Abstract
Background
Chondrosarcomas of bone traditionally have been treated by wide or radical excision, procedures that may result in considerable lifelong disability. Grade 1 chondrosarcomas have little or no metastatic potential and are often difficult to distinguish from painful benign enchondromas. Curettage with adjuvant cryosurgery has been proposed as an alternative therapy for Grade 1 chondrosarcomas given the generally better function after the procedure. However, because it is an intralesional procedure, curettage and cryosurgery may be associated with higher rates of recurrence.
Questions/purposes
We asked whether Grade 1 chondrosarcomas and enchondromas of uncertain malignant potential treated by curettage and cryosurgery are associated with low recurrence rates and high functional scores.
Patients and Methods
We retrospectively reviewed the records of 46 patients with Grade 1 chondrosarcomas and enchondromas of uncertain malignant potential treated by curettage and cryosurgery. Forty-one patients had tumors of the long bones. Patients were followed a minimum of 18 months (average, 47.2. months; range, 18–134 months) for evidence of recurrence and for assessment of Musculoskeletal Tumor Society (MSTS) functional score.
Results
Two of the 46 patients had recurrences in the original tumor site (4.3% recurrence rate), which subsequently were removed by wide excision, and both patients were confirmed to be disease-free 36 and 30 months, respectively, after the second surgery. The mean MSTS score was 27.2 of 30 points (median, 29 points).
Conclusions
Our observations show curettage with cryosurgery is associated with low recurrence of Grade 1 chondrosarcoma and high functional scores. Curettage with cryosurgery is a reasonable alternative to wide or radical excision as the treatment for Grade 1 chondrosarcomas, and allows for more radical surgery in the event of local recurrence.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Chondrosarcomas are malignant cartilage tumors that account for ¼ of primary bone sarcomas [7, 18]. These tumors are divided into low-, intermediate-, and high-grade lesions based on the degree of hypercellularity, pleomorphism, hyperchromatism, and cellular and nuclear atypia [7, 22]. Low-grade (Grade 1) chondrosarcomas have low metastatic potential and are difficult to distinguish from benign enchondromas even after clinical, histologic, and radiologic examination [22, 28, 29]. Patients with Grade 1 chondrosarcomas and enchondromas can have similar radiographic and pathologic findings including endosteal scalloping, positive uptake of radiotracer on bone scan, and similar degrees of histologic abnormality [7, 12, 18, 19]. Grade 1 chondrosarcomas often are differentiated from enchondromas based on growth observed on interval radiographs, pain associated with the lesion, or retrospectively based on local or distant recurrence [7, 18, 19]. In contrast, enchondromas have a very low incidence of recurrence and do not metastasize [19], and often are found at distal locations such as the hands and feet [7, 12]. Two large series suggest that despite their ability to metastasize, Grade 1 chondrosarcomas are slow-growing and have a low rate of metastasis [15, 26]. Currently, surgery is the only effective treatment for chondrosarcomas because these tumors are resistant to chemotherapy and radiotherapy [7, 19, 28].
As with all bone sarcomas, the traditional treatment for chondrosarcoma is surgical resection with a wide margin, in which entire bone segments are excised to ensure total removal of tumor cells [7, 19, 28]. These procedures necessitate complex reconstructions and are associated with a high degree of morbidity, which may lead to lifelong disability and reduced quality of life [11, 13]. The appropriate treatment for cartilaginous tumors is controversial. Painful enchondromas and Grade 1 chondrosarcomas cannot be reliably distinguished on clinical, radiographic, or microscopic findings. Many benign enchondromas are misdiagnosed as Grade 1 chondrosarcomas [7], for which many patients undergo unnecessary wide or radical resections. Conversely, not treating a potential malignant tumor can result in risk of local recurrence and metastasis. Given the low malignant potential of Grade 1 chondrosarcomas and the inevitable inclusion of benign enchondromas in any series of low-grade cartilage lesions, surgeons may consider treating these tumors with an intralesional, bone-preserving approach such as curettage with adjuvant cryosurgery to obtain microscopic kill of tumor cells [5, 32]. This method involves careful curettage followed by three cycles of high-speed burring and liquid nitrogen application with subsequent defect filling with bone cement and internal fixation to prevent fracture and to facilitate rehabilitation [5, 32]. Curettage with cryosurgery avoids the postoperative morbidities of wide and radical resections with reconstruction commonly used for high-grade chondrosarcomas. However, there is concern that this intralesional procedure could result in unacceptable rates of local recurrence resulting from incomplete removal of tumor cells and intraoperative contamination of the surgical site by viable tumor cells. Some studies suggest curettage with adjuvant ablative therapy achieves low local recurrence rates (0%–13.3% at a mean followup of 26–145 months) and high postoperative functional scores (mean MSTS scores 27–30) [1–3, 6, 10, 13, 17, 27, 30, 31]. However, the recurrence rates associated with curettage, as reported in other studies, have been substantially greater (40%–100% recurrence rate) [9, 23, 25].
To address this controversy, we therefore asked whether patients who had undergone curettage and cryosurgery for Grade 1 chondrosarcomas and enchondromas of unknown malignant potential had (1) low recurrence rates; (2) high functional scores; and (3) low postoperative complication rates.
Patients and Methods
We retrospectively reviewed the medical records of 68 patients treated for Grade 1 chondrosarcomas and enchondromas by curettage and cryosurgery between January 1997 and June 2008. Diagnosis was based on clinical history, physical examination, and radiographs. No patient underwent biopsy before definitive surgery. All patients were diagnosed as having a Grade 1 chondrosarcoma, enchondroma, or borderline tumor (possible Grade 1 chondrosarcoma or enchondroma) after evaluation of clinical, radiologic (radiograph, MRI), and histologic results performed by a multidisciplinary group consisting of the primary surgeon (DGM), radiologists (SB, CB, KS), and pathologists (MV, JM, RK) with expertise in musculoskeletal oncology. At our institution, pathologic and radiographic material is reviewed by at least two specialists in their respective fields as part of our multidisciplinary protocols for patients with musculoskeletal tumors. The histologic slides were not reviewed again for this study. The indication for surgery was one or more of the following: persistent pain at the tumor site; radiographic signs of an aggressive tumor such as cortical thinning, endosteal scalloping, or interval increase in tumor size; or patient desire to have the tumor removed. In cases of shoulder pain with evidence of rotator cuff symptoms, an impingement test using a subacromial injection of lidocaine and bupivacaine was routinely performed to discriminate between rotator cuff pain and tumor-specific pain.
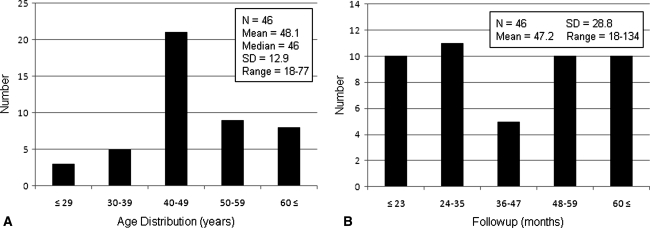
We chose 18 months as a minimum followup based on the median time to local recurrence reported in similar studies [1, 4, 6, 14, 17, 30, 31]. Of the 68 patients, 46 (68%) had at least 18 months followup. Twenty-two patients (32%) were lost to followup. All 46 patients with at least 18 months followup had adequate chart information, and the chart information was supplemented by telephone calls to seven. Patients were followed an average of 47.2 months (Fig. 1) (range, 18–134 months).
Fig. 1A–B.
Distribution of patient demographics is shown with respect to (A) patient age and (B) followup time.
Demographic information included age at surgery, gender, followup time, followup mode (clinic or phone), signs of preoperative pain, radiographic evidence of endosteal scalloping or cortical erosion, tumor size, and tumor anatomic site (humerus, femur, tibia, fibula, radius, pelvis, sacrum, metatarsal) (Tables 1, 2; Fig. 1). Forty-one patients (89%) had tumors located in the long bones (21 humerus, 12 femur, five tibia, two fibula, one radius); and the remaining five patients had tumors located in the metatarsal, ilium, ischium, and sacrum. Mean and median ages at surgery were 48.1 and 46 years, respectively (range, 18–77 years). Thirty-six patients (78%) were followed for 2 years or longer. Male-to-female ratio was 16:30. Final diagnosis was based on what was believed to be most probable after multidisciplinary review of all clinical and diagnostic information. Cases were divided among diagnoses favoring Grade 1 chondrosarcoma (N = 17), enchondroma (N = 16), or borderline tumor (uncertain Grade 1 chondrosarcoma or enchondroma) (N = 13). Preoperative pain was present in 44 patients (96%). The two patients without obvious tumor-specific pain previously had symptoms or injury prompting radiographs of the affected bone. They were concerned about the potential for malignancy and wanted their tumors removed and were included in the study. Preoperative radiographic signs of bone destruction (endosteal scalloping, cortical erosion) were present in 36 patients (78%). Information regarding tumor size was available for 20 patients; the mean tumor size defined as the longest dimension on radiographs or MRI was 5.0 ± 2.0 cm (range, 2.0–8.0 cm).
Table 1.
Complete patient data
| Patient | Age | Gender | Followup (months) | Tumor | Site | Pain | Erode | Recurrence | MSTS Complications | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | F | Clinic, 134 | C | Humerus | Yes | Yes | – | 29 | – |
| 2 | 40 | M | Clinic, 117 | C/E | Femur | Yes | Yes | – | 11 | – |
| 3 | 54 | F | Clinic, 113 | E | Humerus | Yes | Yes | – | 30 | Postoperative fracture |
| 4 | 38 | F | Clinic, 113 | C/E | Tibia | Yes | Yes | – | 30 | – |
| 5 | 63 | F | Clinic, 88 | C | Tibia | Yes | Yes | – | 30 | – |
| 6 | 71 | M | Clinic, 80 | C/E | Humerus | No | Yes | – | 30 | – |
| 7 | 73 | F | Clinic, 75 | C/E | Femur | Yes | Yes | – | 30 | – |
| 8 | 49 | M | Clinic, 61 | C/E | Tibia | Yes | Yes | – | 29 | – |
| 9 | 37 | M | Clinic, 54 | C | Femur | Yes | Yes | Yes* | 26 | Postoperative fracture |
| 10 | 71 | F | Clinic, 54 | C/E | Humerus | Yes | Yes | – | 30 | – |
| 11 | 58 | F | Clinic, 52 | E | Humerus | Yes | No | – | 30 | – |
| 12 | 53 | F | Clinic, 50 | C/E | Humerus | Yes | Yes | – | 29 | – |
| 13 | 49 | M | Clinic, 48 | C | Humerus | Yes | Yes | Yes* | 29 | Leftover tumor |
| 14 | 49 | M | Clinic, 48 | E | Tibia | Yes | No | – | 25 | – |
| 15 | 39 | F | Clinic, 46 | C | Humerus | Yes | Yes | – | 27 | – |
| 16 | 42 | F | Clinic, 45 | C/E | Humerus | Yes | Yes | – | 30 | – |
| 17 | 70 | F | Clinic, 42 | C | Fibula | Yes | Yes | – | 30 | – |
| 18 | 43 | M | Clinic, 37 | C | Humerus | Yes | Yes | – | 28 | – |
| 19 | 46 | F | Clinic, 36 | E | Femur | Yes | Yes | – | 30 | – |
| 20 | 44 | F | Clinic, 35 | C | Femur | No | Yes | – | 30 | – |
| 21 | 45 | F | Clinic, 34 | C | Humerus | Yes | Yes | – | 29 | – |
| 22 | 64 | M | Clinic, 33 | C | Femur | Yes | Yes | – | 30 | – |
| 23 | 45 | F | Clinic, 29 | C | Humerus | Yes | Yes | – | 16 | – |
| 24 | 58 | M | Clinic, 26 | E | Humerus | Yes | Yes | – | 30 | – |
| 25 | 52 | F | Clinic, 23 | E | Femur | Yes | No | – | 30 | – |
| 26 | 18 | M | Clinic, 23 | C | Tibia | Yes | Yes | – | 20 | – |
| 27 | 50 | F | Clinic, 20 | C | Femur | Yes | Yes | – | 29 | – |
| 28 | 45 | F | Clinic, 18 | E | Fibula | Yes | Yes | – | 14 | – |
| 29 | 52 | M | Clinic, 18 | C/E | Humerus | Yes | Yes | – | 29 | – |
| 30 | 35 | M | Clinic, 18 | C | Metatarsal | Yes | Yes | – | 29 | – |
| 31 | 40 | F | Clinic, 18 | C | Humerus | Yes | Yes | – | 30 | – |
| 32 | 46 | F | Clinic, 18 | E | Radius | Yes | Yes | – | 28 | – |
| 33 | 43 | F | Phone, 67 | E | Metatarsal | Yes | No | – | 30 | – |
| 34 | 49 | M | Phone, 66 | E | Humerus | Yes | No | – | 26 | – |
| 35 | 41 | F | Phone, 59 | C/E | Femur | Yes | No | – | 25 | – |
| 36 | 77 | F | Phone, 54 | E | Femur | Yes | Yes | – | 23 | – |
| 37 | 24 | F | Phone, 53 | E | Femur | Yes | Yes | – | 30 | – |
| 38 | 31 | F | Phone, 32 | E | Ischium | Yes | Yes | – | 30 | – |
| 39 | 42 | M | Phone, 18 | E | Humerus | Yes | Yes | – | 19 | – |
| 40 | 20 | F | Clinic, 53 | E | Femur | Yes | No | – | – | – |
| 41 | 49 | F | Clinic, 32 | C/E | Humerus | Yes | Yes | – | – | – |
| 42 | 56 | F | Clinic, 32 | E | Humerus | Yes | No | – | – | Postoperative fracture |
| 43 | 41 | F | Clinic, 29 | C | Sacrum | Yes | Yes | – | – | – |
| 44 | 53 | F | Clinic, 28 | C/E | Humerus | Yes | Yes | – | – | – |
| 45 | 40 | M | Clinic, 25 | C | Ilium | Yes | No | – | – | – |
| 46 | 60 | M | Clinic, 18 | C/E | Humerus | Yes | No | – | – | – |
Age = in years at date of initial surgery; Followup = by clinic or phone interview in months since initial surgery; MSTS = Musculoskeletal Tumor Society functional score out of 30 points; Site = anatomic location of tumor; Pain = signs of preoperative pain; Erode = radiographic evidence of endosteal scalloping or cortical erosion; * both cases of recurrence detected 18 months after initial surgery; Complications = postoperative fracture, leftover tumor from incomplete removal; F = female; M = male; C = chondrosarcoma Grade 1; E = enchondroma; C/E = chondrosarcoma Grade 1 or enchondroma.
Table 2.
Summary of patient demographics
| Category | Outcome 1 | Outcome 2 |
|---|---|---|
| Gender (male/female) | 16 males | 30 females |
| Followup (clinic/phone) | 39 clinic | 7 phone |
| Tumor site (long bone/other) | 41 long bones | 5 other |
| Preoperative pain (yes/no) | 44 yes | 2 no |
| Bone destruction (yes/no) | 36 yes | 10 no |
Long bone = humerus, femur, tibia, fibula, radius; other = metatarsal, sacrum, ilium, ischium; Bone destruction = endosteal scalloping or cortical erosion.
The surgical procedure, as described by Marcove et al. [20, 21], consisted of curettage and three cycles of liquid nitrogen application with burr drilling followed by cementation of the defect and internal fixation to prevent pathologic fracture. After exposure of the involved bone, holes were drilled in the cortex in an oval fashion and this elliptical bone window was removed with an osteotome to expose the underlying tumor. Soft tissues were protected with gauze and the tumor was handled very carefully to avoid touching the soft tissue in the surgical approach site. Broaching anatomic barriers with retractors or drills was avoided to keep the tumor site contained and amenable to radical resection with limb salvage should there be a subsequent local recurrence. Curettage was performed under direct vision throughout the tumor until the opposite cortical bone was encountered and all visible traces of the tumor were removed. Liquid nitrogen of −196°C was sprayed onto the tumor cavity divided into quadrants with application of 30 seconds per quadrant. A Midas Rex® (Medtronic, Minneapolis, MN, USA) then was used to burr drill through frozen tissue, and the cavity was inspected for absence of tumor tissue. This cycle of cryosurgery with burr drilling was performed two more times until margins of the cavity were cleared down to the cortex on the opposite side of the surgical opening. The cavity and soft tissues then were irrigated with hydrogen peroxide. After inspection for absence of tumor tissue, internal fixation of the cavity was accomplished with 6.5-mm titanium screws fixed into the medullary cavity by interference fit or with a titanium locking plate and unicortical screws or a combination of both techniques based on cavity size, anatomic location, and anticipated sports and manual labor demands on the surgically treated bone. Bone cement mixed with antibiotics (Simplex P with Tobramycin®; Stryker-Howmedica, Mahwah, NJ, USA) was used to fill the tumor defect and was contoured to the surface of the existing bone. The surgical approach site was irrigated with hydrogen peroxide and saline and a drain was placed on the bone surface. Overlying muscle and fascia were closed over the tumor site. A sling, splint, or immobilizer was applied to stabilize the surgically treated limb. Resected tumors were sent fresh or in formalin to the pathology department for diagnostic evaluation.
Postoperatively, patients were assessed for ROM of the extremity and started on physical therapy. Weightbearing was permitted as tolerated, but no contact sports or resistance exercises were allowed for 6 to 12 weeks.
Patients were clinically followed at 3- to 4-month intervals during the first 2 years and at 6- to 12-month intervals thereafter. Recurrences were suspected based on pain or a new mass. Functional performance was assessed at each visit using the revised system established by the MSTS and International Society of Limb Salvage [8]. This system measures outcomes in six categories, including pain, function, and emotional acceptance for all patients; use of walking aids, walking ability, and gait for patients with lower limb operations; and hand positioning, dexterity, and lifting ability for patients with upper limb operations. Each parameter is scored 0 to 5 and combined for a possible total score of 30. A score of 23 or greater is considered an excellent result; a score of 15 to 22 points, a good result; a score of 8 to 14 points, a fair result; and a score less than 8, a poor result. Seven patients who could not return to our institution for final followup were contacted by phone by one of the authors (DAM) and asked questions about the results of recent clinical checkups for tumor recurrence and MSTS functional performance. Postoperative complications such as fracture at the operation site were recorded from the records.
At each visit we routinely obtained radiographs with a minimum of two orthogonal views of the extremities or minimum of three views of the pelvis looking for recurrences and MRI scans with and without contrast if there was suspicion of local recurrence. Screening for lung metastasis was by chest radiograph with CT scans reserved for patients with equivocal findings or any local recurrence.
Recurrent tumors were confirmed by pathologic examination of the resected tissue with comparison to the original tumor.
Results
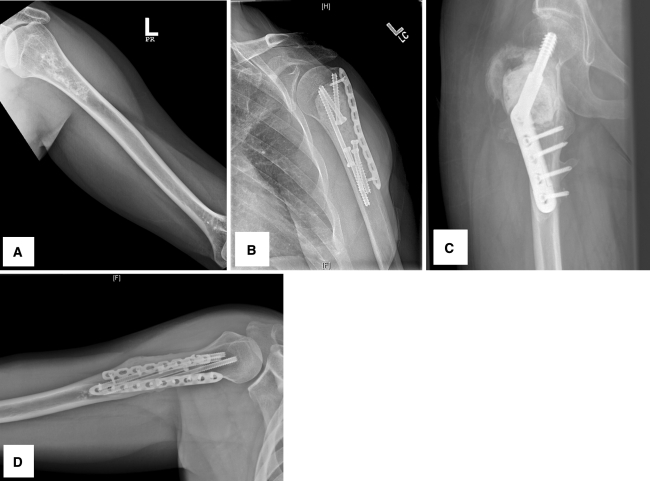
Forty-four of the 46 patients had good postoperative results with no recurrence at the last followup (Fig. 2A–B). Only two of the 46 patients (4.3%) had local recurrence. One patient had a recurrence at the original tumor site, and the second patient had a recurrence in the subcutaneous surgical scar from presumed intraoperative tumor contamination. The patient with the recurrence in the primary tumor site had a 6-cm Grade 1 chondrosarcoma in the greater trochanter of the right femur (Fig. 2C). The recurrence was diagnosed 18 months after the initial surgery and was associated with a nondisplaced greater trochanter avulsion fracture resulting from a fall. The patient subsequently underwent wide excision and endoprosthetic reconstruction of the proximal femur. At last followup 36 months after the second surgery, the patient was disease-free with no evidence of pain or metastasis. The second patient with a Grade 1 chondrosarcoma on the initial curettage had a 1-cm recurrent tumor in his upper arm surgical scar detected 18 months after his initial resection (Fig. 2D). It was removed by wide excision of the soft tissue mass with primary closure, and pathologic evaluation confirmed low-grade chondrosarcoma. The site of his original bone tumor remains with no evidence of recurrence 30 months after his second surgery.
Fig. 2A–D.
The radiographic images show a Grade 1 chondrosarcoma of the proximal humerus (A) before and (B) after removal by curettage and cryosurgery with postoperative good outcome, (C) the local recurrence in the right proximal femur, and (D) the residual tumor (second recurrent case) in the right proximal humerus.
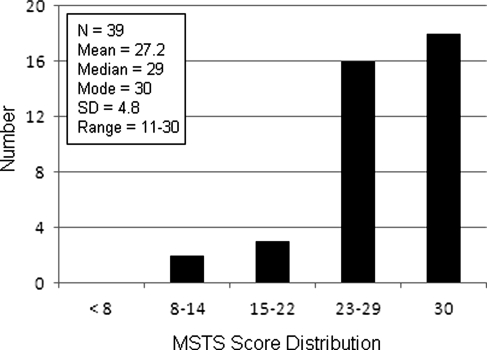
The mean, median, and mode MSTS functional scores of the 39 subjects from whom MSTS scores were obtained were 27.2, 29, and 30, respectively, of 30 points (Fig. 3) (SD 4.8; range, 11–30).
Fig. 3.
Distribution of Musculoskeletal Tumor Society (MSTS) functional scores is shown with respect to categories of ≥ 23 (excellent result), 15–22 (good result), 8–14 (fair result), and < 8 (poor result).
Postoperative complications included three cases of fracture at the surgery site: two were associated with enchondromas in the proximal humerus during the first 6 weeks after surgery, and the third was associated with a Grade 1 chondrosarcoma 18 months after surgery. All patients with fractures had intramedullary interference screw stabilization rather than locking plates. The two proximal humeral fractures occurred when one patient fell onto the shoulder and the second while the patient was in a police restraint hold. The third nondisplaced trochanteric fracture was caused when the patient fell out of bed. This latter patient was one of the two in whom local recurrence of a Grade 1 chondrosarcoma was detected. The proximal humeral fractures healed successfully after open reduction internal fixation, and the patient with local recurrence underwent radical excision and proximal femoral replacement 6 weeks after the fracture incident. Postoperative neural damage was not observed in any of the cases, including the single case of sacral tumor located in segments S1 and S2 of the patient.
Discussion
Differentiation of low-grade chondrosarcomas from enchondromas is difficult and remains a controversial topic in musculoskeletal oncology. Many authors recommend observation as a first line of treatment for cartilaginous bone tumors of uncertain malignant potential [1, 14, 29]. However, in certain cases, because of patient concerns, tumor-specific pain, or radiographic findings suggesting a low-grade expanding lesion, surgery is indicated. Surgery with wide resection margins, which involves removing large segments of bone, followed by prosthetic replacement or segmental reconstruction, is associated with considerable morbidity.
Enchondromas often are misdiagnosed as Grade 1 chondrosarcomas given their clinical, radiographic, and histologic similarities, and the lack of clear diagnostic criteria. Both types of tumors for instance, may have similar clinical presentation, radiographic signs of bone destruction, and similar degrees of histologic abnormality [7, 12, 18, 19]. Many diagnoses of chondrosarcoma therefore are confirmed retrospectively on recurrence, given that Grade 1 chondrosarcomas may recur locally or metastasize, whereas enchondromas rarely recur and do not metastasize [19]. Because the true malignant potential of these tumors on initial diagnosis is uncertain, the risks and morbidity of such aggressive extralesional surgery may be unnecessarily excessive, especially for patients with enchondromas. In addition, Grade 1 chondrosarcomas are slow-growing and have an inherently low rate of metastasis [15, 26]. To determine whether patients may benefit from an intralesional, bone-preserving procedure such as curettage with adjuvant cryosurgery, we retrospectively evaluated the recurrence rates and functional scores of patients with Grade 1 chondrosarcomas or enchondromas treated by this procedure. In this study, we determined that curettage with cryosurgery is associated with a low recurrence rate (4.3%), excellent MSTS functional scores (mean 27.2, median 29 of 30), and low complication rates (only three cases of postoperative fracture).
We recognize limitations to the study. First, given the retrospective cohort design of this study, there is likely a selection bias to include patients with less severe disease. We acknowledge this is an important limitation; however, the diagnostic and treatment decisions made in this study reflect those most surgeons face in everyday practice. We therefore believe the information presented is applicable and will be useful to surgeons caring for similar patients. Second, local recurrence of these tumors is an infrequent event and with our small study population, we likely underestimated the frequency and clinical impact of adverse outcomes associated with local recurrence. Third, seven of the 46 patients could not return to the clinic for followup, and outcomes of recurrence and functional scores were obtained by telephone interview. Data obtained by this method are less accurate because they are not clinically verified by physical and radiographic evaluation. Also, of the 39 patients who returned to the clinic for followup, seven did not have MSTS functional scores. Fourth, a median followup of 18 months, although adequate to detect local recurrence, as in the patient described in this study, is not sufficient to identify late recurrence known to occur in low-grade tumors [26]. However, eight patients were followed for longer than 5 years without any evidence of recurrence (Table 1). Finally, an important limitation is that loss to followup was 32% (22 of the original 68 patients who underwent surgery before June 2008 were unable to complete a minimum followup of 18 months). We do not know whether this would bias the findings toward patients without complications and without recurrences.
Our results agree with those of previous studies that examined recurrence and functional outcomes of Grade 1 chondrosarcomas after curettage and cryosurgery (Table 3). When adjuvants such as cryosurgery, phenol, and methylmethacrylate are used in combination with curettage in treating benign or low-grade malignant tumors, the local recurrence rates are reportedly as low as 0.0% to 6.0% [1–3, 16, 21, 27, 30, 31]. In the largest published series of patients with enchondromas and Grade 1 chondrosarcomas treated with curettage and cryosurgery, van der Geest et al. [31] reported on 123 patients with an average followup of 60 months. Two (1.6%) patients had local recurrences, both of whom were treated with repeat surgery and reportedly were disease-free at 3 and 5 years. Other complications included 18 postsurgical fractures (14%), four of which required surgery, three wound infections, three nerve palsies, one nitrogen gas embolus without permanent sequelae, and six miscellaneous resulting in a total complication rate, including local recurrence, of 26.8%. In the study by Leerapun et al. [17], phenolization was used in place of cryosurgery as the adjunct, and there was only one recurrence among the 13 patients. Etchebehere et al. [10] used cauterization in place of cryosurgery as the adjunct, and the recurrence rate was 0.0%. These studies are common in that (1) a form of adjunctive therapy is used (cryosurgery, phenolization, cauterization); and (2) the surgeries are performed mostly on tumors of the long bones. When these two conditions are met, recurrence rates approach zero. When curettage is not accompanied by a form of surgical adjunct, recurrence rates can be substantially greater (three of three = 100% [25] and one of two = 50% [9]). When curettage is performed on bones of the axial skeleton such as the pelvis or scapula, as reported by Normand et al. [23] (in which all five cases were tumors of the pelvis), recurrence rates also can be substantially greater (two of five [40%]), especially if some form of adjunctive therapy is not used. With respect to functional performance, curettage and cryosurgery yield high functional scores as shown in our study and eight others [1–3, 6, 14, 27, 30, 31], with mean functional scores ranging from 27 to 30.
Table 3.
Summary of studies of intralesional resections of Grade 1 chondrosarcomas and enchondromas
| Study | Date of publication | Treatment | Tumor site | Sample size (number of patients) | Followup (months) | Recurrence (number/%) | MSTS |
|---|---|---|---|---|---|---|---|
| Current study | – | Cu, Cr, Ce | 47L, 2P, 2D, 1Sa | 52 | 40 (13–134) | 2 (3.8%) | 27.2 |
| Souna et al. [30] | 2010 | Cu, Cr | 15L | 15 | 96 (60–132) | 0 (0.0%) | 27.9 |
| Donati et al. [6] | 2010 | Cu, Ph/Ce, Bg | 15L | 15 | 145 (81–251) | 2 (13.3%) | 27 |
| Hanna et al. [14] | 2009 | Cu, Ce | 39L | 39 | 61 (36–104) | 2 (5.1%) | 28.2 |
| Okada et al. [24] | 2009 | Cu, Pa, Bg | 2L | 2 | 132* | 0 (0.0%) | – |
| Aarons et al. [1] | 2009 | Cu, Ca, Ph/Cr/Ce | 17L | 17 | 56 (29–130) | 1 (5.9%) | 29.5 |
| van der Geest et al. [31] | 2008 | Cu, Cr, Ce/Bg | – | 123 | 60 (24–119) | 2 (1.6%) | 28 |
| Leerapun et al. [17] | 2007 | Cu, Ph, Ce/Bg | 13L | 13 | 102 (2–274) | 1 (7.7%) | – |
| Normand et al.[23] | 2007 | Cu, Ce/Bg | 5P | 5 | 69 (14–142) | 2 (40%) | – |
| Ahlmann et al. [3] | 2006 | Cu, Cr, Ce | 7L, 2S, 1P | 10 | 39 (24–60) | 0 (0.0%) | 27 |
| Etchebehere et al. [10] | 2005 | Cu, Ca, Ce | 11L | 11 | 52 (24–108) | 0 (0.0%) | – |
| Kollender et al. [16] | 2003 | Cu, Cr, Ce | 1Sa | 1 | 120 | 0 (0.0%) | – |
| Schreuder et al. [27] | 1998 | Cu, Cr, Bg | 17L, 6D | 23 | 26 (15–40) | 0 (0.0%) | 29 |
| Ozaki et al. [25] | 1996 | Cu | 1L, 1P, 1D | 3 | 45 (24–70) | 3 (100%) | – |
| Bauer et al. [4] | 1995 | Cu, Ce/Bg | 45L, 1D | 46 | 77 (36–156) | 3 (6.5%) | – |
| Aboulafia et al. [2] | 1994 | Cu, Cr, Ce | 1L | 1 | 52 | 0 (0.0%) | 30 |
| Eriksson et al. [9] | 1980 | Cu, Bg | 1L† | 2 | 17† | 1 (50%) | – |
| Marcove et al. [21] | 1977 | Cu, Cr | 4L, 2S, 1P | 7 | 78 (59–104) | 0 (0.0%) | – |
All cases involve Grade 1 chondrosarcomas or enchondromas only; * both subjects had the same followup time; †information given for recurrent case only, nonrecurrent case is not described; MSTS = Musculoskeletal Tumor Society mean functional score (out of 30); Cu = curettage; Cr = cryosurgery; Ce = cementation; Bg = bone graft; Ph = phenolization; Ca = cauterization; Pa = pasteurization; L = long bone; P = pelvis; D = distal (hand, feet); S = scapula; Sa = sacrum.
Curettage with adjuvant cryosurgery is an effective treatment for cartilaginous bone tumors of uncertain malignant potential as observed by the low tumor recurrence rate and high functional scores. We believe the key to success of this treatment approach is meticulous surgical technique to address the entire tumor cavity, avoid contamination of uninvolved anatomy, and minimize tumor seeding of the soft tissues in the surgical approach site. We have incorporated intralesional curettage and cryosurgery with cementation and prophylactic fixation into our treatment protocols for patients with cartilaginous bone tumors of malignant potential. Intralesional surgery with adjuvant treatment such as cryotherapy for treatment of benign and low-grade malignant cartilaginous bone tumors achieves a low local recurrence rate and good function in the short and intermediate term. This treatment strategy may replace wide excision as the preferred initial operation for Grade 1 chondrosarcomas. In the rare event of local recurrence, wide excision or repeat curettage may be considered.
Acknowledgments
We thank Sandeep Biswal MD, Christopher Beaulieu MD, and Kathryn Stevens MD, for radiographic evaluation, and Matt van de Rijn MD, Jesse McKinney MD, and Richard Kempton MD, for pathologic evaluation of the patients in this study.
Footnotes
One of the authors (RC) has received research funding from the Stanford Medical Scholars Research Fellowship Program.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aarons C, Potter BK, Adams SC, Pitcher JD, Jr, Temple HT. Extended intralesional treatment versus resection of low-grade chondrosarcomas. Clin Orthop Relat Res. 2009;467:2105–2111. doi: 10.1007/s11999-008-0691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aboulafia AJ, Rosenbaum DH, Sicard-Rosenbaum L, Jelinek JS, Malawer MM. Treatment of large subchondral tumors of the knee with cryosurgery and composite reconstruction. Clin Orthop Relat Res. 1994;307:189–199. [PubMed] [Google Scholar]
- 3.Ahlmann ER, Menendez LR, Fedenko AN, Learch T. Influence of cryosurgery on treatment outcome of low-grade chondrosarcoma. Clin Orthop Relat Res. 2006;451:201–207. doi: 10.1097/01.blo.0000229293.98850.5d. [DOI] [PubMed] [Google Scholar]
- 4.Bauer HC, Brosjo O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand. 1995;66:283–288. doi: 10.3109/17453679508995543. [DOI] [PubMed] [Google Scholar]
- 5.Bickels J, Meller I, Malawer M. The biology and role of cryosurgery in the treatment of bone tumors. In: Malawer MM, Sugarbaker PH, editors. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Norwell, MA: Kluwer Academic Publishers; 2001. pp. 135–146. [Google Scholar]
- 6.Donati D, Colangeli S, Colangeli M, Di Bella C, Bertoni F. Surgical treatment of grade I central chondrosarcoma. Clin Orthop Relat Res. 2010;468:581–589. doi: 10.1007/s11999-009-1056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorfman HD, Czerniak B. Malignant cartilage tumors. In: Dorfman HD, Czerniak B, editors. Bone Tumors. St Louis, MO: Mosby; 1998. pp. 353–440. [Google Scholar]
- 8.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 9.Eriksson AI, Schiller A, Mankin HJ. The management of chondrosarcoma of bone. Clin Orthop Relat Res. 1980;153:44–66. [PubMed] [Google Scholar]
- 10.Etchebehere M, Camargo OP, Croci AT, Oliveira CR, Baptista AM. Relationship between surgical procedure and outcome for patients with grade 1 chondrosarcomas. Clinics (Sao Paulo) 2005;60:121–126. doi: 10.1590/s1807-59322005000200007. [DOI] [PubMed] [Google Scholar]
- 11.Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res. 2002;397:106–113. doi: 10.1097/00003086-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Gitelis S, Soorapanth C. Benign chondroid tumors. In: Menendez LR, editor. Orthopaedic Knowledge Update: Musculoskeletal Tumors. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2002. pp. 103–111. [Google Scholar]
- 13.Ham SJ, Schraffordt Koops H, Veth RP, Horn JR, Molenaar WM, Hoekstra HJ. Limb salvage surgery for primary bone sarcoma of the lower extremities: long-term consequences of endoprosthetic reconstructions. Ann Surg Oncol. 1998;5:423–436. doi: 10.1007/BF02303861. [DOI] [PubMed] [Google Scholar]
- 14.Hanna SA, Whittingham-Jones P, Sewell MD, Pollock RC, Skinner JA, Saifuddin A, Flanagan A, Cannon SR, Briggs TW. Outcome of intralesional curettage for low-grade chondrosarcoma of long bones. Eur J Surg Oncol. 2009;35:1343–1347. doi: 10.1016/j.ejso.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Henderson ED, Dahlin DC. Chondrosarcoma of bone: a study of two hundred and eighty-eight cases. J Bone Joint Surg Am. 1963;45:1450–1458. [PubMed] [Google Scholar]
- 16.Kollender Y, Meller I, Bickels J, Flusser G, Issakov J, Merimsky O, Marouani N, Nirkin A, Weinbroum AA. Role of adjuvant cryosurgery in intralesional treatment of sacral tumors. Cancer. 2003;97:2830–2838. doi: 10.1002/cncr.11383. [DOI] [PubMed] [Google Scholar]
- 17.Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res. 2007;463:166–172. doi: 10.1097/BLO.0b013e318146830f. [DOI] [PubMed] [Google Scholar]
- 18.Mankin HJ. Chondrosarcoma of bone. In: Menendez LR, editor. Orthopaedic Knowledge Update: Musculoskeletal Tumors. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2002. pp. 187–194. [Google Scholar]
- 19.Marco RA, Gitelis S, Brebach GT, Healey JH. Cartilage tumors: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:292–304. doi: 10.5435/00124635-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Marcove RC. A 17-year review of cryosurgery in the treatment of bone tumors. Clin Orthop Relat Res. 1982;163:231–234. [PubMed] [Google Scholar]
- 21.Marcove RC, Stovell PB, Huvos AG, Bullough PG. The use of cryosurgery in the treatment of low and medium grade chondrosarcoma: a preliminary report. Clin Orthop Relat Res. 1977;122:147–156. [PubMed] [Google Scholar]
- 22.Mirra JM, Gold R, Downs J, Eckardt JJ. A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones: a clinicopathologic analysis of 51 cases. Clin Orthop Relat Res. 1985;201:214–237. [PubMed] [Google Scholar]
- 23.Normand AN, Cannon CP, Lewis VO, Lin PP, Yasko AW. Curettage of biopsy-diagnosed grade 1 periacetabular chondrosarcoma. Clin Orthop Relat Res. 2007;459:146–149. doi: 10.1097/BLO.0b013e3180619554. [DOI] [PubMed] [Google Scholar]
- 24.Okada K, Nagasawa H, Chida S, Nishida J. Curettage with pasteurization in situ for grade 1 chondrosarcoma: long-term follow up study of less invasive surgical procedure. Med Sci Monit. 2009;15:CS44–CS48. [PubMed] [Google Scholar]
- 25.Ozaki T, Lindner N, Hillmann A, Rodl R, Blasius S, Winkelmann W. Influence of intralesional surgery on treatment outcome of chondrosarcoma. Cancer. 1996;77:1292–1297. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1292::AID-CNCR10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Sanerkin NG, Gallagher P. A review of the behaviour of chondrosarcoma of bone. J Bone Joint Surg Br. 1979;61:395–400. doi: 10.1302/0301-620X.61B4.500746. [DOI] [PubMed] [Google Scholar]
- 27.Schreuder HW, Pruszcynski M, Veth RP, Lemmens JA. Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur J Surg Oncol. 1998;24:120–126. doi: 10.1016/S0748-7983(98)91459-7. [DOI] [PubMed] [Google Scholar]
- 28.Shmookler B, Bickels J, Jelinek J, Sugarbaker P, Malawer M. Bone and soft-tissue sarcomas: epidemiology, radiology, pathology and fundamentals of surgical treatment. In: Malawer MM, Sugarbaker PH, editors. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Norwell, MA: Kluwer Academic Publishers; 2001. pp. 3–36. [Google Scholar]
- 29.Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am. 2007;89:2113–2123. doi: 10.2106/JBJS.F.01530. [DOI] [PubMed] [Google Scholar]
- 30.Souna BS, Belot N, Duval H, Langlais F, Thomazeau H. No recurrences in selected patients after curettage with cryotherapy for grade I chondrosarcomas. Clin Orthop Relat Res. 2010 Jan 7. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 31.Geest IC, Valk MH, Rooy JW, Pruszczynski M, Veth RP, Schreuder HW. Oncological and functional results of cryosurgical therapy of enchondromas and chondrosarcomas grade 1. J Surg Oncol. 2008;98:421–426. doi: 10.1002/jso.21122. [DOI] [PubMed] [Google Scholar]
- 32.Veth R, Schreuder B, Beem H, Pruszczynski M, Rooy J. Cryosurgery in aggressive, benign, and low-grade malignant bone tumours. Lancet Oncol. 2005;6:25–34. doi: 10.1016/S1470-2045(04)01710-3. [DOI] [PubMed] [Google Scholar]