Abstract
Background
Rodent lumbar and caudal (tail) spine segments provide useful in vivo and in vitro models for human disc research. In vivo caudal models allow characterization of the effect of static and dynamic loads on disc mechanics of individual animals with time, but the lumbar models have required sacrifice of the animals for in vitro mechanical testing.
Questions/purposes
We therefore developed a novel displacement controlled in vivo lumbar spine noninvasive induced angular displacement (NIAD) test; data obtained with NIAD were used to compare angular displacement between segmental levels (L4/L5, L5/L6 and L6/S1), interobserver radiograph measurement agreement, and intraobserver radiograph measurement repeatability. Measurements from NIAD were compared with angular displacement, bending stiffness, and moment to failure measured by an in vitro test.
Methods
Anesthetized Lewis rats were xrayed in a 90° angled fixture, and NIAD was measured at lumbar levels L4 to S1 by two independent and blinded observers. After euthanasia, in vitro angular displacement (IVAD), stiffness, and failure moment were measured for the combined L4-L6 segment in four-point bending.
Results
NIAD was greater at L4/L5 and L5/L6 than at L6/S1. Combined coronal NIAD for L4-L6 was 42.8° ± 5.3° and for IVAD was 61.5° ± 3.8°. Reliability assessed by intraclass correlation coefficient (ICC) was 0.905 and 0.937 for intraobserver radiograph measurements, and interobserver ICCs ranged from 0.387 to 0.653 for individual levels. The interobserver ICC was 0.911 for combined data from all levels. Reliability for test-retest NIAD measurements had an ICC of 0.932. In vitro failure moment correlated with NIAD left bending.
Conclusions
The NIAD method yielded reproducible and reliable rat lumbar spine angular displacement measurements without required euthanasia, and allows repetitive monitoring of animals with time. For lumbar spine research studies performed during a course of time, the NIAD method may reduce animal numbers required by providing serial angular displacement measurements without euthanasia.
Clinical Relevance
Improved methods to assess comparative models for disease or aging may permit enhanced clinical treatments and improved patient care.
Introduction
Intervertebral discs play a critical role in spine flexibility and ROM. Additional determinants of spine flexibility and ROM include spine ligaments, intrinsic spine muscles, and trunk musculature and articulations with the axial and appendicular skeleton. Intervertebral disc disorders can alter ROM and cause back pain, as evidenced by multiple clinical etiologies including spondyloarthropathy [11, 15], disc infection [3, 16], and aging and disc degeneration [21, 35]. Comparative rodent models of these spinal disease states have offered mechanisms for better understanding of these conditions, and suggestions to improve clinical care [18, 33, 37, 38, 43]. Through enhanced overall understanding of the mechanics, physiology, and pathology of the intervertebral disc, additional preventative and curative clinical strategies may be developed [1].
Spine and intervertebral disc mechanics have been studied extensively in comparative rodent models, using the lumbar and caudal (tail) spine segments. Caudal models have used numerous mechanical, pharmacologic, and control treatments delivered in vivo and in vitro [13, 14, 22, 23, 27–29, 31, 32, 36, 42], and biomechanical testing in vivo [23, 28, 29, 42] and in vitro [13, 22, 27, 30, 36]. These various caudal in vivo models use invasive external fixators to deliver forces and assess force-displacement characteristics. Lumbar models have been used to deliver disc treatments in vivo [4] and in vitro [5, 6], but the biomechanical consequences were reported only for samples assessed in vitro after animal sacrifice [4–6]. Methods to noninvasively assess lumbar spine angular displacement or other biomechanical end points in vivo have not been described, possibly owing to technical difficulty for quantitative delivery of forces to the spine during load-controlled experiments.
We therefore raised the following questions: (1) Could lumbar angular displacement be reproducibly noninvasively quantified in vivo? Reproducibility was assessed by interobserver and intraobserver tests and test-retest repeatability; and (2) would displacement controlled in vivo measurements correlate with in vitro load controlled tests?
Materials and Methods
In this observational study of rat lumbar spine angular displacement, we used bending radiographs of living animals under anesthesia to measure and compare segmental NIAD, and explanted lumbar specimens from euthanized animals to generate comparative IVAD measurements (Fig. 1). We used 78 male Lewis rats (275–300 gm) that had been obtained by other investigators for unrelated projects: (1) on arrival to the facility and before surgery (n = 53), or (2) postoperatively from limb surgery and immediately before euthanasia (n = 25). The first group of 53 animals was used to quantify segmental lumbar NIAD, and to determine intraobserver and interobserver agreement for measurement of the radiographs. Fifteen animals from the second group were used to measure NIAD test-retest repeatability, and for dual testing with NIAD and IVAD methods for paired sample induced angular displacement measurement comparisons; the remaining 10 animals from the second group were tested only for IVAD, and in vitro stiffness and failure moment. We had prior approval for our protocol from our Institutional Animal Care and Use Committee, and permission from the other investigators for use of their animals.
Fig. 1.
A flowchart for the study design is depicted. The left column shows how interobserver and intraobserver radiographic measurements were assessed, and where the ANOVA assessments were made to compare segmental angular displacement for each observer and between observers. The right column shows how specimens were used for the test-retest, paired in vivo/in vitro, and in vitro biomechanics assessments.
All animals were anesthetized with isoflurane (1.5% to 3%) anesthesia for NIAD testing. High-resolution standardized radiographs were obtained using a Faxitron model MX-20 with DC-4 digital camera (Faxitron X-Ray Corporation, Wheeling, IL, USA). Faxitron imaging was performed at 35 kV and 10-second maximal exposure. Animals were maintained in supine right and left bending positions in the AP plane with a radiolucent 90° bracket (Fig. 2A). Flexion and extension bending radiographs were not obtained. In vivo sagittal ROM was not measured owing to technical difficulties reproducibly immobilizing the pelvic girdle of the animals when in the lumbar flexed position, and inability to generate a three-point fulcrum by positioning in the 90° bracket. Digital xray images were repeated if pelvic rotation, assessed by symmetry of sacroiliac joints (posterior-inferior iliac spine) and pubic symphysis to the center of the sacrum, was not neutral and if three-point force was not in contact with the iliac wing and low flank (Fig. 2B). To assess NIAD test-retest reproducibility, animals were xrayed in the right-bending position, repositioned in the left-bending position, and xrayed, and then this process was repeated to obtain triplicate sets of radiographs (n = 15 animals) while being maintained under a single anesthesia.
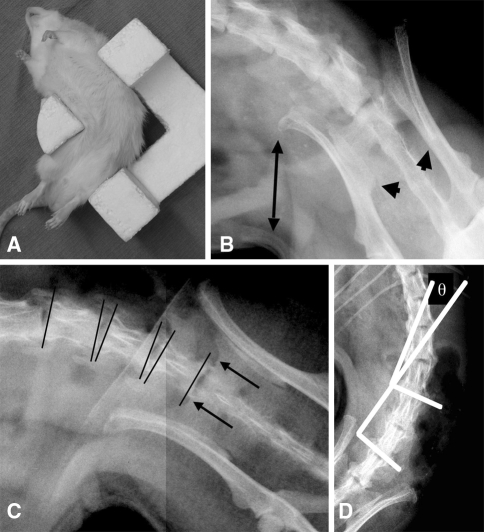
Fig. 2A–D.
(A) An anesthetized Lewis rat is shown in the bending bracket positioned for right-bending radiograph. (B) This high-definition digital radiograph shows inappropriate positioning of the animal in the bracket for right bend assessment with lack of correct force application on the iliac wing (double-ended arrow) and unacceptable rotation judged by the asymmetry of the posterior inferior iliac spines (arrowheads). (C) Bony landmarks for the pelvic measurements (arrows point to the bilateral sacral ala sulci, the most caudal location of the proximal sacral ridge) and vertebral end plate line placement (lines) are shown. The animal is well positioned with symmetric posterior inferior iliac spines and good contact of the iliac wing with the positioning bracket. (D) Cobb end plate measurement (θ) for the L4/L5 segment is shown.
Two of us (MEC, SB) used a modified Cobb technique to measure angles between the lumbar vertebrae (n = 53, Question 1; n = 15, Question 2). Digital measurements were taken directly from the Faxitron images (Image-J Freeware, Version 1.37 h; National Institutes of Health, Bethesda, MD). Both observers were blinded and measured identical images. Measurements were performed by defining the proximal and distal end plates for L4, L5, L6, and a line connecting the sulci of the S1 sacral ala (Fig. 2C). To normalize between images, the end plates (referenced to the unique horizontal of each image by the software) were converted to reference from the sacrum that was defined as 0° based on methods reported in clinical studies [40, 41]. Sacrum-referenced end-plate positions were used to measure angulation differences between the proximal vertebra upper end plate and distal vertebra lower end plate (Fig. 2D), according to the Cobb method [10]. Angles between the L4/L5, L5/L6, L4-L6, and L6/S1 vertebrae were measured for each bending direction and summed for each animal to obtain segmental coronal NIAD (coronal NIAD = right bend NIAD + left bend NIAD). As a result of out-of-plane coupled movement mediated by the spine posterior elements, quantifications above L4 were not possible.
Spine specimens were potted and tested in vitro in nondestructive and destructive bending (n = 25, Question 2). Animals were euthanized by carbon dioxide asphyxiation, spines explanted intact from L3 to S1, and muscle and ligaments were removed from the posterior elements preserving the discs, facets, and bony elements (n = 25). When necessary, samples were stored in saline-soaked gauze at −20°C. One sample was damaged during preparation, and was excluded. The proximal (L3 and half of L4) and distal (S1 and half of L6) ends of each segment were fixed with acrylic bone cement (COE Tray Plastic; GC America, Chicago, IL, USA) in custom rectangular aluminum fixtures, allowing free movement of the L4/L5 and L5/L6 levels. Four-point bending was performed with a 50-mm outer support span and inner support span of 22.5 mm with loads applied at 0.5 N/second (ELF 3200; EnduraTec, Eden Prairie, MN, USA) (Fig. 3A). Rotation of the rectangular fixtures allowed the samples to be positioned in four orthogonal anatomic directions. Hydration was maintained by wrapping the specimens in saline-soaked gauze. Samples were tested nondestructively in right and left lateral bending, extension, and flexion in random order and then tested to failure in extension over the combined L4-L6 segment. Flexion and extension testing were included in the in vitro testing to provide a more complete description of the mechanics in four-directions. The maximum load and number of cycles for the nondestructive testing were determined empirically from a series of practice samples and set at 4 N, corresponding to a bending moment of 55 N-mm. Each sample was loaded for five cycles (Fig. 3B). Stiffness was measured from the fifth cycle, after preconditioning of the sample had led to nearly identical load-displacement traces for cycles 2 through 5 (Fig. 3C). At maximal deflection during the final nondestructive testing cycle, the sample angular deformation also was recorded photographically (Nikon D70, Melville, NY, USA). Composite L4-L6 segment IVAD was measured relative to the fixture edges in each loading direction from the photographs (Image-J Freeware) (Fig. 3A). Coronal IVAD was defined as the sum of the right and left IVAD measurements (as described previously) and sagittal IVAD as the sum of the flexion and extension IVADs. For the destructive tests (Fig. 3D), the failure bending moment and failure location (L4/L5 or L5/L6 disc) were recorded.
Fig. 3A−D.
(A) This photograph of the four-point bending fixture shows the specimen in maximal deflection in left lateral bending with angular deformation (θ) indicated. Displacement is in the downgoing vertical direction and the load cell is beneath the bottom fixture. (B) Continuous cycling data for a sample tested in flexion are shown. Displacement is graphed as decreasing from right to left during cycling reflecting the apparatus construction and applied force is negative by convention. (C) Load-displacement traces for a representative sample in the fifth loading cycle for each of the specified directions are shown. Stiffness is the slope of curve between 2 and 4 N in the loading phase. The plots for each of the directions were superposed and are illustrated after translation to avoid overlap. (D) A representative bending to failure curve is shown; the arrow marks the failure load (20 N).
Bending stiffness (EI) for each of the four directions was calculated using the formula for four-point bending mechanics [7]. Variables are as follows: ∆F = force applied, y = ½ inner support separation distance, x = ½ (outer support separation − inner support separation), d = displacement of sample during test, and EI is the four-point bending flexural stiffness. The complete equation is: d = (1/2 ∆F x2 * y)/EIsample + (1/2 ∆F x3)/3EIsupports. As reported previously [12], if the latter term in this formula is insignificant as a result of a large value of the EI supports term, then the formula simplifies to the following: stiffness (EIsample, N-mm2) = (1/16*(∆F/d)*(outer support separation – inner support separation)2 * (inner support length)). Testing in bending, rather than torsion or axial loading, was chosen as most comparable to the loading delivered in the NIAD testing method. Four-point bending was chosen over three-point assessment because a constant moment is applied to samples during measurement, and loads are not applied directly to the sample, each factor avoiding potential sources of error.
The potential effect that different observers impart to NIAD measurements, as this relates to application of the modified Cobb measurement technique described above, was quantified through independent blinded observers (MEC, SB) making measurements on identical digital radiographs (n = 53, Question 1). Segmental radiographic measurements were assessed for agreement between observers (interobserver measurement reliability) using the ICC for two independent observers taking single measures. After a delay to prevent measurement bias, the two observers performed repeat measurements of the same digital radiographs and intraobserver measurement agreement was assessed by ICC as above. ICC calculations were performed using the two-way mixed single-measure formula for consistency (SPSS Version 16; SPSS Inc., Chicago, IL, USA).
Reproducibility for application of the NIAD measurement method was quantified using ICC testing of NIAD measurements obtained from serial (triplicate) test applications during a single anesthesia, with radiographs obtained and digital measurements performed by one observer (MEC). Triplicate NIAD data sets were compared for measurement agreement using ICC as described. Correlations between the NIAD, IVAD, and in vitro biomechanics measurements were assessed by linear regression to estimate correlation (Pearson’s r). One-factor ANOVA with Bonferroni post hoc testing was used to compare NIAD measurement means between levels for each rater, and one-factor ANOVA was used to compare segmental radiographic measurements between raters for each level.
Results
Displacement controlled NIAD measurements showed different segmental motion by level for each observer, reliable intraobserver radiographic measurements, an observer effect on digital radiograph measurements, and reproducible NIAD measurements with serial test application. Average coronal lumbar spine NIAD was similar between the L4/L5 bodies and the L5/L6 bodies for each observer (Table 1) (p > 0.05). Average NIAD was less at the L6/S1 junction as compared with the L4/L5 or L5/L6 levels (p < 0.001 for both raters for each comparison). Radiographic measurements performed on identical digital radiographs were different between raters (p < 0.001 at each segment), suggesting an observer effect when applying the modified Cobb measurement technique. The ICC intraobserver radiograph measurement reliability was 0.905 and 0.937 for each observer, and the interobserver ICCs ranged from 0.387 to 0.653 for measurements from individual segments (Table 1) and overall was 0.911 for measurements pooled and assessed as a single data set. NIAD test-retest reliability ICC was 0.932 for all segments assessed as a single data set, and average segmental ROM did not show progression during serial NIAD test applications (data not shown).
Table 1.
Lumbar in vivo noninvasive induced angular displacement
| Spine level | NIAD | Interobserver | ||
|---|---|---|---|---|
| Observer 1 (degrees) | Observer 2 (degrees) | ICC | ANOVA | |
| L4/L5 | 24.6 ± 4.6 | 19.7 ± 4.6 | 0.387 | p < 0.001 |
| L5/L6 | 22.3 ± 4.2 | 18.6 ± 4 | 0.517 | p < 0.001 |
| L6/S1 | 17.4 ± 4.8 | 13.7 ± 3.6 | 0.552 | p < 0.001 |
| L4-L6 | 42.8 ± 5.3 | 37.9 ± 5.6 | 0.653 | p < 0.001 |
| Intersegmental ANOVA | ||||
| L4/L5 vs L5/L6 | p = 0.28 | p = 0.452 | ||
| L4/L5 vs L6/S1 | p < 0.001 | p < 0.001 | ||
| L5/L6 vs L6/S1 | p < 0.001 | p < 0.001 | ||
NIAD = noninvasive induced angular displacement; ICC = intraclass correlation coefficient; ANOVA – analysis of variance.
Comparison between NIAD and in vitro testing methods showed correlation between NIAD left bending and in vitro extension moment to failure. Average IVAD in four directions and in vitro bending stiffness in four directions were quantified (Table 2). In comparison to NIAD over the combined L4-L6 segment, IVAD measurements were greater in each specimen assessed by both methods (compared with Observer 1, measurement differences averaged 13.7° ± 6.9°; range 2.2°–24.5°; n = 14), and this also was shown by comparison of group averages for the techniques that had 12.8° more motion in the in vitro samples (Table 2). For the samples tested with in vivo and in vitro methods, we observed no correlation between NIAD and IVAD, and no correlation between NIAD or IVAD with in vitro stiffness (Table 2). Specimens loaded to failure in extension failed at the discs, with 14 of 25 (56%) occurring at the L5/L6 level, and an average failure moment of 138.2 ± 46.1 N-mm. For the samples tested with in vivo and in vitro methods, in vitro failure moment correlated with left bending NIAD only (Table 2). Comparing all available in vitro tested samples (n = 24), we observed a correlation between extension IVAD and failure moment (r = 0.44, p < 0.05) and sagittal IVAD and failure moment (r = 0.41, p < 0.05). No correlations were found for the (n = 24) in vitro sample comparisons for stiffness in any direction and failure moment (p = 0.35 to 0.82), or IVAD with stiffness in any direction (p = 0.26 to 0.55).
Table 2.
Lumbar in vitro biomechanics assessments and correlations with angular displacement
| Testing direction | L4-L6 segment in vitro measurements | NIAD vs IVAD angular displacement§ | Failure moment correlations§ | In vitro stiffness correlations§ | |||
|---|---|---|---|---|---|---|---|
| IVAD (degrees) | Bending stiffness (N-mm2) | vs NIAD | vs IVAD | vs NIAD | vs IVAD | ||
| Right bending | 27.8 ± 3.8 | 11,036 ± 3,474 | p = 0.34 (r = −0.27) | p = 0.83 (r = 0.049) | p = 0.86 (r = 0.05) | p = 0.71 (r = 0.11) | p = 0.23 (r = 0.35) |
| Left bending | 27.8 ± 3.8 | 11,746 ± 3,220 | p = 0.79 (r = 0.08) | p = 0.027* (r = 0.59) | p = 0.62 (r = 0.15) | p = 0.69 (r = −0.12) | p = 0.24 (r = 0.33) |
| Total coronal | 55.6 ± 5.8 | NM | p = 0.84 (r = −0.06) | p = 0.101 (r = 0.46) | p = 0.67 (r = 0.13) | NM | NM |
| Extension | 22.4 ± 3.6 | 10,464 ± 3,125 | NM | NM | p = 0.07 (r = 0.5) | NM | p = 0.42 (r = −0.24) |
| Flexion | 39.1 ± 1.7 | 20,498 ± 11,189 | NM | NM | p = 0.76 (r = −0.09) | NM | p = 0.61 (r = −0.15) |
| Total sagittal | 61.5 ± 3.8 | NM | NM | NM | p = 0.14 (r = 0.41) | NM | NM |
§Correlations for samples tested by in vivo and in vitro methods (n = 14, Pearson’s r); * significant correlation (p < 0.05); NIAD = noninvasive induced angular displacement; IVAD = in vitro angular displacement; NM = not measured.
Discussion
In vitro biomechanical tests have been described in numerous studies using comparative rodent lumbar spine models [2, 6, 13, 14], but noninvasive in vivo lumbar mechanical testing methods for rodents have not been reported. We sought to develop a displacement controlled test to measure in vivo lumbar spine induced angular displacement (NIAD), and to assess the reliability of the measurements made by single observers, between observers, and by repeated application of the test. We also determined how the in vivo measurement method compared with standard in vitro biomechanics tests: angular displacement (IVAD), stiffness, and load to failure.
Our study has some limitations. First, it is limited by the use of displacement-controlled rather than load-controlled force application during the NIAD assessment. Displacement control applies a fixed displacement and delivers a variable force magnitude to the spines of individual animals, and would be expected to generate more data scatter and measurement error than load-controlled tests. Despite larger relative standard deviations for the NIAD measurements, test-retest measurement reliability was high, suggesting soft tissue displacement dissipation and variation of forces applied to the spine did not dramatically affect the measurements. Second, NIAD radiograph measurements for lumbar motion between observers were different, suggesting an observer effect. These differences were noted despite observers making blinded measurements from identical radiographic images; however, the measurement differences did not affect the conclusions drawn regarding relative segmental NIAD. Therefore, interpretation of measurement data using the NIAD method should be performed for each observer independently. Third, we had a relatively small number of animals used in the direct comparisons of in vivo and in vitro measurements (n = 14), which may have led to the limited correlations between the techniques in addition to the technical differences. Use of larger sample sizes, as was seen here in the comparisons of all in vitro specimens (n = 24), may improve correlations in future studies by providing a source for larger range in the data set. Several factors contributed to differences between the two methods: in vivo tests used the entire spine with soft tissues intact, whereas in vitro specimens used a two-disc segment without soft tissues; tests performed in different anatomic directions may limit comparisons; in vivo animal positioning was controlled but subject to movement error, whereas in vitro testing was rigorously maintained by the fixture; and different boundary conditions for in vivo displacement-controlled and in vitro load-controlled tests. Finally, although four-point bending is a strength of our approach, few published reports exist for comparison to our data. Despite these limitations, our findings suggest the in vivo lumbar NIAD measurement technique is a reliable method for comparative rat spine research.
The displacement controlled NIAD method we described for the lumbar spine has precedent in techniques reported in the literature for clinical spine deformity. In either normal volunteers [20], or patients with scoliosis [19, 39], induced translation and bending of the spine can be generated with displacements of the trunk made with reference to the pelvic girdle. Analogous, although load-controlled, experiments delivering unilateral static bending in a rodent caudal model have been done to study experimental scoliosis [23, 31, 32, 36], with unidirectional initial angles averaging 18.1° to 26° over a two-disc segment [23, 32]. Although these caudal deformation assessments would compare favorably with our lumbar NIAD observations, differences between lumbar and caudal biomechanics have been reported for axial [13] and torsional [14] testing, thereby limiting validity for direct comparison of the caudal data to our lumbar measurements. Direct comparisons for our findings do not appear in the clinical or experimental literature. Regarding the accuracy agreement of the in vivo measurement method, angular deformation quantifications were generated using a modified Cobb technique. The Cobb method is the gold standard for angular measurement between vertebrae in clinical scoliosis and spine care, with 95% of interrater measurements for idiopathic scoliosis within 5° to 8° of one another when measured graphically [8, 17], or within 2.6° to 4.8° when measured digitally [9, 24]. The in vivo segmental NIAD averages had interobserver agreement within 4.9° for each measured segment. Digital Cobb interobserver measurement reliability as reported in clinical applications, evaluated by ICC, varies widely by the radiographic parameter assessed, with low values (0.25 to 0.33) associated with small measurement magnitudes and not easily identified landmarks, and high values (0.93 to 1) associated with larger measurement magnitude and more easily identified landmarks [25]. The relatively small magnitude of segmental measurements made by the in vivo method (13.7° to 24.6°) suggests that the low to moderate ICC values reflect what would have been expected, as was the higher ICC obtained for the larger magnitude combined L4-L6 segment measurement. These comparisons suggest the in vivo quantification technique is comparable in accuracy and precision to the Cobb method of measuring spinal deformity clinically.
Comparison of in vivo NIAD to in vitro end points revealed correlation only for in vivo left bending and in vitro extension moment to failure. The lack of correlation between NIAD and IVAD, and either of these with other parameters measured by the in vitro testing method was thought to represent the relatively small sample used for the investigation, and lack of sufficient data nonhomogeneity to strengthen observed associations. Measurement data for the two methods were each normally distributed (data not shown), and when orthogonally graphed formed rounded scatterplots. Further study to test correlation between the measurement techniques is warranted with larger study populations, and populations containing subjects with altered lumbar spine mobility to provide larger data ranges.
Regarding comparison of the in vitro lumbar spine testing data to that contained in the literature, no rodent lumbar spine four-point bending data currently exist. Four-point bending has been reported for a murine caudal model [27], but with the established differences between lumbar and caudal mechanics in axial and torsion testing [13, 14], direct comparison to the murine data is difficult to make even when corrected for relative size [1, 2]. Four-point bending measurements were reported from rabbit and canine cadaver spines using a similar method to assess in vitro stiffness [12]. The stiffness of rabbit and dog spines was in the range of 4 to 6 N-m2 with little difference seen between the species. Direct comparison of our current and prior data yields approximately 250-fold greater stiffness in the larger animals. Approximating the disc and vertebral body geometry of the spine as a rigid rod, spine stiffness as a function of specimen size would be expected to relate to the spine radius squared. If relative animal mass is assumed to be proportional to vertebral body size, then a typical 4-kg New Zealand White rabbit is approximately 14-fold larger than the rats (0.275–0.3 kg) used in this study and would be expected to have average spine stiffness approximately 200-fold larger than the rodents. Using this rationale, the data between the species are comparable.
The in vivo and in vitro lumbar spine tests described in this study can be used to objectively quantify spine mechanics. The noninvasive in vivo method can be used to measure spine angular displacement longitudinally at multiple times on one animal and thus may be useful for testing experimental animals with time without requirement for euthanasia if absolute biomechanical stiffness and ultimate load to failure data are not required to answer the research question. After euthanasia, the in vitro method to test bending angular displacement and stiffness in four anatomic directions and failure moment in extension provides additional objective means to measure spine end points. Used in this way, the methods are complementary and may limit or prevent the need for euthanasia at multiple times to assess mechanics, as has been described [26, 34], thereby limiting the number of animals required in complex study designs.
Acknowledgments
We thank Dr. Thomas Lawhorne for assistance with data collection and Dr. Bernard A. Rawlins for helpful discussions.
Footnotes
One or more of the authors has received funding from: Arthritis Foundation New York Chapter (MEC), Cobb Scoliosis Research Fund (OBA), North American Spine Society (MEC), and Musculoskeletal Repair and Regeneration Core Center NIH P30-AR46121 (MvdM and MEC).
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine (Phila Pa 1976) 2008;33:E166–173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 3.Bettini N, Girardo M, Dema E, Cervellati S. Evaluation of conservative treatment of non specific spondylodiscitis. Eur Spine J. 2009;18(suppl 1):143–150. doi: 10.1007/s00586-009-0979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boxberger JI, Auerbach JD, Sen S, Elliott DM. An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine (Phila Pa 1976) 2008;33:146–154. doi: 10.1097/BRS.0b013e31816054f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boxberger JI, Orlansky AS, Sen S, Elliott DM. Reduced nucleus pulposus glycosaminoglycan content alters intervertebral disc dynamic viscoelastic mechanics. J Biomech. 2009;42:1941–1946. doi: 10.1016/j.jbiomech.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boxberger JI, Sen S, Yerramalli CS, Elliott DM. Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. J Orthop Res. 2006;24:1906–1915. doi: 10.1002/jor.20221. [DOI] [PubMed] [Google Scholar]
- 7.Bramer JA, Barentsen RH, vd Elst M, Lange ES, Patka P, Haarman HJ. Representative assessment of long bone shaft biomechanical properties: an optimized testing method. J Biomech. 1998;31:741–745. doi: 10.1016/S0021-9290(98)00101-8. [DOI] [PubMed] [Google Scholar]
- 8.Carman DL, Browne RH, Birch JG. Measurement of scoliosis and kyphosis radiographs: intraobserver and interobserver variation. J Bone Joint Surg Am. 1990;72:328–333. [PubMed] [Google Scholar]
- 9.Cheung J, Wever DJ, Veldhuizen AG, Klein JP, Verdonck B, Nijlunsing R, Cool JC, Horn JR. The reliability of quantitative analysis on digital images of the scoliotic spine. Eur Spine J. 2002;11:535–542. doi: 10.1007/s00586-001-0381-7. [DOI] [PubMed] [Google Scholar]
- 10.Cobb JR. Outline for the study of scoliosis. In: Thomson JEM, Boount WP, eds. The American Academy of Orthopaedic Surgeons.Instructional Course Lectures. Ann Arbor, MI: JW Edwards; 1948:5:261–275.
- 11.Collantes E, Zarco P, Munoz E, Juanola X, Mulero J, Fernandez-Sueiro JL, Torre-Alonso JC, Gratacos J, Gonzalez C, Batlle E, Fernandez P, Linares LF, Brito E, Carmona L. Disease pattern of spondyloarthropathies in Spain: description of the first national registry (REGISPONSER) extended report. Rheumatology (Oxford) 2007;46:1309–1315. doi: 10.1093/rheumatology/kem084. [DOI] [PubMed] [Google Scholar]
- 12.Cottrell JM, Meulen MC, Lane JM, Myers ER. Assessing the stiffness of spinal fusion in animal models. HSS J. 2006;2:12–18. doi: 10.1007/s11420-005-5123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott DM, Sarver JJ. Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine (Phila Pa 1976) 2004;29:713–722. doi: 10.1097/01.brs.0000116982.19331.ea. [DOI] [PubMed] [Google Scholar]
- 14.Espinoza Orias AA, Malhotra NR, Elliott DM. Rat disc torsional mechanics: effect of lumbar and caudal levels and axial compression load. Spine J. 2009;9:204–209. doi: 10.1016/j.spinee.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fendler C, Braun J. Clinical measures in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009;27(4 suppl 55):S80–S82. [PubMed] [Google Scholar]
- 16.Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics. 2000;105:1299–1304. doi: 10.1542/peds.105.6.1299. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg MS, Poitras B, Mayo NE, Labelle H, Bourassa R, Cloutier R. Observer variation in assessing spinal curvature and skeletal development in adolescent idiopathic scoliosis. Spine. 1988;13:1371–1377. doi: 10.1097/00007632-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Gruber HE, Gordon B, Williams C, Norton HJ, Hanley EN., Jr Vertebral endplate and disc changes in the aging sand rat lumbar spine: cross-sectional analyses of a large male and female population. Spine (Phila Pa 1976) 2007;32:2529–2536. doi: 10.1097/BRS.0b013e318158cd69. [DOI] [PubMed] [Google Scholar]
- 19.Hamzaoglu A, Talu U, Tezer M, Mirzanli C, Domanic U, Goksan SB. Assessment of curve flexibility in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2005;30:1637–1642. doi: 10.1097/01.brs.0000170580.92177.d2. [DOI] [PubMed] [Google Scholar]
- 20.Harrison DE, Betz JW, Cailliet R, Colloca CJ, Harrison DD, Haas JW, Janik TJ. Radiographic pseudoscoliosis in healthy male subjects following voluntary lateral translation (side glide) of the thoracic spine. Arch Phys Med Rehabil. 2006;87:117–122. doi: 10.1016/j.apmr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Hicks GE, Morone N, Weiner DK. Degenerative lumbar disc and facet disease in older adults: prevalence and clinical correlates. Spine (Phila Pa 1976) 2009;34:1301–1306. doi: 10.1097/BRS.0b013e3181a18263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh AH, Hwang D, Ryan DA, Freeman AK, Kim H. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine (Phila Pa 1976) 2009;34:998–1005. doi: 10.1097/BRS.0b013e31819c09c4. [DOI] [PubMed] [Google Scholar]
- 23.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine (Phila Pa 1976) 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Jeffries BF, Tarlton M, Smet AA, Dwyer SJ, III, Brower AC. Computerized measurement and analysis of scoliosis: a more accurate representation of the shape of the curve. Radiology. 1980;134:381–385. doi: 10.1148/radiology.134.2.6986054. [DOI] [PubMed] [Google Scholar]
- 25.Kuklo TR, Potter BK, O’Brien MF, Schroeder TM, Lenke LG, Polly DW, Jr, Spinal Deformity Study Group Reliability analysis for digital adolescent idiopathic scoliosis measurements. J Spinal Disord Tech. 2005;18:152–159. doi: 10.1097/01.bsd.0000148094.75219.b0. [DOI] [PubMed] [Google Scholar]
- 26.Lee YP, Jo M, Luna M, Chien B, Lieberman JR, Wang JC. The efficacy of different commercially available demineralized bone matrix substances in an athymic rat model. J Spinal Disord Tech. 2005;18:439–444. doi: 10.1097/01.bsd.0000175696.66049.f7. [DOI] [PubMed] [Google Scholar]
- 27.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine (Phila Pa 1976) 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine (Phila Pa 1976) 2003;28:973–981. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 30.Masuoka K, Michalek AJ, MacLean JJ, Stokes IA, Iatridis JC. Different effects of static versus cyclic compressive loading on rat intervertebral disc height and water loss in vitro. Spine (Phila Pa 1976) 2007;32:1974–1979. doi: 10.1097/BRS.0b013e318133d591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mente PL, Aronsson DD, Stokes IA, Iatridis JC. Mechanical modulation of growth for the correction of vertebral wedge deformities. J Orthop Res. 1999;17:518–524. doi: 10.1002/jor.1100170409. [DOI] [PubMed] [Google Scholar]
- 32.Mente PL, Stokes IA, Spence H, Aronsson DD. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine (Phila Pa 1976) 1997;22:1292–1296. doi: 10.1097/00007632-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Onda A, Yabuki S, Kikuchi S. Effects of neutralizing antibodies to tumor necrosis factor-alpha on nucleus pulposus-induced abnormal nociresponses in rat dorsal horn neurons. Spine (Phila Pa 1976) 2003;28:967–972. doi: 10.1097/01.BRS.0000061984.08703.0C. [DOI] [PubMed] [Google Scholar]
- 34.Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. Osteoinductivity of commercially available demineralized bone matrix: preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86:2243–2250. doi: 10.2106/00004623-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Salminen JJ, Erkintalo MO, Pentti J, Oksanen A, Kormano MJ. Recurrent low back pain and early disc degeneration in the young. Spine (Phila Pa 1976) 1999;24:1316–1321. doi: 10.1097/00007632-199907010-00008. [DOI] [PubMed] [Google Scholar]
- 36.Stokes IA, McBride CA, Aronsson DD. Intervertebral disc changes in an animal model representing altered mechanics in scoliosis. Stud Health Technol Inform. 2008;140:273–277. [PubMed] [Google Scholar]
- 37.Sugiura A, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Norimoto M, Orita S, Eguchi Y, Takahashi Y, Watanabe TS, Ochiai N, Takaso M, Takahashi K. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Phila Pa 1976) 2008;33:2047–2051. doi: 10.1097/BRS.0b013e31817f8d58. [DOI] [PubMed] [Google Scholar]
- 38.Uei H, Matsuzaki H, Oda H, Nakajima S, Tokuhashi Y, Esumi M. Gene expression changes in an early stage of intervertebral disc degeneration induced by passive cigarette smoking. Spine (Phila Pa 1976) 2006;31:510–514. doi: 10.1097/01.brs.0000201304.81875.cc. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan JJ, Winter RB, Lonstein JE. Comparison of the use of supine bending and traction radiographs in the selection of the fusion area in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 1996;21:2469–2473. doi: 10.1097/00007632-199611010-00012. [DOI] [PubMed] [Google Scholar]
- 40.Wood KB, Olsewski JM, Schendel MJ, Boachie-Adjei O, Gupta M. Rotational changes of the vertebral pelvic axis after sublaminar instrumentation in adolescent idiopathic scoliosis. Spine. 1997;22:51–57. doi: 10.1097/00007632-199701010-00009. [DOI] [PubMed] [Google Scholar]
- 41.Wood KB, Transfeldt EE, Ogilvie JW, Schendel MJ, Bradford DS. Rotational changes of the vertebral-pelvic axis following Cotrel-Dubousset instrumentation. Spine. 1991;16(8 suppl):S404–S408. [PubMed] [Google Scholar]
- 42.Wuertz K, Godburn K, MacLean JJ, Barbir A, Donnelly JS, Roughley PJ, Alini M, Iatridis JC. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res. 2009;27:1235–1242. doi: 10.1002/jor.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang KB, Zheng ZM, Liu H, Liu XG. The effects of punctured nucleus pulposus on lumbar radicular pain in rats: a behavioral and immunohistochemical study. J Neurosurg Spine. 2009;11:492–500. doi: 10.3171/2009.4.SPINE08744. [DOI] [PubMed] [Google Scholar]