Abstract
Background
TKA with conventional metal-backed tibial implants subjects the tibial metaphysis to stress shielding, with resultant loss of bone density.
Questions/purposes
We hypothesized tibial bone mineral density in patients with porous tantalum (trabecular metal) tibial baseplates would (1) more closely parallel tibial bone mineral density in the nonoperative control limb and (2) be better maintained than in conventional historical controls.
Patients and Methods
We prospectively followed 41 patients (35 men, six women) 60 years of age or younger undergoing TKA with uncemented trabecular metal tibial components. Patients underwent dual-energy xray absorptiometry scans of both proximal tibiae preoperatively and at 2 months, 1 year, and 2 years postoperatively. We determined bone mineral density in three selected regions of interest (Zone 1, between the pegs; Zone 2, beneath the pegs; Zone 3, directly below entire baseplate). Precision analysis revealed a precision error of 4% or less for each region of interest, indicating adequate power to detect bone mineral density changes of 8% or greater.
Results
Bone mineral density percent change was different between the operative and nonoperative knees only in Zone 3 and only at 2 months. There was no change in bone mineral density in any zone in the nonoperative knee at any time. Only in Zone 3 did the bone mineral density decrease at 2 months in the operative knee.
Conclusions
Trabecular metal implants appear to maintain tibial bone mineral density in a parallel fashion to the nonoperative limb in this population and better than historical controls.
Introduction
The number of TKAs performed in the United States continues to increase, with the rate of TKAs tripling from 1990 to 2002 [12]. The incidence of osteoarthritis of the knee is increasing in a younger patient population, with TKA more commonly performed for active patients in their 50s [13, 22]. These patients present multiple challenges for orthopaedic surgeons, as their activity level and life expectancy demand better long-term implant performance.
The surgical challenge and decreased survival of revision knee arthroplasty are well documented [2]. The severity of bone loss encountered at the time of revision arthroplasty plays a major role in the challenges faced with revision TKA. Bauman et al. reported a 10-year survival rate of 75.9% for revision TKA with structural allograft for bone loss [2]. Others have reported a lower survival rate of revision TKA in younger patient populations [7, 23]. With the increasing number of younger patients receiving TKAs, the need for bone-preserving implants becomes clear [6].
Previous studies have documented the pattern of bone mineral density (BMD) changes about TKA implants, with some studies reporting a marked decrease in BMD beneath tibial baseplates of various designs [14, 21, 26]. This decreased BMD is presumably in response to altered stress concentration, which may be time and design dependent [5, 14, 15]. Implants with a lower modulus of elasticity approximating that of native bone may decrease or eliminate this bone loss.
Cementless tibial implants rely on bone ingrowth and remodeling around the implant for secure fixation and implant longevity. Tantalum has a unique modulus of elasticity similar to that of host bone [9]. Tantalum tibial implants therefore might result in increased loading of the bone in contact with the whole implant and minimize stress shielding seen about the tibial implant. As marked bone ingrowth has been observed at the time of revisions of this implant [11, 24], and because bone ingrowth presumably is related to durability, this sort of implant may result in fewer revisions for aseptic loosening. However, long-term survival of this implant has not been clinically confirmed.
We hypothesized tibial BMD in patients with porous tantalum (trabecular metal [TM]) tibial baseplates would (1) more closely parallel tibial BMD in the nonoperative control limb and (2) be maintained better than in conventional historical controls.
Patients and Methods
We considered patients younger than 62 years undergoing primary TKA between 2005 and 2007 as candidates for this study. During this time, we performed TKAs in 458 patients. We excluded patients with known metabolic bone disease, those taking medications affecting BMD such as bisphosphonates, calcitonin, and hormone replacement (calcium and vitamin D were not excluded), and those whose preoperative deformity was judged to require modular stems, augmentation, or a degree of constraint not offered in the studied design. Patients with a prior contralateral TKA were not excluded. During the study time 41 patients (35 men, six women) who met the criteria underwent TKA with an uncemented TM tibial component (Zimmer NexGen®; Zimmer Inc, Warsaw, IN) (Fig. 1) and cemented femoral and all-polyethylene patellar components. Two patients had a contralateral TKA before the study and one had staged bilateral TKAs performed during the course of the study. Forty-two operative knees and 39 nonoperative knees therefore were available for analysis. Power analysis indicated 40 patients would be needed to detect a 35% difference in bone density (consistent with another study [15] and at a level that may be clinically relevant), assuming an alpha of 0.05 and a power of 80%. The mean age of the patients was 56.0 years (range, 42.9–62.6 years; SD, 4.3 years) (Table 1). IRB approval was obtained and all patients provided informed consent.
Fig. 1.
The uncemented tantalum TM tibial component used in the study (Zimmer Nex-Gen® TM) is shown.
Table 1.
Patient demographics
| Variable | Value |
|---|---|
| Number of patients | 41 |
| Age of patient preoperatively (years)* | 56.0 ± 4.3 (42.9–62.6) |
| Number of males/females | 35/6 |
| Number of Caucasian/African-American/Asian | 37/3/1 |
| Body mass index of patients preoperatively* | 32.6 ± 4.4 (24.8–43.5) |
| Number of normal weight/overweight/obese | 2/10/29 |
| Number with osteoporosis/low bone mass/normal bone mass | 0/16/24 |
* Values are expressed as mean ± SD, with range in parentheses.
Standard operative techniques were used; the femoral component was aligned in 3° external rotation relative to the posterior condylar axis or in neutral rotation relative to the transepicondylar axis with use of an intramedullary guide, and the tibial cuts were aligned with an extramedullary system. All operations were performed by or under the direct supervision of the principal investigator or one of three other adult reconstructive surgeons, each of whom supervised similar numbers of procedures throughout this trial. We recognize tibial bone density is greatest closest to the joint and may vary as the implant is placed at differing levels from the joint line. Although it would be difficult to ensure the implant was placed at the exact level in each patient, our surgical technique typically was based on a tibial resection of 0 to 2 mm below joint level of the most deficient compartment.
All perioperative and postoperative treatments were identical. All patients were allowed immediate postoperative weightbearing and progressed from using assistive devices as tolerated. Each patient received 24 hours of perioperative antibiotic prophylaxis and routine postoperative deep vein thrombosis (DVT) prophylaxis. Patients without additional risk factors for DVT or a history of DVT/pulmonary embolism received aspirin, 162 mg daily for 6 weeks. Patients with additional risk factors were given low-molecular-weight heparin until discharge and then treated with aspirin for 6 weeks. All patients also received mechanical prophylaxis with foot and ankle pneumatic compression devices until discharge.
Routine clinical followup included radiographs and validated scoring instruments (Knee Society score [10], WOMAC score [3], SF-36 score [17, 18]) at scheduled intervals of 2 weeks, 2 months, 6 months, and 1 and 2 years.
Patients underwent dual-energy xray absorptiometry (DEXA) scans of the bilateral proximal tibiae with designated precision knee software (GE Lunar Prodigy Advance®, v.11.1; GE Medical Systems, Milwaukee, WI) preoperatively and at 2 months, 1 year, and 2 years postoperatively. Fourteen of the patients (12 men, two women) were measured three times while they were in the supine position, repositioning between scans, to calculate the coefficient of variation and determine precision error measurements. Three selected regions of interest (ROIs) (Zone 1, between the pegs; Zone 2, beneath the pegs; Zone 3, directly below the entire baseplate, encompassing Zone 1) (Fig. 2) were chosen to evaluate BMD using a standardized protocol. Additional ROIs were used to help align the composite template on the nonoperated knee using similar methodology to that used by Abu-Rajab et al. [1]. Lumbar spine and hip BMD values also were obtained preoperatively and at 2 years to characterize patients as having osteoporosis, low bone mass, or normal bone mass. Some patients did not return for their 1- and 2-year scan appointments despite frequent reminders and rescheduled appointments, leaving smaller data cohorts at these periods.
Fig. 2A–B.
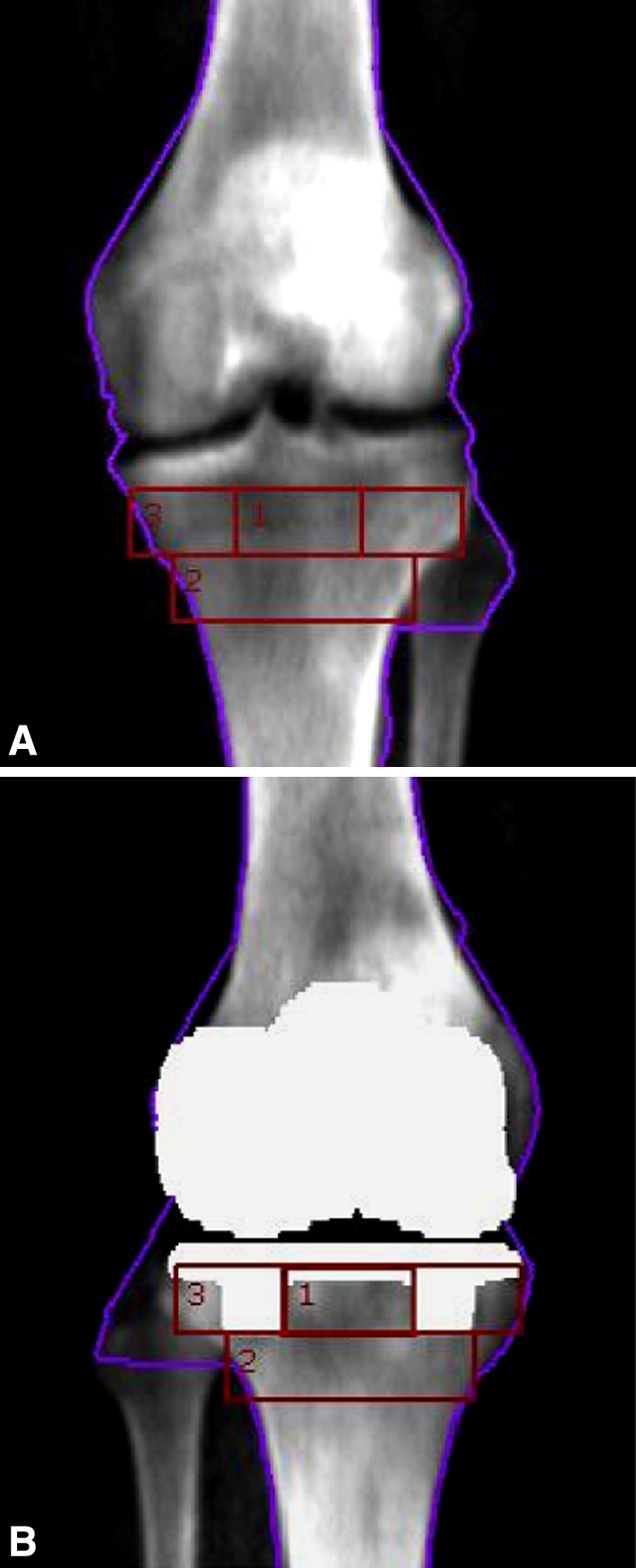
The ROIs are shown superimposed on (A) the nonoperative knee and (B) the operative knee. Zone 1 is between the pegs and is contained in Zone 3.
BMD and precision (reproducibility) analyses were completed using GE Lunar Prodigy Advance® (v.10.5) software. Fifteen patients had each knee scanned three times and BMD was calculated for each scan. The BMD precision error (reproducibility), which was measured using the root mean square method, ranged from 3.0% to 3.6% for each of the three ROIs, with a precision error of 2.8% for the three sites combined. As a result, BMD changes of 8% or greater for individual patients could be considered real changes and not artifacts of precision error. In comparison, age-related bone loss in trabecular bone is reportedly approximately 1% annually [16]. The percent change in BMD from the preoperative scans on the operative and nonoperative knees at the three designated ROIs to scans at 2 months, 12 months, and 24 months was analyzed using Student’s paired t test. BMD preoperatively and at 2 months, 12 months, and 24 months also was compared for operative and nonoperative knees at the three designated ROIs using Student’s t test or the Mann-Whitney test depending on the normality of the data, to determine if there were differences in BMD between the knees. Because of multiple comparisons in each zone, the Bonferroni correction method was used and p values less than 0.016 were considered significant. SPSS® v15.0 (SPSS Inc, Chicago, IL) was used for all analyses.
Results
The percent change in BMD was similar in the operative and nonoperative knees except in Zone 3 where it was reduced (p = 0.001) only at 2 months (Table 2). There was no change in BMD in the nonoperative knee for any ROI at any time. In Zone 1, the area between the pegs immediately beneath the baseplate, we found no change in BMD in the operative and nonoperative knees at any time (Table 3). In Zone 2, the region immediately beneath the pegs, we again found no change in BMD in the operative or nonoperative knee at any time (Table 4). Only in Zone 3 did the BMD decrease in the operative knee (Table 5). The mean BMD decreased (p = 0.005) in Zone 3 from 1.12 (SD, 0.19) preoperatively to 1.04 (SD, 0.22) at 2 months in the operative knee. There was no difference in BMD at 1 and 2 years in this ROI in the operative knee (p = 0.06 and p = 0.136, respectively).
Table 2.
Comparison in percent change in BMD for operative and nonoperative knees for all ROIs
| ROI/times of measurement | Percent change in BMD* | p Value† | |
|---|---|---|---|
| Operated knees | Nonoperated knees | ||
| Zone 1 | |||
| Δ between preoperative and 2 months | −0.6% ± 25.2% | 0.6% ± 12.9% | 0.08 |
| Δ between preoperative and 12 months | −3.0% ± 27.3% | −1.2% ± 14.4% | 0.75 |
| Δ between preoperative and 24 months | 2.0% ± 25.6% | −2.2% ± 10.0% | 0.98 |
| Zone 2 | |||
| Δ between preoperative and 2 months | −2.0% ± 15.8% | −0.8% ± 8.9% | 0.36 |
| Δ between preoperative and 12 months | −1.6% ± 13.6% | −0.7% ± 10.2% | 0.78 |
| Δ between preoperative and 24 months | 0.2% ± 14.1% | 1.7% ± 8.2% | 0.62 |
| Zone 3 | |||
| Δ between preoperative and 2 months | −6.7% ± 18.0% | −0.5% ± 9.6% | 0.001 |
| Δ between preoperative and 12 months | −8.4% ± 25.3% | −1.1% ± 11.3% | 0.14 |
| Δ between preoperative and 24 months | −6.6% ± 21.5% | −1.2% ± 9.6% | 0.31 |
* Values are expressed as mean ± SD; †paired t test; BMD = bone mineral density; ROI = region of interest.
Table 3.
Comparison of preoperative and postoperative BMD in Zone 1
| Knee/time of measurement | Number | BMD value* | p Value† |
|---|---|---|---|
| Operated knee | |||
| Preoperative | 42 | 1.03 (0.22) | 0.46 |
| 2 months | 42 | (0.24) | |
| Preoperative | 34 | 1.03 (0.23) | 0.35 |
| 12 months | 34 | 0.98 (0.30) | |
| Preoperative | 21 | 1.05 (0.28) | 0.59 |
| 24 months | 21 | 1.02 (0.34) | |
| Nonoperated knee | |||
| Preoperative | 39 | 1.04 (0.18) | 0.94 |
| 2 months | 39 | 1.04 (0.18) | |
| Preoperative | 30 | 1.04 (0.18) | 0.47 |
| 12 months | 30 | 1.03 (0.19) | |
| Preoperative | 18 | 1.05 (0.22) | 0.26 |
| 24 months | 18 | 1.01 (0.19) | |
* Values are expressed as mean, with SD in parentheses; †paired t test; BMD = bone mineral density.
Table 4.
Comparison of preoperative and postoperative BMD in Zone 2
| Knee/time of measurement | Number | BMD value* | p Value† |
|---|---|---|---|
| Operated knee | |||
| Preoperative | 42 | 1.19 (0.20) | 0.24 |
| 2 months | 42 | 1.15 (0.22) | |
| Preoperative | 34 | 1.19 (0.22) | 0.35 |
| 12 months | 34 | 1.17 (0.24) | |
| Preoperative | 21 | 1.22 (0.25) | 0.98 |
| 24 months | 21 | 1.22 (0.28) | |
| Nonoperated knee | |||
| Preoperative | 39 | 1.20 (0.17) | 0.42 |
| 2 months | 39 | 1.19 (0.17) | |
| Preoperative | 30 | 1.20 (0.18) | 0.55 |
| 12 months | 30 | 1.19 (0.18) | |
| Preoperative | 18 | 1.23 (0.19) | 0.30 |
| 24 months | 18 | 1.20 (0.18) | |
* Values are expressed as mean, with SD in parentheses; †paired t test; BMD = bone mineral density.
Table 5.
Comparison of preoperative and postoperative BMD in Zone 3
| Knee/time of measurement | Number | BMD value* | p Value† |
|---|---|---|---|
| Operated knee | |||
| Preoperative | 42 | 1.12 (0.19) | 0.005 |
| 2 months | 42 | 1.04 (0.22) | |
| Preoperative | 34 | 1.12 (0.20) | 0.06 |
| 12 months | 34 | 1.02 (0.31) | |
| Preoperative | 21 | 1.14 (0.24) | 0.136 |
| 24 months | 21 | 1.06 (0.31) | |
| Nonoperated knee | |||
| Preoperative | 39 | 1.12 (0.16) | 0.52 |
| 2 months | 39 | 1.11 (0.15) | |
| Preoperative | 30 | 1.13 (0.15) | 0.47 |
| 12 months | 30 | 1.12 (0.17) | |
| Preoperative | 18 | 1.12 (0.17) | 0.47 |
| 24 months | 18 | 1.10 (0.17) | |
* Values are expressed as mean, with SD in parentheses; †paired t test; BMD = bone mineral density.
Discussion
Bone loss or poor bone quality is a major concern for any orthopaedic surgeon performing revision TKAs. As an increasingly younger patient population undergoes TKA, the orthopaedic surgery community must consider techniques and implants that may preserve bone for a potential revision TKA. We sought to evaluate the BMD about a new uncemented tibial implant with a lower modulus of elasticity to determine if this implant might limit the stress shielding seen with more traditional tibial baseplates.
There are some limitations to this study. First, the majority of the participants were male, which, although characteristic of a Veterans Administration Medical Center patient population, is not representative of other TKA populations. However, this controlled population did allow us to better control for variations in BMD based on age and gender. Second, no statements can be made regarding BMD changes beneath the TM tibial baseplate beyond 2 years. As survival of most TKA designs is excellent for the first few years after TKA, a longer-term evaluation might prove valuable. At least one longer-term followup study has shown BMD loss of as much as 36.4% at 8 years after TKA [14]. Third, BMD changes after TKA may be most pronounced (and therefore most clinically relevant) in the distal femur [1, 14, 25, 26], but we did not examine this, as no tantalum ingrowth primary femoral component was available.
Accurately measuring and comparing bone density is challenging given the many variables that may affect bone density measurement and comparison. Osteoarthritis may alter the preoperative bone density of the operative and nonoperative knees, and pain secondary to osteoarthritis may limit the patient’s weightbearing, likewise affecting the bone density of either limb. Additionally, changes in anatomy and the weightbearing axis of the affected knee may increase density of the medial or lateral compartment in varus or valgus knees, respectively. Postoperatively, bone density may be affected similarly by weightbearing activity of patients and alterations in the mechanical axis of the limb. Finally, a potentially useful comparison to different TKA designs in the same patient was impractical as the sample size was small and the different implant design in the contralateral limb made zone modeling difficult. We acknowledge these limitations but believe the study population was too small to analyze multiple variables. Considering the logistics, patient commitment, and expense involved in this study of 41 patients (one of the larger single-design BMD studies), larger studies may prove impractical.
Quantifying BMD can be accomplished by numerous techniques such as ultrasound, quantitative CT, and DEXA. DEXA has the benefit of decreased radiation compared with CT. Also, recent software advances with DEXA technology allows for accurate measurement around arthroplasty implants. The software we used in this study to measure BMD can subtract the region occupied by the implant, measuring only the bone in question. The density is reported for the area as mg/cm2, not as total density, effectively negating the area occupied by the implant pegs.
Because of the unique design characteristics of low modulus and excellent bone ingrowth, it has been suggested a monoblock TM tibial component may be associated with a lower rate of osteolysis and component loosening, thereby leading to improved implant survivorship. A recent midterm study of this implant showed stable components with no evidence of osteolysis or loosening in 125 arthroplasties at a minimum 5-year followup [19]. Additionally, a recent radiostereophotogrammetric analysis of this implant compared with a cemented tibial component showed cessation of subsidence after 3 months in the TM component, suggesting improved stability compared with previous metal-backed cementless designs [8].
Previous studies examining BMD surrounding TKA tibial components have shown variable changes, although comparisons are limited by varying followups and implant designs. Seitz et al. [25] reported a small decrease in uncemented tibial BMD 1 month postoperatively with a stemmed tibial component, with density returning to the immediate postsurgery level by 1 year. Bohr and Lund [4] reported a 15% decrease in BMD 6 months after surgery, which returned to 90% of the immediate postoperative level by 3.5 years after the operation. Levitz et al. [14] reported on seven patients followed for 8 years and observed inconsistent declines in tibial BMD in the first 6 weeks to 6 months after TKA, a return to the immediate postoperative density by 1 year, and a mean 36.4% decrease in BMD of the proximal tibia 8 years after TKA. In a more recent study reviewing BMD beneath an uncemented tibial implant, Petersen found BMD decreased 22% at 3 years [20]. His study proposed alteration of the mechanical axis to a prearthrosis alignment may play a role in the decreased BMD noted postoperatively [20]. Unlike these previous studies, we found the BMD unchanged from the preoperative measurement in two of the three ROIs examined. We believe the unique properties of the TM tibial implant may be responsible for maintaining the BMD in Zones 1 and 2. The material characteristics of tantalum closely match that of bone (cancellous 10–1723 MPa; cortical 12,000–18,000 MPa) with a modulus of elasticity of 5200 MPa [9]. With biomaterial properties similar to that of host bone, stress shielding is minimized and load transfer across the implant should be more uniform. This may result in an increase in the loading of the bone in contact with the whole implant and minimize the stress shielding seen about the tibial implant that was reported in other studies [5, 14, 15].
Some studies have sought to determine the role design of the tibial baseplate (stemmed versus pegged) or method of fixation (cemented versus ingrowth) might play in the changes seen in BMD. Lonner et al. [15] and Bourne and Finlay [5] found centrally stemmed tibial components had a greater impact on stress shielding of the proximal tibia than those without stems or with short pegs. In another study, changes in BMD beneath cemented and uncemented stemmed baseplates were compared. The authors found no difference in BMD between the two methods of fixation [1].
The ROIs in our study were designed specifically for the implant in question. Zones 1, 2, and 3 were chosen because they might sustain stress shielding that could be clinically important with time. We believed Zone 3 was an especially important area as it represents all the bone immediately adjacent to and supporting the implant. The BMD in Zone 3 was seen to decrease substantially at 2 months in the operative knee. This is an interesting finding as Zone 3 includes Zone 1, with only the addition of small regions of bone outside the pegs, and Zone 1 did not have a similar decrease in BMD in the operative knee. As Zone 3 would include the more sclerotic medial/lateral plateau region characteristic of varus/valgus gonarthrosis, perhaps BMD decreases enough with the postsurgical removal of this bone to impact the ROI. Although the percent change in Zone 3 BMD from preoperatively to 2 months postoperatively differed between the operative and nonoperative knees, BMD did not increase with time in the nonoperative knee in this ROI. Possible explanations for this finding are diminished BMD with age or improved bilateral weight transfer after successful TKA. We also examined each ROI with time for each patient. Although the average BMD did not change in Zones 1 and 2 in the operative knee, we found the BMD increased in some patients and decreased in others. This is consistent with other studies of BMD of the proximal tibia after TKA showing broad ranges in BMD with large SDs. Abu-Rajab et al. [1] reported SDs from 9.2% to 23.1% and Wang et al. [27] reported SDs from 8.8% to 14.1% with BMD percent changes ranging from −30.9% to +36.5%. Our study showed SDs from 9.0% to 32.1% and BMD percent changes ranging from −68.8% to +100.3%. This shows the importance of analyzing percent change in BMD rather than an absolute value and reflects the number of variables that may impact proximal tibial BMD.
The potential for implants that prevent stress shielding and therefore potential bone loss has long-term implications for arthroplasty revisions. Bone loss attributable to stress shielding in periprosthetic bone has been described in multiple studies involving the distal femur and proximal tibia [1, 5, 7, 12–14, 21, 23, 26]. Results of our study suggest BMD may be better preserved beneath a TM tibial baseplate compared with previous implant designs. Whether this will positively impact the anticipated TKA revision in the younger patient remains unanswered. Continued research of implants that minimize stress shielding and emphasize bone preservation is necessary as an increasingly younger patient population is considered for TKA.
Acknowledgments
We thank Patricia Knickerbocker and Mary Schoeller, RT(R), CDT, for assistance with this study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Minneapolis Veterans Administration Medical Center and HealthEast Osteoporosis Center, St Paul, MN.
References
- 1.Abu-Rajab RB, Watson WS, Walker B, Roberts J, Gallacher SJ, Meek RM. Peri-prosthetic bone mineral density after total knee arthroplasty: cemented versus cementless fixation. J Bone Joint Surg Br. 2006;88:606–613. doi: 10.1302/0301-620X.88B5.16893. [DOI] [PubMed] [Google Scholar]
- 2.Bauman RD, Lewallen DG, Hanssen AD. Limitations of structural allograft in revision total knee arthroplasty. Clin Orthop Relat Res. 2009;467:818–824. doi: 10.1007/s11999-008-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 4.Bohr HH, Lund B. Bone mineral density of the proximal tibia following uncemented arthroplasty. J Arthroplasty. 1987;2:309–312. doi: 10.1016/S0883-5403(87)80064-5. [DOI] [PubMed] [Google Scholar]
- 5.Bourne RB, Finlay JB. The influence of tibial component intramedullary stems and implant-cortex contact on the strain distribution of the proximal tibia following total knee arthroplasty: an in vitro study. Clin Orthop Relat Res. 1986;208:95–99. [PubMed] [Google Scholar]
- 6.Gioe TJ, Novak C, Sinner P, Ma W, Mehle S. Knee arthroplasty in the young patient: survival in a community registry. Clin Orthop Relat Res. 2007;464:83–87. doi: 10.1097/BLO.0b013e31812f79a9. [DOI] [PubMed] [Google Scholar]
- 7.Harrysson OL, Robertsson O, Nayfeh JF. Higher cumulative revision rate of knee arthroplasties in younger patients with osteoarthritis. Clin Orthop Relat Res. 2004;421:162–168. doi: 10.1097/01.blo.0000127115.05754.ce. [DOI] [PubMed] [Google Scholar]
- 8.Henricson A, Linder L, Nilsson KG. A trabecular metal tibial component in total knee replacement in patients younger than 60 years: a two-year radiostereophotogrammetric analysis. J Bone Joint Surg Br. 2008;90:1585–1593. doi: 10.1302/0301-620X.90B12.20797. [DOI] [PubMed] [Google Scholar]
- 9.Hedrocel: A Structural Biomaterial. Allendale, NJ: Implex Corp; 1997. [Google Scholar]
- 10.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 11.Klein GR, Levine HB, Hartzband MA. Removal of a well-fixed trabecular metal monoblock tibial component. J Arthroplasty. 2008;23:619–622. doi: 10.1016/j.arth.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 13.Laskin RS. Session III: Total knee replacement in young patients. Clin Orthop Relat Res. 2002;404:100–101. doi: 10.1097/00003086-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Levitz CL, Lotke PA, Karp JS. Long-term changes in bone mineral density following total knee replacement. Clin Orthop Relat Res. 1995;321:68–72. [PubMed] [Google Scholar]
- 15.Lonner JH, Klotz M, Levitz C, Lotke PA. Changes in bone density after cemented total knee arthroplasty: influence of stem design. J Arthroplasty. 2001;16:107–111. doi: 10.1054/arth.2001.16486. [DOI] [PubMed] [Google Scholar]
- 16.Mazess RB. On aging bone loss. Clin Orthop Relat Res. 1982;165:239–252. [PubMed] [Google Scholar]
- 17.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 18.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 19.O’Keefe TJ, Winter S, Lewallen DG, Robertson DD, Poggie RA. Clinical and radiographic evaluation of a monoblock tibial component. J Arthroplasty. 2009 July 27 [Epub ahead of print]. [DOI] [PubMed]
- 20.Petersen MM. Bone mineral measurements at the knee using dual photon and dual energy x-ray absorptiometry: methodological evaluation and clinical studies focusing on adaptive bone remodeling following lower extremity fracture, total knee arthroplasty, and partial versus total meniscectomy. Acta Orthop Scand Suppl. 2000;293:1–37. [PubMed] [Google Scholar]
- 21.Petersen MM, Nielsen PT, Lauritzen JB, Lund B. Changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty: a 3-year follow-up of 25 knees. Acta Orthop Scand. 1995;66:513–516. doi: 10.3109/17453679509002305. [DOI] [PubMed] [Google Scholar]
- 22.Robertsson O, Dunbar MJ, Knutson K, Lidgren L. Past incidence and future demand for knee arthroplasty in Sweden: a report from the Swedish Knee Arthroplasty Register regarding the effect of past and future population changes on the number of arthroplasties performed. Acta Orthop Scand. 2000;71:376–380. doi: 10.1080/000164700317393376. [DOI] [PubMed] [Google Scholar]
- 23.Robertsson O, Knutson K, Lewold S, Lidgren L. The Swedish Knee Arthroplasty Register 1975–1997: an update with special emphasis on 41, 223 knees operated on in 1988–1997. Acta Orthop Scand. 2001;72:503–513. doi: 10.1080/000164701753532853. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez Marquez JM, Del Sel N, Leali A, Gonzalez Della Valle A. Case reports: Tantalum debris dispersion during revision of a tibial component for TKA. Clin Orthop Relat Res. 2009;467:1107–1110. doi: 10.1007/s11999-008-0586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz P, Ruegsegger P, Gschwend N, Dubs L. Changes in local bone density after knee arthroplasty: the use of quantitative computed tomography. J Bone Joint Surg Br. 1987;69:407–411. doi: 10.1302/0301-620X.69B3.3584195. [DOI] [PubMed] [Google Scholar]
- 26.Soininvaara TA, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM, Kroger HP. Periprosthetic tibial bone mineral density changes after total knee arthroplasty: one-year follow-up study of 69 patients. Acta Orthop Scand. 2004;75:600–605. doi: 10.1080/00016470410001493. [DOI] [PubMed] [Google Scholar]
- 27.Wang CJ, Wang JW, Ko JY, Weng LH, Huang CC. Three-year changes in bone mineral density around the knee after a six-month course of oral alendronate following total knee arthroplasty: a prospective, randomized study. J Bone Joint Surg Am. 2006;88:267–272. doi: 10.2106/JBJS.E.00051. [DOI] [PubMed] [Google Scholar]



