0). Mid-band and LDL subfractions migrated at various Rfs between VLDL and HDL. Lipoware computer software (Quantimetrix Corporation) was used to analyze the gel images. The bands were partitioned into discrete segments, and the relative area under the curve calculated for each lipoprotein band. The program also calculated the cholesterol concentration for each lipoprotein fraction using a total cholesterol value obtained for each sample by the routine assay mentioned under analysis.
Using the Lipoprint method, VLDL remained at the origin (Rf = 0.0), whereas HDL migrated at the front (Rf = 1.0). In between, several bands could be detected, ie, mid-bands C, B, and A, which corresponded mainly to intermediate-density lipoproteins, as well as up to seven LDL bands. The LDL1 and LDL2 bands corresponded to large buoyant LDL particles, whereas bands LDL3–7 corresponded to small dense LDL particles. According to the LDL electrophoretic profile, two phenotypes are defined, ie, A and non-A. The LDL particle size was estimated using the Lipoprint System by means of the algorithm developed by Kazumi et al.25 The size cutoff was ≥26.8 nm for phenotype A (normal LDL size) and less than this for non-A (designated as phenotype B). It is likely that the temperature gradient gel electrophoresis method overestimated LDL particle size in comparison with other methods and, therefore, this method’s size cutoff is probably equivalent to the gradient gel electrophoresis cutoff of 25.7 nm for small dense LDL.26
Quality control
The internal quality of routine analyses was monitored at two levels of concentration, ie, normal and pathological. Glucose and lipid parameters were included in the Kuwait National and Biorad external quality assurance schemes. For LDL subclass analysis, the quality control material (Liposure) used was obtained from Quantimetrix Corporation, the manufacturers of the Lipoprint gel tube electrophoresis system.
Data analysis
Assessment of differences between the means, correlations between chosen parameters, and regression analyses were carried out using SPSS 15 for Windows. Significance was set at the level of P < 0.05.
Results
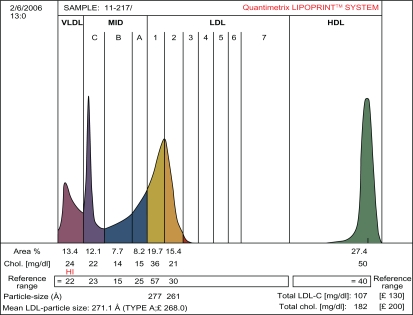
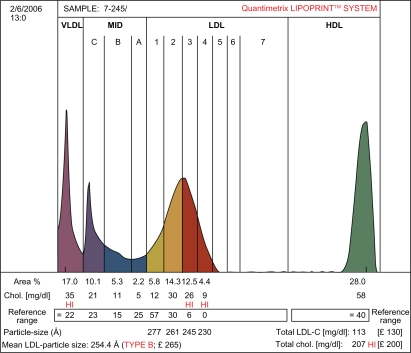
Table 1 shows that patients with SLE and healthy controls were well matched with respect to age, gender, hypertension, BMI, and fasting blood sugar. This was done to eliminate these parameters as confounding variables in the analysis of the lipid data. Compared with controls, patients with SLE had significantly higher plasma total cholesterol (6.62 ± 0.84 versus 5.24 ± 0.64 mmol/L), LDL cholesterol (4.72 ± 0.68 versus 3.30 ± 0.53 mmol/L), triglycerides (2.53 ± 0.24 versus 1.85 ± 0.18 mmol/L), Apo B (0.96 ±0.18 versus 0.73 ±0.10 mmol/L), and triglyceride:HDL cholesterol ratio (3.29 ± 0.42 versus 1.68 ± 0.31), but had significantly lower plasma HDL cholesterol (0.77 ± 0.22 versus 1.10 ± 0.23 mmol/L) and LDL particle size (24.8 ± 1.23 versus 26.1 ± 1.31 nm) than healthy controls. The LDL subclass analysis showed that 26 of the 50 patients with SLE were phenotype B, while only 10 of the 50 healthy controls were phenotype B. The remaining 24 of the SLE patients and 40 of the healthy controls were phenotype A. Thus, the prevalence of the LDL phenotype B among patients with SLE was 52% and was only 20% among healthy controls. This difference was statistically significant (P < 0.01). Figures 1 and 2 represent pattern A and B, respectively.
Table 1.
Demographic and metabolic characteristics of patients with SLE and healthy controls
| Characteristics | Patients with SLE (mean ± SD) | Control (mean ± SD) | Pvalue |
|---|---|---|---|
| Age (SD) years | 50.3 ± 8.6 | 49.6 ± 7.2 | NS |
| Hypertension, % | 10 | 0 | NS |
| Fasting plasma glucose (SD), mmol/L | 6.4 ± 0.38 | 6.2 ± 0.35 | NS |
| Plasma total cholesterol (SD), mmol/L | 6.62 ± 0.84 | 5.24 ± 0.64 | NS |
| Plasma HDL-C (mmol/L) | 0.77 ± 0.22 | 1.10 ± 0.23 | <0.01 |
| Plasma LDL-C (mmol/L) | 4.72 ± 0.68 | 3.30 ± 0.53 | <0.01 |
| Plasma triglycerides (SD) mmol/L | 2.53 ± 0.24 | 1.85 ± 0.18 | <0.05 |
| Plasma Apoprotein B (mmol/L) | 0.96 ± 0.18 | 0.73 ± 0.10 | <0.05 |
| Triglyceride/HDL-C ratio | 3.29 ± 0.42 | 1.68 ± 0.31 | <0.001 |
| LDL particle size (nm) | 24.8 ± 1.23 | 26.1 ± 1.31 | <0.01 |
| Plasma creatinine (μmol/L) | 76.53 ± 8.2 | 66.1 ± 1.31 | NS |
Abbreviations: SD, standard deviation; SLE, systemic lupus erythematosus; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NS, not significant.
Figure 1.
The densitometric scan of LDL subfraction (Pattern A) obtained in controls.
Figure 2.
The densitometric scan of LDL subfractions (Pattern B) obtained in patients with SLE.
Discussion
We have demonstrated in this study that our patients with SLE had significantly higher plasma concentrations of VLDL, triglycerides, LDL, and ApoB, but lower plasma HDL than healthy controls. Two distinct types of dyslipoproteinemia in SLE have been described in the literature. The first type is characterized by high levels of VLDL triglycerides and VLDL cholesterol, and low levels of HDL. This lipid profile is usually seen in untreated or inactive SLE, but can also be seen in patients with active disease.27–30 Our patients fall into this category. Little is known about the mechanisms behind this lipid disorder, although decreased activity of endothelial lipoprotein lipase and impaired chylomicron removal from plasma29 are two factors present in SLE patients which may contribute to elevations of triglycerides. Antibodies to ApoA1 are also detected in some SLE patients and may contribute to low levels of HDL.30,31 However, we did not estimate antibodies to ApoA1 in our patients. The second pattern of lipid disturbance often seen in SLE arises as a complication of steroids.32 Steroid treatment induces an increase in total cholesterol (including both LDL and HDL) and a more modest elevation of triglycerides.33 Patients with renal disease also have increased levels of total and LDL cholesterol, together with triglycerides and HDL. It is unlikely that our patients had renal disease because none of them had proteinuria or a plasma creatinine concentration >120 μmol/L.
We have demonstrated using the Lipoprint LDL system that patients with SLE have a smaller denser LDL particle size than healthy controls. The generation of small dense LDL particles occurs due to elevation of mild to moderate hyperglyceridemia.34 Under these conditions, triglycerolrich VLDL increases due to overproduction in the liver or defective clearance from the circulation. Cholesteryl ester transfer protein removes cholesteryl ester and replaces it with triacylglycerol as the protein shuttles between VLDL, LDL, and HDL particles. Triacylglycerol-enriched LDL is a better substrate for hepatic lipase, which removes triacylglycerol from small lipoprotein particles. When the hepatic lipase activity is high enough, lipolysis generates smaller and denser LDL particles. LDL oxidation is an important factor in atherosclerosis, and small dense LDL particles are easier to oxidize than larger, less dense particles. As a result of oxidation, a variety of immunogenic neoepitopes are formed on the oxidized LDL. For example, oxidation of phosphorylcholine containing phospholipids renders them antigenic. Oxidized LDL forms ligands on oxidized LDL particles recognized by macrophages, leading to enhanced uptake of oxidized LDL particles and foam cell formation.36–38 Through this route, macrophages become lipid-laden and develop into the characteristic foam cells of atherosclerotic lesions. Oxidized LDL is also chemotactic, immunostimulatory, and has toxic properties that promote local inflammatory processes in atherosclerotic lesions.39,40 Furthermore, oxidized LDL elicits a humoral immune response with production of autoantibodies to oxidation-specific epitopes of oxidized LDL. Therefore, the presence of small dense LDL subfractions in patients with SLE may be an independent risk factor for the development of premature atherosclerosis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 2.Petri M, Spence D, Bone LR, Hochberg MC. Coronary artery disease risk factors in the Johns Hopkins Lupus Cohort: Prevalence, recognition by patients and preventive practices. Medicine. 1992;71:291–302. doi: 10.1097/00005792-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Bruce IN, Urowitz MB, Gladman DD, Hallett DC. Natural history of hypercholesterolemia in systemic lupus erythematosus. J Rheumatol. 1999;26:2137–2143. [PubMed] [Google Scholar]
- 5.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 2002;46:2010–2019. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93:198–200. doi: 10.1016/j.amjcard.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 7.Borba EF, Bonfa E. Dyslipoproteinemia in systemic lupus erythematosus: Influence of disease activity and anticardiolipin antibodies. Lupus. 1997;6:533–539. doi: 10.1177/096120339700600610. [DOI] [PubMed] [Google Scholar]
- 8.Borba EF, Santos RD, Bonfa E, et al. Lipoprotein (a) levels in systemic lupus erythematosus. J Rheumatol. 1994;21:220–223. [PubMed] [Google Scholar]
- 9.Martínez-Berriotxoa A, Ruiz-Irastorza G, Egurbide MV, Rueda M, Aguirre C. Homocysteine, antiphospholipid antibodies and risk of thrombosis in patients with systemic lupus erythematosus. Lupus. 2004;13:927–933. doi: 10.1191/0961203304lu2035oa. [DOI] [PubMed] [Google Scholar]
- 10.Frostegard J, Svenungssen R, Wu R, et al. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 11.Selzer F, Sutton-Tyrrell K, Fitzgerald SG, et al. Comparison of risk factors for vascular disease in the carotid artery and aorta in women with systemic lupus erythematosus. Arthritis Rheum. 2004;50:151–159. doi: 10.1002/art.11418. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren FT, Jensen LC, Wills RD, Freeman NK. Flotation rates, molecular weights and hydrated densities of the low density lipoproteins. Lipids. 1969;4:337–344. doi: 10.1007/BF02531003. [DOI] [PubMed] [Google Scholar]
- 13.Adams GH, Schumaker VN. Polydispersity of human low-density lipoproteins. Ann N Y Acad Sci. 1969;164:130–146. doi: 10.1111/j.1749-6632.1969.tb14036.x. [DOI] [PubMed] [Google Scholar]
- 14.Hammond MG, Fisher WR. The characterisation of a discrete series of low-density lipoproteins in the disease, hyper-pre β-lipoproteinemia. J Biol Chem. 1971;246:5454–5455. [PubMed] [Google Scholar]
- 15.Lee DM. Isolation and characterization of low-density lipoproteins. In: Day CE, Levy RS, editors. Low Density Lipoproteins. New York, NY: Plenum Press; 1976. [Google Scholar]
- 16.Shen MS, Krauss RM, Lindgreeen FT, Forte TM. Heterogeneity of serum low-density lipoproteins in normal human subjects. J Lipid Res. 1981;22:236–244. [PubMed] [Google Scholar]
- 17.Baurowich GM, Dash J, Hensley WJ, Turtle JR. Gradient gel electrophoresis of human plasma lipoproteins. Clin Chem. 1973;19:415–418. [PubMed] [Google Scholar]
- 18.Campos H, Granest JJ, Jr, Bilijievents E, et al. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb. 1992;12:187–195. doi: 10.1161/01.atv.12.2.187. [DOI] [PubMed] [Google Scholar]
- 19.Coresh J, Kwiteroviwich PO, Jr, Smith HH, Bachorik PS. Association of plasma triglyceride concentration and LDL particle diameter, density and chemical composition with premature coronary artery disease in men and women. J Lipid Res. 1993;34:1887–1897. [PubMed] [Google Scholar]
- 20.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willet WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–1921. [PubMed] [Google Scholar]
- 21.Chung CP, Oeser A, Raggi P, et al. Lipoprotein subclasses and particle size determined by nuclear magnetic resonance spectroscopy in systemic lupus erythematosus. J Rheumatol. 2009;37:1633–1638. doi: 10.1007/s10067-008-0890-4. [DOI] [PubMed] [Google Scholar]
- 22.Hua X, Su J, Svenungsson E, et al. Dyslipidaemia and lipoprotein pattern in systemic lupus erthematosus (SLE) and SLE-related cardiovascular disease. Scand J Rheumatol. 2009;38:184–189. doi: 10.1080/03009740802541470. [DOI] [PubMed] [Google Scholar]
- 23.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Hoefner DM, Hodel SD, O’Brien JF, et al. Development of a rapid, quantitative method for LDL subfractionation with the use of the Quantimetrix Lipoprint LDL System. Clin Chem. 2001;47:266–274. [PubMed] [Google Scholar]
- 25.Kazumi T, Kawaguchi A, Hozumi T, et al. Low density lipoprotein particle diameter in young, nonobese, normolipidemic Japanese men. Atherosclerosis. 1999;142:113–119. doi: 10.1016/s0021-9150(98)00201-9. [DOI] [PubMed] [Google Scholar]
- 26.Muniz N, Duncan D, Neyer G. Normal reference ranges for serum lipoproteins and their subfractions for the Lipoprint™ LDL System. Presented at the American Association of Clinical Chemistry, 53rd Annual Meeting; Chicago, IL. July 29–August 2, 2001. [Google Scholar]
- 27.Borba EF, Bonfa E. Dyslipoproteinemias in systemic lupus erythematosus: Influence of disease, activity, and anticardiolipin antibodies. Lupus. 1997;6:533–539. doi: 10.1177/096120339700600610. [DOI] [PubMed] [Google Scholar]
- 28.Ilowite NT, Samuel P, Ginzler E, Jacobson MS. Dyslipoproteinemia in pediatric systemic lupus erythematosus. Arthritis Rheum. 1988;31:859–863. doi: 10.1002/art.1780310706. [DOI] [PubMed] [Google Scholar]
- 29.Borba EF, Bonfa E, Vinagre CG, Ramires JA, Maranhao RC. Chylomicron metabolism is markedly altered in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1033–1040. doi: 10.1002/1529-0131(200005)43:5<1033::AID-ANR11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.Merrill JT, Rivkin E, Shen C, Lahita RG. Selection of a gene for apolipoprotein A1 using autoantibodies from a patient with systemic lupus erythematosus. Arthritis Rheum. 1995;38:1655–1659. doi: 10.1002/art.1780381118. [DOI] [PubMed] [Google Scholar]
- 31.Dinu A, Merrill J, Shen C, Antonov I, Myones B, Lahita R. Frequency of antibodies to the cholesterol transport protein apolipoprotein A1 in patients with SLE. Lupus. 1998;7:355–360. doi: 10.1191/096120398678920262. [DOI] [PubMed] [Google Scholar]
- 32.Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: A longitudinal data analysis. Am J Med. 1994;96:254–259. doi: 10.1016/0002-9343(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 33.Ettinger WH, Goldberg AP, Applebaum-Bowden D, Hazzard WR. Dyslipoproteinemia in systemic lupus erythematosus: Effect of corticosteroids. Am J Med. 1987;21:1264–1267. doi: 10.1016/0002-9343(87)90762-5. [DOI] [PubMed] [Google Scholar]
- 34.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 35.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- 36.Wu R, Huang YH, Elinder LS, Frostgard J. Lysophosphatidyl choline is involved in the antigenicity of oxidized LDH. Arterioscler Thromb Vasc Biol. 1998;18:626–630. doi: 10.1161/01.atv.18.4.626. [DOI] [PubMed] [Google Scholar]
- 37.Binder CJ, Chang MK, Shaw PX, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 38.Horkko S, Bird DA, Miller E, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipids-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberg D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 40.Glass CK, Witztum JL. Atherosclerosis: The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]