Abstract
L-Serine is required for the synthesis of glycine and D-serine, both of which are NMDA receptor co-agonists. Although roles for D-serine and glycine have been suggested in schizophrenia, little is known about the role of the L-serine synthesizing cascade in schizophrenia or related psychiatric conditions. Here we report a patient with schizophrenia carrying a balanced chromosomal translocation with the breakpoints localized to 3q13.12 and 9q21.2. We examined this proband and her son with schizotypal personality disorder for chromosomal abnormalities, molecular expression profiles, and serum amino acids. Marked decrease of L-serine and glutamate was observed in the sera of the patient and her son, compared with those in normal controls. Interestingly, expression of PSAT1 gene, which is located next to the breakpoint and encodes one of the enzymes in the L-serine synthesizing cascade, was reduced in both patient and her son. Direct effect of impaired PSAT1 gene expression on decreased serum L-serine level was strongly implicated by rat astrocyte experiments. In summary, we propose an idea that PSAT1 may be implicated in altered serine metabolism and schizophrenia spectrum conditions.
Keywords: schizophrenia, balanced chromosomal translocation, PSAT1, L-serine, D-serine, glycine, glutamate, expression
Introduction
Disturbance of serine metabolism causes many disorders, including hereditary disorders due to mutations in the genes that regulate biosynthesis of serine and related amino acids (Jaeken et al., 1996, Jaeken et al., 1997, de Koning et al., 1998, Pineda et al., 2000, de Koning and Klomp, 2004, Veiga-da-Cunha et al., 2004, Hart et al., 2007). L-Serine is biosynthesized from 3-phosphoglycerate, a metabolite of glucose in the brain, and requires three enzymatic steps catalyzed by 3-phosphohydroxypyruvate dehydrogenase (3-PGDH), phosphoserine aminotransferase 1 (PSAT1), and lastly phosphoserine phosphatase (PSP) (de Koning and Klomp, 2004). From L-serine, other bioactive amino acids, such as D-serine and glycine are generated (de Koning and Klomp, 2004). In a previous report, patients with deficiencies in any of these enzymes presented early with severe neurological manifestations shortly after birth, including seizures, microcephaly, and psychomotor retardation, that were either fatal or required serine supplementation for survival (Jaeken et al., 1996, Jaeken et al., 1997, de Koning et al., 1998, Pineda et al., 2000, Veiga-da-Cunha et al., 2004, Hart et al., 2007).
Schizophrenia is a major psychiatric disorder in which disturbance of glutamate and D-serine has been suggested (Hashimoto et al., 2003, Bendikov et al., 2007, Sawa, 2009). D-serine is an endogenous co-agonist for the NMDA-type glutamate receptor and is believed to underlie the disease pathophysiology via its action on NMDA receptor (Labrie and Roder, 2010). Furthermore, genes coding for D-serine synthesis and degradation, including serine racemase, PICK1, D-amino acid oxidase, and G72 have been genetically associated with the disease (Chumakov et al., 2002, Schumacher et al., 2004, Fujii et al., 2006, Morita et al., 2007). Although synaptic D-serine is dependent on the de novo biosynthesis of L-serine, a role for the L-serine biosynthesis cascade in schizophrenia remains to be determined.
Here we report on two individuals carrying a hereditary balanced chromosomal translocation. The mother was diagnosed with schizophrenia, while her son was diagnosed with schizotypal personality disorder. Resolving chromosome translocation breakpoints has proved a successful means to identify susceptibility genes for psychiatric disorders (Muir et al., 2006). In the present study, this approach suggests a role for the PSAT1 gene in schizophrenia spectrum conditions, which is strengthened by a decreased expression of PSAT1 and reduced serum levels of L-serine in both subjects.
Methods and Materials
Clinical history and neuropsychological assessment
The Japanese proband, recently deceased, was born with no marked medical history perinatally or in childhood, and married at 18 years old after graduating from high school. After the birth of her son (her only child) shortly after marriage, she was diagnosed with schizophrenia, presenting with delusions and hallucinations, and was hospitalized at age 19. Despite extensive treatment with neuroleptics, her positive symptoms did not improve significantly, while her negative symptoms (poverty of thought and thought disorder) worsened steadily. The Positive and Negative Syndrome Scale (PANSS) score was 106 (positive: 32, negative: 28). Because of her deteriorating mental status, further neuropsychological tests could not be performed except the verbal fluency assessment in which she said only one word in each test. Neuropsychological tests were performed in March 2006. The son completed university with no prior psychiatric history, but has had persistent poor social functioning. At age 57, he is currently employed as a manual laborer. He is diagnosed to have schizotypal personality disorder with thought disturbance, inappropriate affect, peculiar behavior, lack of close friends, and excessive social anxiety. On the Wechsler Adult Intelligence Scale-Revised (WAIS-R), he scored 115 in global (FIQ), 110 in visual (VIQ), and 119 in performance (PIQ) intellect. On the Wechsler Memory Scale-Revised (WMS-R), he scored within normal range for verbal memory (110), general memory (97), attention and concentration (112), and delayed recall (90), but was subnormal in visual memory (72). He scored poorly on the Wisconsin Card Sorting Test (WCST), not being able to complete a single category and could not arrive at the correct answers even after the test rules were revealed to him. This study was approved by the Institutional Review Boards of National Center of Neurology and Psychiatry and Chiba University. The controls were healthy volunteers recruited from hospital staff and their associates. All subjects were biologically unrelated Japanese. They were interviewed with the Japanese edition of the mini international neuropsychiatric interview (MINI) (Sheehan et al., 1998, Otsubo et al., 2005), and those who had a current or past history of psychiatric treatment were not enrolled in the study. Documented informed consent was obtained from the participants. We recruited 26 controls (13 male and 13 female) for the establishment of lymphoblastoid cell lines and real-time RT-PCR analysis for PSAT1 mRNA expression (age: 49.0±15.7). Ten controls were recruited for the establishment of lymphoblastoid cells lines and real-time RT-PCR analysis for mRNA expression of other genes (age: 61.7±11.3). For serum amino acid measurement, 16 controls were recruited (male 6 and female 10, age: 66.8±12.9).
Lymphoblastoid cell lines from subjects
Mononuclear cells were isolated from peripheral blood by Ficoll-Paque gradient centrifugation. Lymphoblastoid cell lines were established by transforming B lymphocytes by Epstein–Barr virus, and grown in RPMI-1640 supplemented with 10% fetal bovine serum and penicillin/streptomycin.
Fluorescence in situ hybridization (FISH)
Lymphoblastoid cells were treated with colcemid for 1 h followed by a conventional fixing procedure. Fixed chromosomes were dropped onto microscope slides and stored for 1 week prior to use. BAC and FOSMID clones were selected from the UCSC genome browser and obtained from the Wellcome Trust Sanger Institute. Purified clone DNA labeled with digoxigenin or biotin was hybridized to metaphase spreads and detected with fluorescent secondary antibodies. Images were captured on a Zeiss Axioskop 2 fluorescence microscope by using Digital Scientific SmartCapture 2.1 software.
Total RNA extraction, cDNA synthesis and quantitative real-time RT-PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. After DNAse treatment by use of the TURBO DNA-free Kit (Ambion), the quality and quantity of all RNA samples were checked with NanoDrop (NanoDrop Technologies). cDNA was synthesized from 1 μg of total RNA by using MultiScribe Reverse Transcriptase High-Capacity cDNA Reverse Transcript Kits (Applied Biosystems). Quantitative real-time PCR was performed with TaqMan Gene Expression Assays and ABI 7900HT (Applied Biosystems). Assay IDs for each target were as follows: BBX: Hs00329131_m1, CCDC54: Hs00540426_s1, CEP78: Hs00397220_m1, GNAQ Hs00387073_m1, and PSAT1: Hs00253548_m1. Primers and probe for BC036229 gene, which is located in the breakpoint and encodes a non-coding RNA, were designed as 5’-gaaagatccacctacgacctaagg-3’ (forward), 5’-atctaccagatgaactcttccatgg-3’ (reverse), and FAM-caccaatttcaaatccgaaat-MGB (labeled TaqMan probe). PCR conditions were as follows: 95oC for 2 min, 50 cycles of 95oC for 15 sec, and 60oC for 1 min. The data were presented as expression level relative to glyceraledehyde-3-phosphate dehydrogenase (GAPDH) expression.
Primary rat astrocyte culture
Primary rat astrocyte cultures were prepared from cortices of P2 pups of Sprague-Dawley rats (Charles River Laboratories). Single cell suspensions were obtained by serial trituration of cerebral cortices with 20G and 26G needles with a 10-ml syringe, followed by the removal of cell debris with a Cell strainer (BD bioscience). Cells were suspended in NM-15 medium [MEM medium supplemented with L-glutamine, 15% FBS (both from Invitrogen), 6 mg/mL D-glucose (Sigma), and penicillin/streptomycin (Invitrogen)], and seeded on poly-D-lysine-coated T-75 flasks (Corning) with two brains/flask. Medium was changed on day 3, and every other day thereafter. On day 10-12, the flasks were sealed and shaken at 37°C overnight. Following an additional shake with D-PBS and complete removal of loosely-attached cells (oligodendrocytes and microglia), adherent cells (astrocytes) were dissociated with 0.05% trypsin and collected in NM-15 medium for further use. Typically, we collect >95% GFAP-positive astrocytes by this method.
RNAi knockdown of PSAT1 in primary rat astrocyte culture
Knockdown of Psat1 gene expression was performed by transfection of Psat1 siRNA or control siRNA (ON-TARGETplus SMARTpool® siRNA, Dharmacon) by using Oligofectamine (Invitrogen).
Measurement of amino acids
Amino acids levels were measured by an HPLC system as described previously (Hashimoto et al., 2005, Yamada et al., 2005). The subject's serum samples were collected between 11AM and noon. Cell culture supernatants were collected 24 h after the last medium change. Samples were kept frozen at -80°C. The serum levels of glutamate, glutamine, and glycine were measured using an HPLC system, as reported previously (Hashimoto et al., 2005). D- and L-Serine were measured with a column-switching HPLC system as reported previously (Fukushima et al., 2004, Yamada et al., 2005). Briefly, 20 μL of human serum were homogenized in 180 μl of methanol (HPLC grade). The homogenates were centrifuged at 4500 g for 10 min, and 20 μl of supernatant were evaporated to dryness at 40°C. To the residue, 20 μL of H2O (HPLC grade), 20 μL of 0.1 M borate buffer (pH 8.0), and 60 μL of 50 mM 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F; Tokyo Kasei Kogyo Co., Ltd.) in CH3CN (HPLC grade) were added. The reaction mixture was then heated at 60°C for 1 min, and immediately supplemented with 100 μL of H2O/CH3CN (90/10) containing 0.1 % trifluoroacetic acid (TFA) to quench the reaction. Ten microlitters of the resultant solution were injected into the HPLC system.
Western blotting
Astrocytes were lysed in RIPA buffer and sonicated. Equal amounts of whole cell lysates were loaded into each lane of 4-12% NuPAGE Novex Bis-Tris mini gels (Invitrogen). Electrophoresis, transfer to a polyvinylidene difluoride (PVDF) membrane (Millipore), and visualization by ECL system was done by a standard method. Rabbit anti-PSAT1 antibody was a gift from Drs. Do Youn Jun and Young Ho Kim (Korea), and used to detect endogenous PSAT1 expression.
Statistical analysis
Mann-Whitney U-test was used for the analysis of subject data. L-serine level in astrocyte culture supernatants were analyzed by Student-t-test.
Results
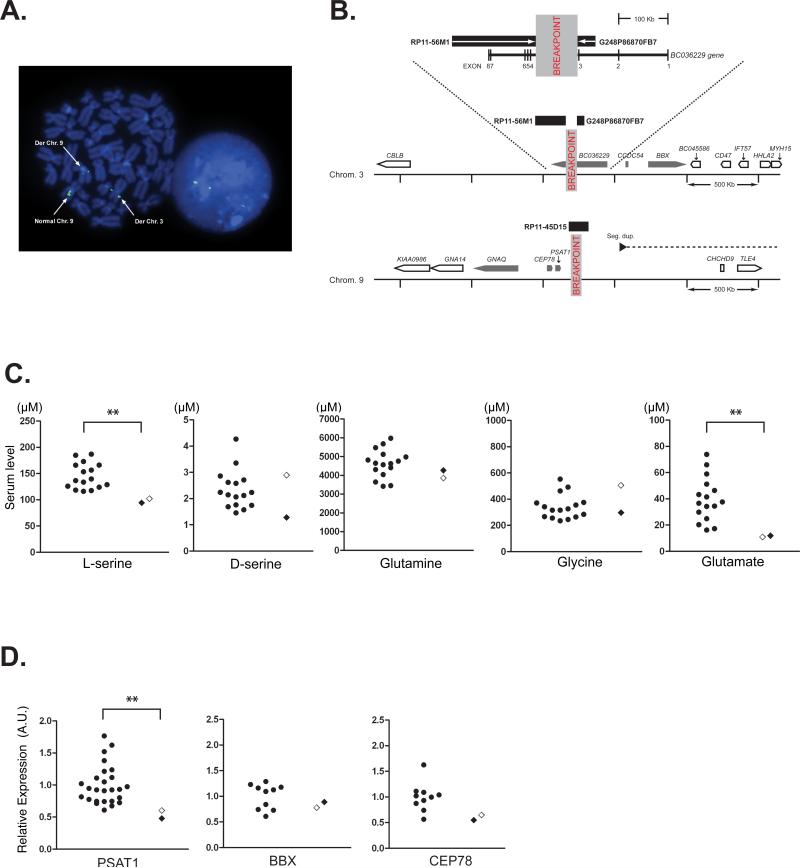
The proband was known to possess a chromosomal abnormality: however, detailed analysis on the breakpoint had not been performed. We hypothesized that the uncharacterized chromosomal abnormality might account for the schizophrenia spectrum diagnoses in both the proband and her son. In the proband lymphoblastoid cells, FISH localized the breakpoints to 3q13.12 and 9q21.2 (Fig. 1A and B). On chromosome 3, the breakpoint was positioned between flanking probes BAC RP11-56M1 and Fosmid G248P86870FB7, defining its location to a window of approximately 100 kb. This corresponds to the third intron of 2.7 kb non-coding transcript BC036229. The 5’ end of the BC036229 transcript is associated with a CpG island that also appears to drive expression of the overlapping, but shorter transcript LOC100302640 and a transcript with opposite orientation, LOC344595. The CpG island is poorly conserved among species and the transcripts are all non-coding and lack non-human orthologs. Therefore, we suggest that these three transcripts are non-functional transcriptional ‘noise’. On chromosome 9, probe RP11-45D15, spanned the breakpoint (Fig. 1A). This restricted the breakpoint to approximately 140 kb but the intensities of the probe on the two derived chromosomes suggested that the breakpoint was located towards the centromeric end of this window. This places the chromosome 9 breakpoint approximately 100 kb downstream of, and telomeric to, the phosphoserine aminotransferase 1 gene (PSAT1). Detailed analysis of translocation was performed only in the proband although the G-banding test identified the similar chromosomal translocation in her son (data not shown).
Figure 1.
Chromosomal translocation and molecular profiles of the proband and her son. A) Fluorescent in situ hybridization positions the chromosome 9q21.2 breakpoint. A metaphase spread (left) and interphase nucleus (right) stained with 4',6-diamidino-2-phenylindole (DAPI) (blue). A green fluorescently labeled probe generated from RP11-45D15 BAC clone DNA is shown hybridized to the normal chromosome 9 and derived chromosomes 3 and 9 on a metaphase spread (left) stained blue with DAPI. This triple signal, also seen in an interphase nucleus (right), indicates that the DNA probe sequence spans the breakpoint. The relative proportions of signals on the two derived chromosomes suggest that the breakpoint is located towards the centromeric end of the DNA probe sequence. B) Schematic representation of the two breakpoint loci. The BAC and fosmid clones flanking the chromosome 3 breakpoint within the BC036229 transcript are shown as black rectangles. The magnified genomic context of the disruption is shown below including the location and orientation of nearby genes (nearest genes, grey block arrows: other genes, white block arrows). The chromosome 9 breakpoint does not directly disrupt a gene but is located close to PSAT1. The proximity to a segmental duplication pair (the black arrow indicates the centromeric component and the dashed line indicates the direction of the telomeric component ~1.8 Mb downstream) may indicate potential chromosome instability in this region. C) Serum level of L-serine, D-serine, and other relevant amino acids determined by HPLC. *p=0.013, Mann-Whitney U-test. Control, n=16. The open diamond indicates the proband. D) mRNA expression of the genes that are adjacent to the breakpoints. Expression level was determined by quantitative real-time RT-PCR and presented as expression relative to GAPDH mRNA. PSAT1 mRNA expression level was decreased significantly in the subjects (proband and her son) (**p=0.0053, Mann-Whitney U-test). No significant difference was observed for bobby sox homolog (Drosophila) (BBX) and centrosomal protein 78 kDa (CEP78) mRNA expression. Control: n=26 for PSAT1, n=10 for BBX and CEP78. The open diamond indicates the proband. mRNA expression of coiled-coil domain containing 54 (CCDC54), guanine nucleotide binding protein q polypeptide (GNAQ), and BC036229 was not detected in either subject or control lymphoblastoid cells. See Figure 1B for the location of each gene.
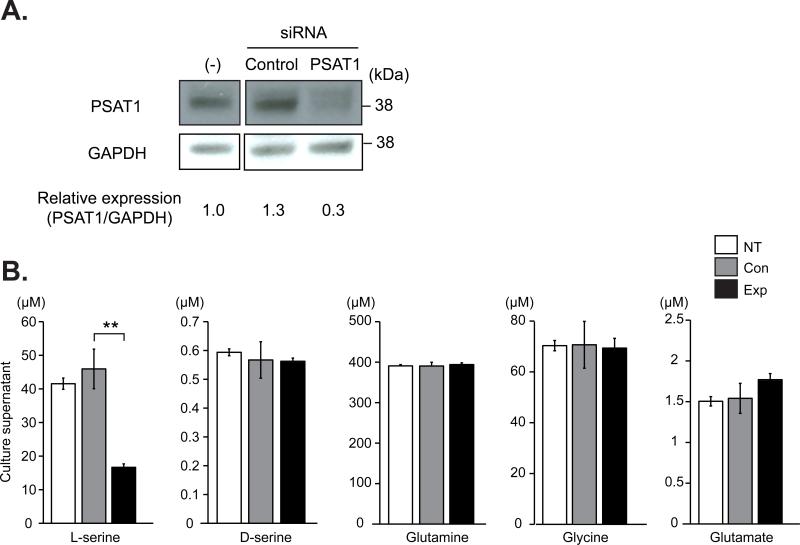
In the initial screening of possible molecular changes in these subjects, we measured serum levels of several amino acids that may play a role in the pathophysiology of schizophrenia and related disorders (Fig. 1C). Intriguingly, levels of L-serine and glutamate in the subjects (proband and her son) were significantly lower than normal Japanese controls. In contrast, we did not observe differences in the levels of glutamine between the subjects and normal controls. Therefore, we hypothesized that these alterations in the levels of amino acids are associated with the chromosomal abnormality. While no protein-coding gene is likely to be disrupted by the breakpoints, we searched for cis-effects of the breakpoints on expression levels of all the annotated genes with an open reading frame within 500 kb of the breakpoints in lymphoblastoid cells by quantitative RT-PCR (Fig. 1D). Among the genes examined, significant and selective decrease in expression was only observed for PSAT1 in both the proband and her son (Fig. 1D). There were no significant changes in bobby sox homolog (BBX) and centrosomal protein 78kDa (CEP78) mRNAs, and no mRNA expression was detected in lymphoblasoid cells for coiled-coil domain containing 54 (CCDC54) and guanine nucleotide binding protein q polypeptide (GNAQ) genes. To examine whether decreased PSAT1 expression results in decreased L-serine production by brain cells, we knocked down PSAT1 by RNAi in rat primary astrocytes in which major serine signaling takes place in the brain (Fig. 2A). We observed decreased L-serine levels in supernatants from these astrocyte cultures while we did not see any significant changes in the levels of other amino acids including D-serine, glutamine, glutamate, and glycine (Fig. 2B).
Figure 2.
Effects of PSAT1 knockdown by RNAi on L-serine level. A) Successful knockdown of PSAT1 by siRNA in primary rat astrocytes. Primary rat astrocytes were transfected with siRNA against PSAT1 or control siRNA. Cells were harvested on day 7 and examined for PSAT1 protein expression by Western blot analysis by using anti-PSAT1 antibody. GAPDH expression level was examined for internal control. (-); no transfection. Bar graph shows PSAT1 expression level relative to GAPDH. B) Amino acids concentration in culture supernatants of primary rat astrocytes with PSAT1 siRNA. Astrocytes transfected with either PSAT1 siRNA or control siRNA was determined at 24 h after the last medium change (day 7 post-transfection) by a high-performance liquid chromatography (HPLC) system. Error bars represent standard deviation. NT; non-treated, Con; treated with control siRNA, and Exp; treated with PSAT1 siRNA. **p=0.00175, Student t-test. Data are representative of three independent experiments.
Discussion
Here we report a case of a proband and her son suffering from schizophrenia spectrum conditions and carrying a chromosomal translocation. The 9q21 locus where the PSAT1 candidate gene lies adjacent to one of the breakpoints has moderate support for a role in psychiatric illness from two genome-wide linkage studies (Hovatta et al., 1999, McInnis et al., 2003). Our data on expression of genes adjacent to the translocation breakpoints suggested a potential role for PSAT1 in the pathophysiology of their conditions. This is in line with many previous studies that have shown long-range (up to 1.3 Mb) regional perturbation of transcription levels by chromosome abnormalities causing illness, even in the absence of direct gene disruption (Kleinjan and Coutinho, 2009). This phenomenon most likely results from altered regional chromatin state or separation of up- or down-stream genomic regulatory elements from the main body of the gene. In agreement with this, and in contrast to recessive PSAT1 mutations (Hart et al., 2007), we observe only partially reduced PSAT1 expression and speculate that this mild decrease may be associated with psychiatric conditions without major developmental deficits. Similar correlations between mutation allele and severity of phenotype have been reported for many other disease genes including NPAS3 which can present as a psychiatric diagnosis (Huang et al., 2010), a psychiatric diagnosis co-morbid with mild mental retardation (Kamnasaran et al., 2003, Pickard et al., 2005) or severe mental retardation/Sotos syndrome (Visser et al., 2010).
We fully acknowledge various limitations of this study. First, no karyotypically normal family members were available to act as negative controls in the serum studies or to define the statistical significance of the co-segregation of chromosomal translocation with illness. Additionally, no further experimental interventions such as the administration of L- or D-serine are planned for the surviving son because approval from the ethical committee and subject is unlikely. Even for the subjects studied, no further clinical information can be expected although all possible clinical assessment and tissue acquisition approved by the ethical committee has been completed. Second, the selective downregulation of PSAT1 and L-serine reasonably suggests a mechanistic link between this chromosomal translocation and schizophrenia spectrum conditions that are known to associate with disturbed glutamatergic neurotransmission, but it is impossible to prove the causal link in clinical studies. However, small pedigree cytogenetic and sporadic copy number findings are proving incredibly important to the biological description of neuropsychiatric disease processes such as those responsible for autism and schizophrenia (Gill et al., 2010, Pinto et al., 2010). This paper seeks to add to this knowledge by provoking further testing of the candidacy of the PSAT1 gene. For example, although beyond the scope of this report, generation of Psat1 knockout mice and their characterization (especially the heterozygote knockout mice) would provide us with a more definitive picture of how this gene contributes to brain function and psychiatric disorders, especially schizophrenia. We do not know the reason for the discrepancy in the levels of D-serine and glycine between the proband and son, but this may be a reflection of age, gender, individual genetic background, physiological state or medication. For these very reasons, the observed statistical differences in metabolites between carrier and control groups are of greater importance than individual values.
In addition to reducing L-serine concentrations, deficits in PSAT1 activity may have an alternative pathological effect through reduction of its direct product, L-Serine-o-phosphate (L-SOP). L-SOP is a potent agonist of group III metabotropic glutamate receptors in the brain (Antflick et al., 2009). Through these receptors L-SOP has been shown to modulate adult neurogenesis in the hippocampus: reducing neural proliferation and promoting maturation and survival of newly generated neurons (Nakano et al., 2007, Saxe et al., 2007). One abundant group III metabotropic glutamate receptor stimulated by L-SOP is mGluR7 (Okamoto et al., 1994), encoded by the GRM7 gene. The GRM7 gene has been linked by several genome-wide association and copy number variation studies to increased risk of schizophrenia (Walsh et al., 2008), bipolar disorder (WTCCC, 2007, Baum et al., 2008, Ferreira et al., 2008, Zhang et al., 2009) and attention-deficit hyperactivity disorder (Elia et al., 2009).
Accumulating evidence suggests therapeutic potentials in the supplementation or intervention with amino acids to patients with schizophrenia (Buchanan et al., 2007). In particular, strategies that target glutamatergic neurotransmission have been showing promising results. These include the supplementation of D-serine, D-cycloserine, D-alanine, or glycine, as well as the modulation of their transporters (Kantrowitz et al., 2010, Cascella et al., 1994, Heresco-Levy et al., 2004, Heresco-Levy and Javitt, 2004, Javitt, 2004, Heresco-Levy et al., 2005, Lane et al., 2005, Lane et al., 2006, Tsai et al., 2006, Javitt, 2008). Although it is not clear from our current data whether L-serine is simply utilized to synthesize D-serine, they suggest the potential of L-serine supplementation for the treatment of patients with schizophrenia.
Acknowledgements
We thank Ms. K. Yuasa, supervisor of guardian of adult for the patient, for supporting our project; Dr. M. Honda for introducing us to the patient; Drs. Do Youn Jun and Young Ho Kim for PSAT1 antibody; Ms. Yukiko Lema for organizing the manuscript; and Dr. Pamela Talalay for critically reading the manuscript. This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japanese Society for the Promotion of Science (JSPS) KAKENHI21591492 (Y.O.), U.S. Public Heath Service Grant MH-069853 (A.S.), Silvio O. Conte Center grant MH-084018 (A.S.), MH-085226 (A.S.), MH-088753 (A.S.), and foundation grants from Stanley (A.S.), S-R (A.S.), RUSK (A.S.), as well as NARSAD (A.S). B.P. held a Sim Fellowship from the Royal College of Physicians in Edinburgh. The cytogenetics component of the work was supported by a grant from the Chief Scientist Office of the Scottish Government. S.K. was supported by Uehara Memorial Foundation and Kanae Foundataion for the Promotion of Medical Science, and is currently a JSPS Postdoctoral Fellow for Research Abroad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antflick JE, Vetiska S, Baizer JS, Yao Y, Baker GB, Hampson DR. L-Serine-O-phosphate in the central nervous system. Brain Res. 2009;1300:1–13. doi: 10.1016/j.brainres.2009.08.087. [DOI] [PubMed] [Google Scholar]
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophrenia research. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull. 2007;33:1120–1130. doi: 10.1093/schbul/sbm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella NG, Macciardi F, Cavallini C, Smeraldi E. d-cycloserine adjuvant therapy to conventional neuroleptic treatment in schizophrenia: an open-label study. J Neural Transm Gen Sect. 1994;95:105–111. doi: 10.1007/BF01276429. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning TJ, Duran M, Dorland L, Gooskens R, Van Schaftingen E, Jaeken J, Blau N, Berger R, Poll-The BT. Beneficial effects of L-serine and glycine in the management of seizures in 3-phosphoglycerate dehydrogenase deficiency. Annals of Neurology. 1998;44:261–265. doi: 10.1002/ana.410440219. [DOI] [PubMed] [Google Scholar]
- de Koning TJ, Klomp LW. Serine-deficiency syndromes. Current Opinion in Neurology. 2004;17:197–204. doi: 10.1097/00019052-200404000-00019. [DOI] [PubMed] [Google Scholar]
- Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, D'Arcy M, Deberardinis R, Frackelton E, Kim C, Lantieri F, Muganga BM, Wang L, Takeda T, Rappaport EF, Grant SF, Berrettini W, Devoto M, Shaikh TH, Hakonarson H, White PS. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol. Psychiatry. 2009 doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Maeda K, Hikida T, Mustafa AK, Balkissoon R, Xia J, Yamada T, Ozeki Y, Kawahara R, Okawa M, Huganir RL, Ujike H, Snyder SH, Sawa A. Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol. Psychiatry. 2006;11:150–157. doi: 10.1038/sj.mp.4001776. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Kawai J, Imai K, Toyo'oka T. Simultaneous determination of D- and L-serine in rat brain microdialysis sample using a column-switching HPLC with fluorimetric detection. Biomed. Chromatogr. 2004;18:813–819. doi: 10.1002/bmc.394. [DOI] [PubMed] [Google Scholar]
- Gill M, Donohoe G, Corvin A. What have the genomics ever done for the psychoses? Psychol Med. 2010;40:529–540. doi: 10.1017/S0033291709991139. [DOI] [PubMed] [Google Scholar]
- Hart CE, Race V, Achouri Y, Wiame E, Sharrard M, Olpin SE, Watkinson J, Bonham JR, Jaeken J, Matthijs G, Van Schaftingen E. Phosphoserine aminotransferase deficiency: a novel disorder of the serine biosynthesis pathway. American Journal of Human Genetics. 2007;80:931–937. doi: 10.1086/517888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindström L, Iyo M. Elevated glutamine/glutamate ratio in cerebrospinal fluid of first episode and drug naive schizophrenic patients. BMC Psychiatry. 2005;5:6. doi: 10.1186/1471-244X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Archives of general psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 2004;55:165–171. doi: 10.1016/s0006-3223(03)00707-8. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC. Comparative effects of glycine and D-cycloserine on persistent negative symptoms in schizophrenia: a retrospective analysis. Schizophr Res. 2004;66:89–96. doi: 10.1016/S0920-9964(03)00129-4. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, Catinari S, Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Varilo T, Suvisaari J, Terwilliger JD, Ollikainen V, Arajarvi R, Juvonen H, Kokko-Sahin ML, Vaisanen L, Mannila H, Lonnqvist J, Peltonen L. A genomewide screen for schizophrenia genes in an isolated Finnish subpopulation, suggesting multiple susceptibility loci. Am J Hum Genet. 1999;65:1114–1124. doi: 10.1086/302567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Perlis RH, Lee PH, Rush AJ, Fava M, Sachs GS, Lieberman J, Hamilton SP, Sullivan P, Sklar P, Purcell S, Smoller JW. Cross-Disorder Genomewide Analysis of Schizophrenia, Bipolar Disorder, and Depression. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09091335. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J, Detheux M, Fryns JP, Collet JF, Alliet P, Van Schaftingen E. Phosphoserine phosphatase deficiency in a patient with Williams syndrome. Journal of Medical Genetics. 1997;34:594–596. doi: 10.1136/jmg.34.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J, Detheux M, Van Maldergem L, Foulon M, Carchon H, Van Schaftingen E. 3-Phosphoglycerate dehydrogenase deficiency: an inborn error of serine biosynthesis. Archives of Disease in Childhood. 1996;74:542–545. doi: 10.1136/adc.74.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glycine transport inhibitors and the treatment of schizophrenia. Biol Psychiatry. 2008;63:6–8. doi: 10.1016/j.biopsych.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Kamnasaran D, Muir WJ, Ferguson-Smith MA, Cox DW. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J Med Genet. 2003;40:325–332. doi: 10.1136/jmg.40.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, D'Souza C, Saksa J, Woods SW, Javitt DC. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121:125–130. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan DJ, Coutinho P. Cis-ruption mechanisms: disruption of cis-regulatory control as a cause of human genetic disease. Brief Funct Genomic Proteomic. 2009;8:317–332. doi: 10.1093/bfgp/elp022. [DOI] [PubMed] [Google Scholar]
- Labrie V, Roder JC. The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci. Biobehav. Rev. 2010;34:351–372. doi: 10.1016/j.neubiorev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:1196–1204. doi: 10.1001/archpsyc.62.11.1196. [DOI] [PubMed] [Google Scholar]
- Lane HY, Huang CL, Wu PL, Liu YC, Chang YC, Lin PY, Chen PW, Tsai G. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol Psychiatry. 2006;60:645–649. doi: 10.1016/j.biopsych.2006.04.005. [DOI] [PubMed] [Google Scholar]
- McInnis MG, Dick DM, Willour VL, Avramopoulos D, MacKinnon DF, Simpson SG, Potash JB, Edenberg HJ, Bowman ES, McMahon FJ, Smiley C, Chellis JL, Huo Y, Diggs T, Meyer ET, Miller M, Matteini AT, Rau NL, DePaulo JR, Gershon ES, Badner JA, Rice JP, Goate AM, Detera-Wadleigh SD, Nurnberger JI, Reich T, Zandi PP, Foroud TM. Genome-wide scan and conditional analysis in bipolar disorder: evidence for genomic interaction in the National Institute of Mental Health genetics initiative bipolar pedigrees. Biol Psychiatry. 2003;54:1265–1273. doi: 10.1016/j.biopsych.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, Hamase K, Zaitsu K, Kuroda S. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol. Psychiatry. 2007;61:1200–1203. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Muir WJ, Pickard BS, Blackwood DH. Chromosomal abnormalities and psychosis. Br. J. Psychiatry. 2006;188:501–503. doi: 10.1192/bjp.bp.106.023895. [DOI] [PubMed] [Google Scholar]
- Nakano I, Dougherty JD, Kim K, Klement I, Geschwind DH, Kornblum HI. Phosphoserine phosphatase is expressed in the neural stem cell niche and regulates neural stem and progenitor cell proliferation. Stem Cells. 2007;25:1975–1984. doi: 10.1634/stemcells.2007-0046. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J. Biol. Chem. 1994;269:1231–1236. [PubMed] [Google Scholar]
- Otsubo T, Tanaka K, Koda R, Shinoda J, Sano N, Tanaka S, Aoyama H, Mimura M, Kamijima K. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59:517–526. doi: 10.1111/j.1440-1819.2005.01408.x. [DOI] [PubMed] [Google Scholar]
- Pickard BS, Malloy MP, Porteous DJ, Blackwood DH, Muir WJ. Disruption of a brain transcription factor, NPAS3, is associated with schizophrenia and learning disability. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:26–32. doi: 10.1002/ajmg.b.30204. [DOI] [PubMed] [Google Scholar]
- Pineda M, Vilaseca MA, Artuch R, Santos S, Garcia Gonzalez MM, Aracil A, Van Schaftingen E, Jaeken J. 3-phosphoglycerate dehydrogenase deficiency in a patient with West syndrome. Developmental Medicine and Child Neurology. 2000;42:629–633. doi: 10.1017/s0012162200001171. [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M, Piven J, Ponting CP, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Sequeira AF, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stein O, Sykes N, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Webber C, Weksberg R, Wing K, Wittemeyer K, Wood S, Wu J, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Devlin B, Ennis S, Gallagher L, Geschwind DH, Gill M, Haines JL, Hallmayer J, Miller J, Monaco AP, Nurnberger JI, Jr., Paterson AD, Pericak-Vance MA, Schellenberg GD, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Scherer SW, Sutcliffe JS, Betancur C. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa A. Cortical development and glutamatergic dysregulation in schizophrenia. Biol. Psychiatry. 2009;66:530–532. doi: 10.1016/j.biopsych.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe JP, Wu H, Kelly TK, Phelps ME, Sun YE, Kornblum HI, Huang J. A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation. Chem. Biol. 2007;14:1019–1030. doi: 10.1016/j.chembiol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Freudenberg J, Becker T, Ohlraun S, Otte AC, Tullius M, Kovalenko S, Bogaert AV, Maier W, Rietschel M, Propping P, Nothen MM, Cichon S. Examination of G72 and D-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol. Psychiatry. 2004;9:203–207. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chang YC, Chong MY. D-alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2006;59:230–234. doi: 10.1016/j.biopsych.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Veiga-da-Cunha M, Collet JF, Prieur B, Jaeken J, Peeraer Y, Rabbijns A, Van Schaftingen E. Mutations responsible for 3-phosphoserine phosphatase deficiency. European Journal of Human Genetics. 2004;12:163–166. doi: 10.1038/sj.ejhg.5201083. [DOI] [PubMed] [Google Scholar]
- Visser R, Gijsbers A, Ruivenkamp C, Karperien M, Reeser HM, Breuning MH, Kant SG, Wit JM. Genome-wide SNP array analysis in patients with features of sotos syndrome. Horm Res Paediatr. 2010;73:265–274. doi: 10.1159/000284391. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- WTCCC Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, Nakamura K, Shimizu E, Itokawa M, Mori N, Iyo M, Yoshikawa T. Identification of Multiple Serine Racemase (SRR) mRNA Isoforms and Genetic Analyses of SRR and DAO in Schizophrenia and d-Serine Levels. Biol. Psychiatry. 2005;57:1493–1503. doi: 10.1016/j.biopsych.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang D, Cheng L, Qian Y, Alliey-Rodriguez N, Kelsoe JR, Greenwood T, Nievergelt C, Barrett TB, McKinney R, Schork N, Smith EN, Bloss C, Nurnberger J, Edenberg HJ, Foroud T, Sheftner W, Lawson WB, Nwulia EA, Hipolito M, Coryell W, Rice J, Byerley W, McMahon F, Schulze TG, Berrettini W, Potash JB, Belmonte PL, Zandi PP, McInnis MG, Zollner S, Craig D, Szelinger S, Koller D, Christian SL, Liu C, Gershon ES. Singleton deletions throughout the genome increase risk of bipolar disorder. Mol. Psychiatry. 2009;14:376–380. doi: 10.1038/mp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]