Abstract
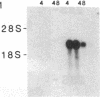
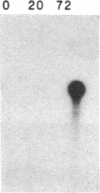
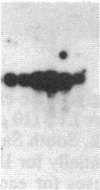
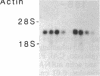
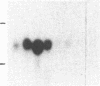
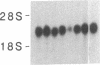
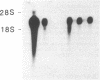
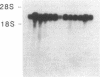
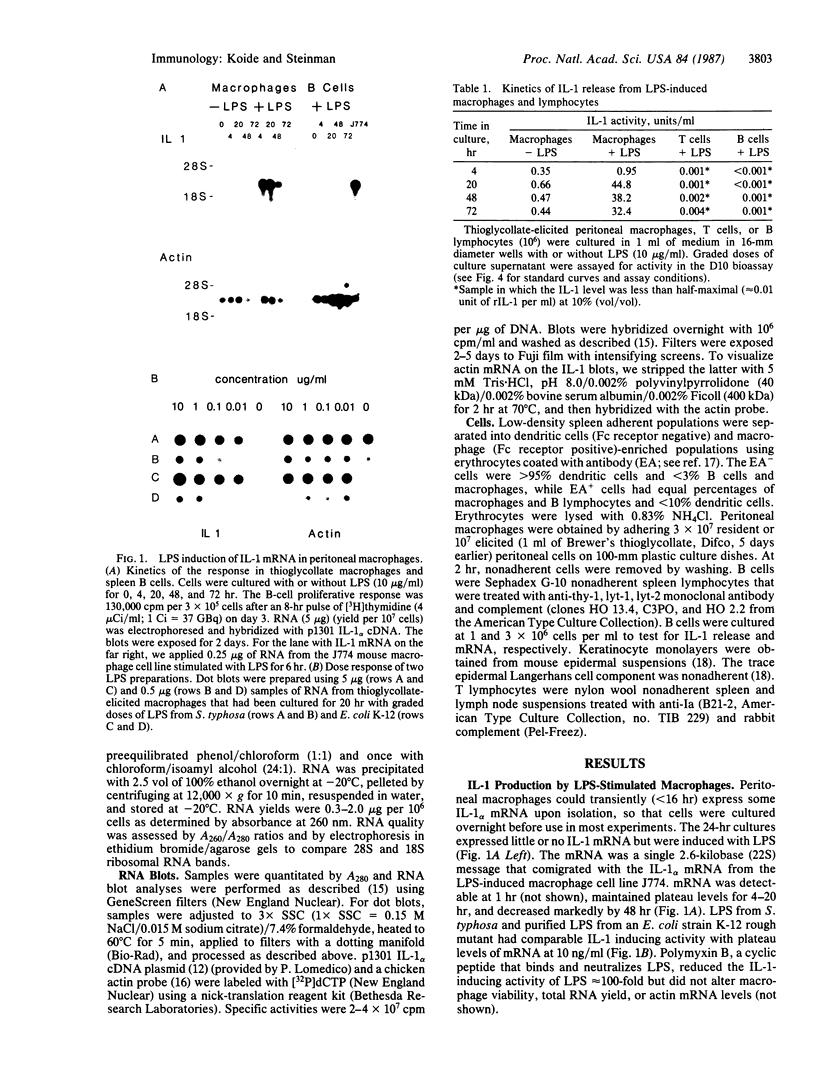
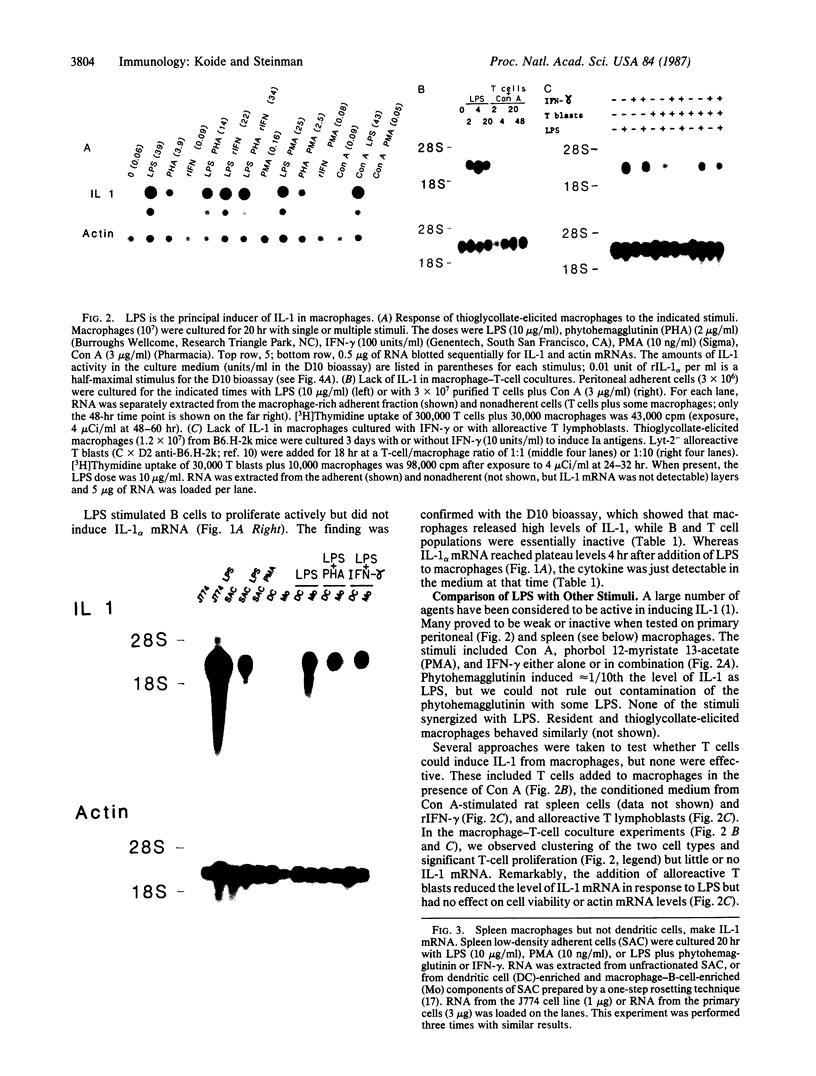
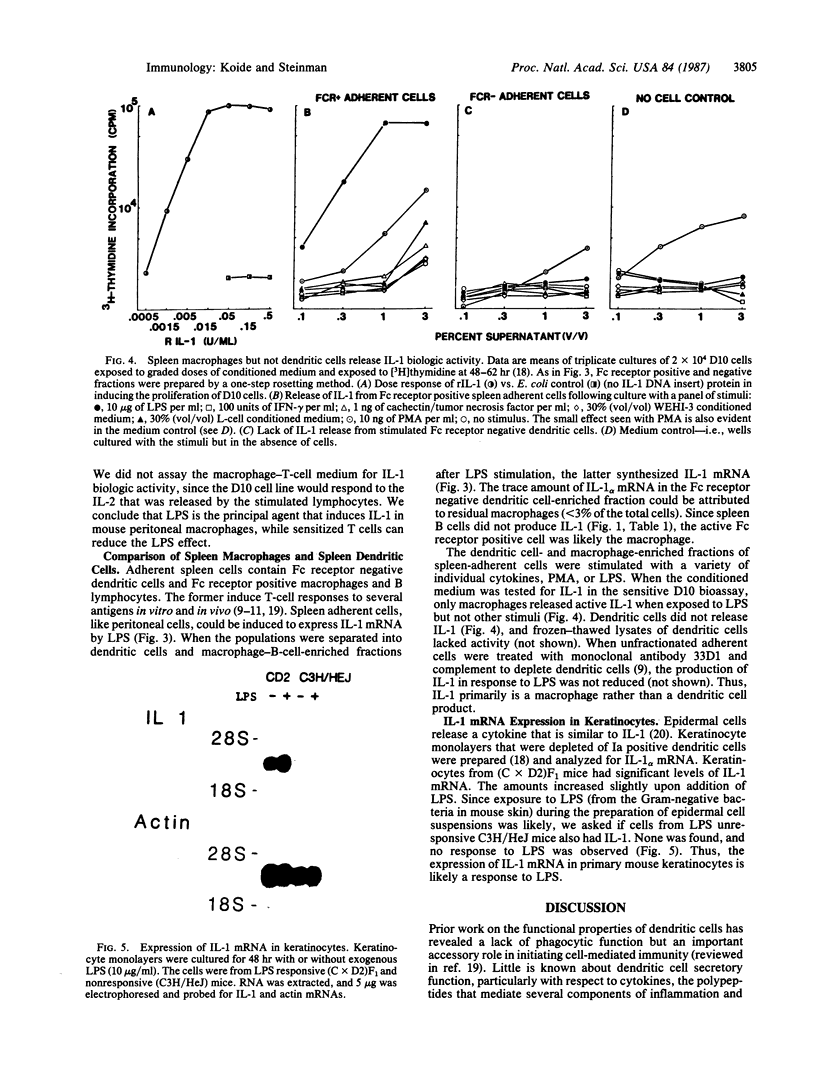
An interleukin 1 alpha (IL-1 alpha) cDNA probe and an IL-1 responsive T-cell clone (D10.G4; half-maximal stimulation, 0.1-1 pM) have been used to study the production of IL-1 by primary murine cell populations, particularly macrophages and dendritic cells. Spleen and peritoneal macrophages produced IL-1 mRNA and released biologically active IL-1 when challenged with lipopolysaccharide (LPS). Induction of IL-1 was evident over a dose range of 0.01-10 micrograms of LPS per ml, and maximal mRNA levels were maintained from 4 to 20 hr. Several other stimuli did not induce IL-1 in cultured macrophages, including phorbol 12-myristate 13-acetate, gamma-interferon, Con A, macrophage colony-stimulating factor, IL-3, cachectin, and activated T cells. Activated T cells could markedly reduce the response of peritoneal macrophages to LPS. When other cell types were compared with macrophages, keratinocytes had high levels of IL-1 mRNA, apparently in response to endogenous LPS. However B and T lymphocytes did not yield detectable IL-1 during proliferative responses to LPS and Con A, respectively, while dendritic cells produced little or no IL-1 when challenged with a battery of stimuli. Therefore, IL-1 may not be required for the potent accessory function of dendritic cells in lymphocyte mitogenesis. The results indicate that macrophages and dendritic cells have different secretory capacities. The macrophage is the principal leukocyte that synthesizes IL-1, and select stimuli increase and decrease the levels of macrophage IL-1 mRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayne E. K., Rupp E. A., Limjuco G., Chin J., Schmidt J. A. Immunocytochemical detection of interleukin 1 within stimulated human monocytes. J Exp Med. 1986 May 1;163(5):1267–1280. doi: 10.1084/jem.163.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Andrus L., Steinman R. M. Lymphokine and nonlymphokine mRNA levels in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1986 Apr 1;163(4):922–937. doi: 10.1084/jem.163.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. F., Murphy P. A., Windle B. E. Failure of rabbit neutrophils to secrete endogenous pyrogen when stimulated with staphylococci. J Exp Med. 1980 Jun 1;151(6):1360–1371. doi: 10.1084/jem.151.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science. 1985 Aug 2;229(4712):475–479. doi: 10.1126/science.3160115. [DOI] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Mathieson B. J., Mage M., Schmidt J. A., Oppenheim J. J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982 May;128(5):2147–2152. [PubMed] [Google Scholar]
- Matsushima K., Procopio A., Abe H., Scala G., Ortaldo J. R., Oppenheim J. J. Production of interleukin 1 activity by normal human peripheral blood B lymphocytes. J Immunol. 1985 Aug;135(2):1132–1136. [PubMed] [Google Scholar]
- Pereira R. A., King N. J., Blanden R. V. Comparison of functional properties of thymic and splenic dendritic cells. Cell Immunol. 1986 Oct 1;102(1):152–167. doi: 10.1016/0008-8749(86)90334-5. [DOI] [PubMed] [Google Scholar]
- Pistoia V., Cozzolino F., Rubartelli A., Torcia M., Roncella S., Ferrarini M. In vitro production of interleukin 1 by normal and malignant human B lymphocytes. J Immunol. 1986 Mar 1;136(5):1688–1692. [PubMed] [Google Scholar]
- Röllinghoff M., Pfizenmaier K., Wagner H. T-T cell interactions during cytotoxic T cell responses. IV. Murine lymphoid dendritic cells are powerful stimulators for helper T lymphocytes. Eur J Immunol. 1982 Apr;12(4):337–342. doi: 10.1002/eji.1830120415. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakovsky B., Kovacs E. J., Takacs L., Durum S. K. T cell clone producing an IL 1-like activity after stimulation by antigen-presenting B cells. J Immunol. 1986 Jul 1;137(1):160–166. [PubMed] [Google Scholar]