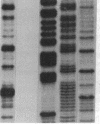
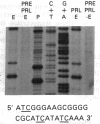
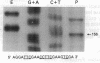
Abstract
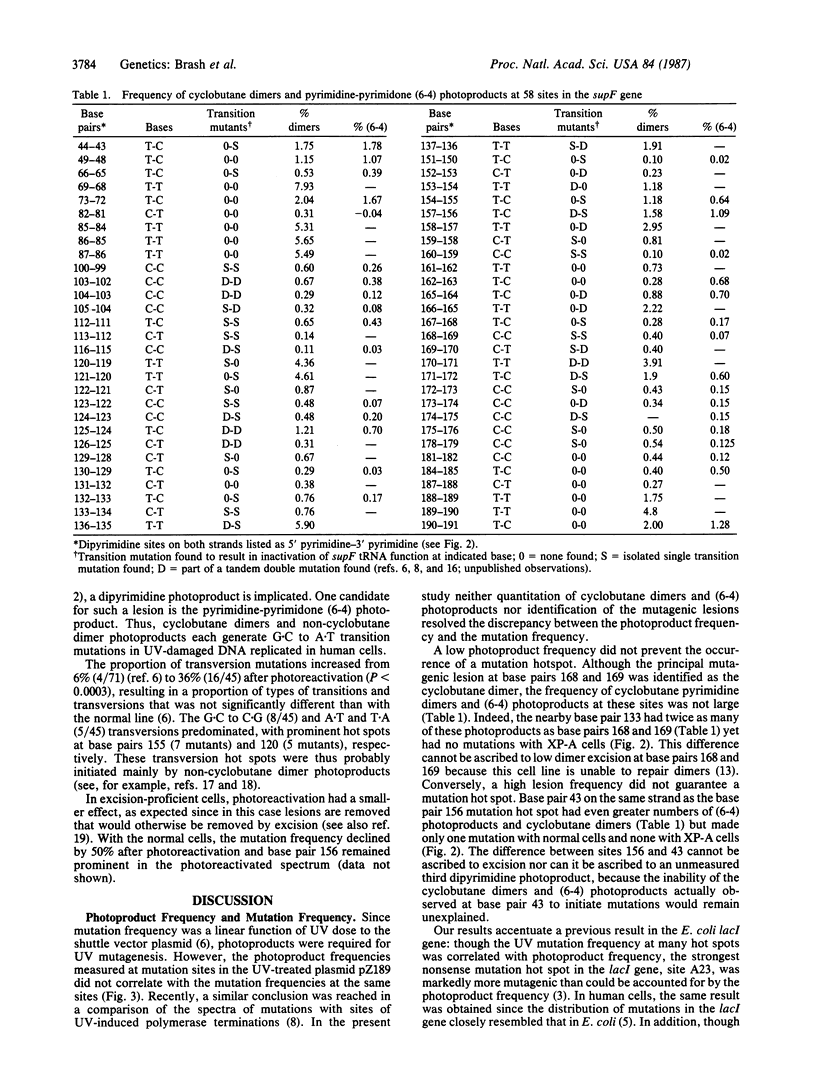
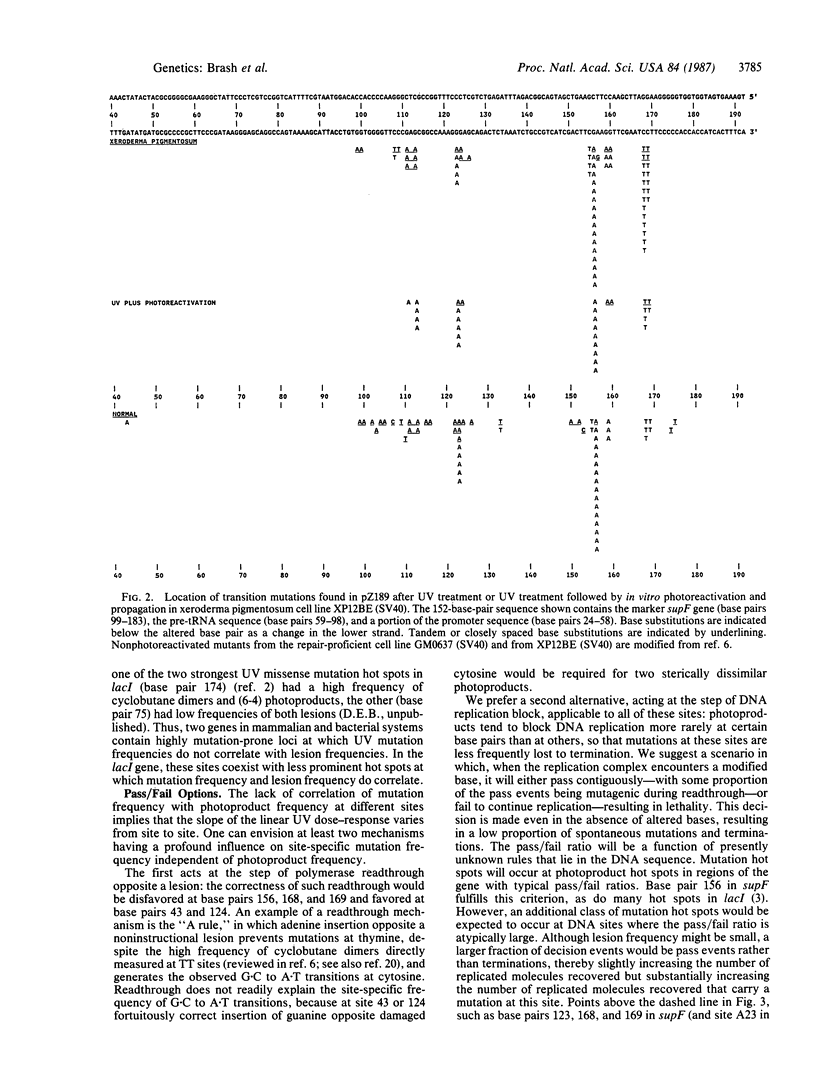
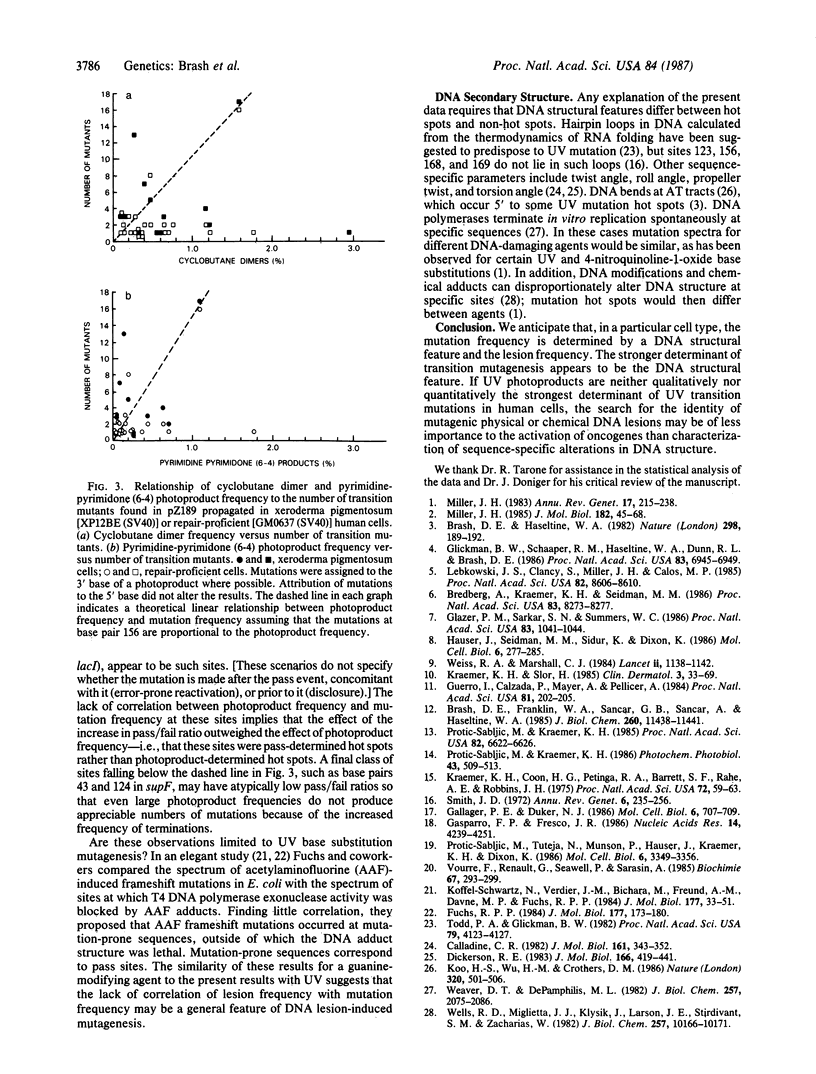
The role of UV radiation-induced photoproducts in initiating base substitution mutations in human cells was examined by measuring photoproduct frequency distributions and mutations in a supF tRNA gene on a shuttle vector plasmid transfected into DNA repair-deficient cells (xeroderma pigmentosum, complementation group A) and into normal cells. Frequencies of cyclobutane dimers and pyrimidine-pyrimidone (6-4) photoproducts varied by as much as 80-fold at different dipyrimidine sites within the gene. All transition mutations occurred at dipyrimidine sites, predominantly at cytosine, with a 17-fold variation in mutation frequency between different sites. Removal of greater than 99% of the cyclobutane dimers by in vitro photoreactivation before transfection reduced the mutation frequency while preserving the mutation distribution, indicating that (i) cytosine-containing cyclobutane dimers were the major mutagenic lesions at these sites and (ii) cytosine-containing non-cyclobutane dimer photoproducts were also mutagenic lesions. However, at individual dipyrimidine sites neither the frequency of cyclobutane dimers nor the frequency of pyrimidine-pyrimidone (6-4) photoproducts correlated with the mutation frequency, even in the absence of excision repair. Mutation hot spots occurred at sites with low or high frequency of photoproduct formation and mutation cold spots occurred at sites with many photoproducts. These results suggest that although photoproducts are required for UV mutagenesis, the prominence of most mutation hot spots and cold spots is primarily determined by DNA structural features rather than by the frequency of DNA photoproducts.
Full text
PDF
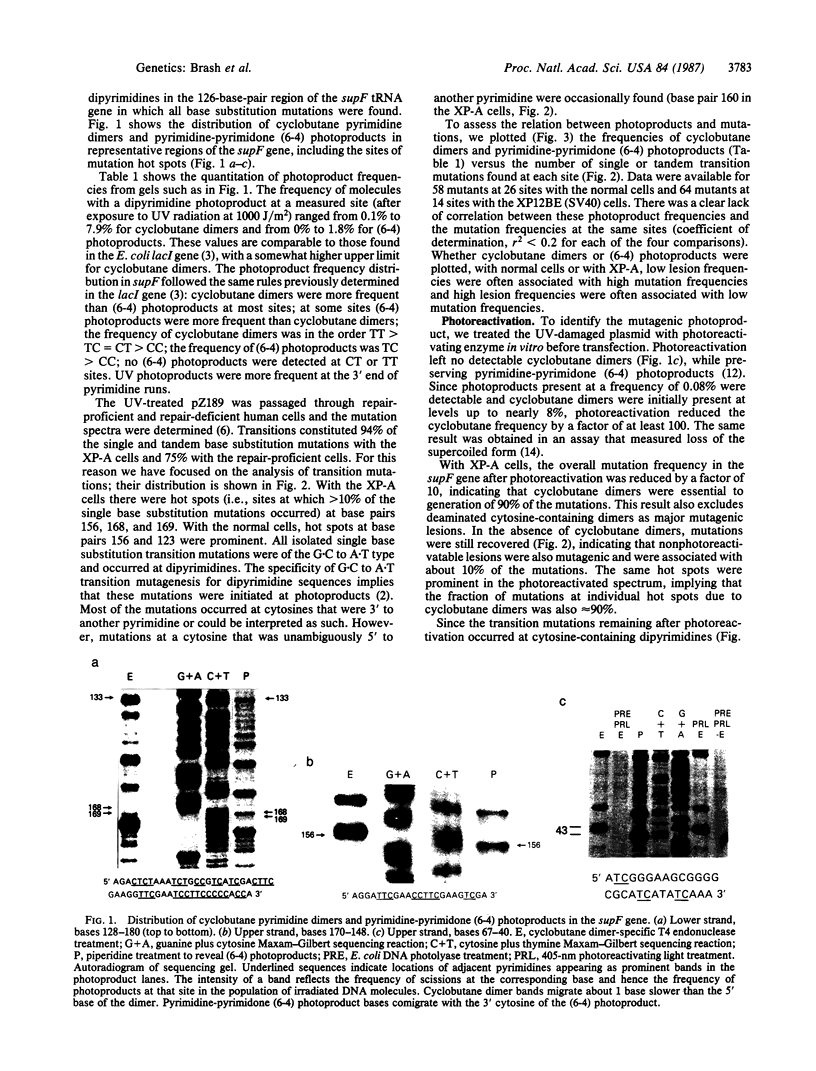
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourre F., Renault G., Seawell P. C., Sarasin A. Distribution of ultraviolet-induced lesions in simian virus 40 DNA. Biochimie. 1985 Mar-Apr;67(3-4):293–299. doi: 10.1016/s0300-9084(85)80071-7. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Franklin W. A., Sancar G. B., Sancar A., Haseltine W. A. Escherichia coli DNA photolyase reverses cyclobutane pyrimidine dimers but not pyrimidine-pyrimidone (6-4) photoproducts. J Biol Chem. 1985 Sep 25;260(21):11438–11441. [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calladine C. R. Mechanics of sequence-dependent stacking of bases in B-DNA. J Mol Biol. 1982 Oct 25;161(2):343–352. doi: 10.1016/0022-2836(82)90157-7. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. Base sequence and helix structure variation in B and A DNA. J Mol Biol. 1983 May 25;166(3):419–441. doi: 10.1016/s0022-2836(83)80093-x. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Gallagher P. E., Duker N. J. Detection of UV purine photoproducts in a defined sequence of human DNA. Mol Cell Biol. 1986 Feb;6(2):707–709. doi: 10.1128/mcb.6.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparro F. P., Fresco J. R. Ultraviolet-induced 8,8-adenine dehydrodimers in oligo- and polynucleotides. Nucleic Acids Res. 1986 May 27;14(10):4239–4251. doi: 10.1093/nar/14.10.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer P. M., Sarkar S. N., Summers W. C. Detection and analysis of UV-induced mutations in mammalian cell DNA using a lambda phage shuttle vector. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1041–1044. doi: 10.1073/pnas.83.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman B. W., Schaaper R. M., Haseltine W. A., Dunn R. L., Brash D. E. The C-C (6-4) UV photoproduct is mutagenic in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6945–6949. doi: 10.1073/pnas.83.18.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser J., Seidman M. M., Sidur K., Dixon K. Sequence specificity of point mutations induced during passage of a UV-irradiated shuttle vector plasmid in monkey cells. Mol Cell Biol. 1986 Jan;6(1):277–285. doi: 10.1128/mcb.6.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Coon H. G., Petinga R. A., Barrett S. F., Rahe A. E., Robbins J. H. National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20014, USA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer K. H., Slor H. Xeroderma pigmentosum. Clin Dermatol. 1985 Jan-Mar;3(1):33–69. doi: 10.1016/0738-081x(85)90096-3. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Clancy S., Miller J. H., Calos M. P. The lacI shuttle: rapid analysis of the mutagenic specificity of ultraviolet light in human cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8606–8610. doi: 10.1073/pnas.82.24.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Albertini A., Hofer M., Combépine C. Construction of plasmids carrying lacI mutations. J Mol Biol. 1985 Mar 5;182(1):65–68. doi: 10.1016/0022-2836(85)90027-0. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutational specificity in bacteria. Annu Rev Genet. 1983;17:215–238. doi: 10.1146/annurev.ge.17.120183.001243. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. Reduced repair of non-dimer photoproducts in a gene transfected into xeroderma pigmentosum cells. Photochem Photobiol. 1986 May;43(5):509–513. doi: 10.1111/j.1751-1097.1986.tb09528.x. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Tuteja N., Munson P. J., Hauser J., Kraemer K. H., Dixon K. UV light-induced cyclobutane pyrimidine dimers are mutagenic in mammalian cells. Mol Cell Biol. 1986 Oct;6(10):3349–3356. doi: 10.1128/mcb.6.10.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D. Gentics of transfer RNA. Annu Rev Genet. 1972;6:235–256. doi: 10.1146/annurev.ge.06.120172.001315. [DOI] [PubMed] [Google Scholar]
- Todd P. A., Glickman B. W. Mutational specificity of UV light in Escherichia coli: indications for a role of DNA secondary structure. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4123–4127. doi: 10.1073/pnas.79.13.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. T., DePamphilis M. L. Specific sequences in native DNA that arrest synthesis by DNA polymerase alpha. J Biol Chem. 1982 Feb 25;257(4):2075–2086. [PubMed] [Google Scholar]
- Weiss R. A., Marshall C. J. DNA in medicine. Oncogenes. Lancet. 1984 Nov 17;2(8412):1138–1142. doi: 10.1016/s0140-6736(84)91566-6. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]