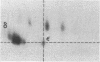
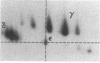
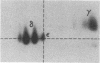
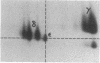
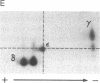
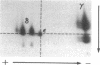
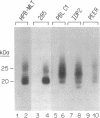
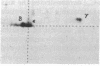
Abstract
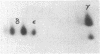
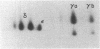
The T-cell receptor (TCR) gamma gene product occurs in association with T3 (CD3) polypeptides on the surface of human T lymphocytes. TCR gamma lymphocytes express arrays of T3 polypeptides distinct from those typically observed on TCR alpha beta lymphocytes. This report demonstrates that identical T3 gamma, delta, and epsilon polypeptides are synthesized by TCR gamma lymphocytes and TCR alpha beta lymphocytes. However, the processing of T3 delta oligosaccharides is distinct in the two cell types. This observation may suggest distinct quaternary structures of these receptor complexes. Despite these structural differences, the T3 molecule on TCR gamma lymphocytes is functional. It is associated with and comodulates with TCR gamma and it serves as a substrate for protein kinase C-mediated phosphorylation. Anti-T3 monoclonal antibodies induce a rapid increase in cytoplasmic free calcium, indicating that the receptor complex is involved in signal transduction and triggering of TCR gamma lymphocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando I., Hariri G., Wallace D., Beverley P. Tumor promoter phorbol esters induce unresponsiveness to antigen and expression of interleukin 2 receptor on T cells. Eur J Immunol. 1985 Feb;15(2):196–199. doi: 10.1002/eji.1830150217. [DOI] [PubMed] [Google Scholar]
- Bank I., DePinho R. A., Brenner M. B., Cassimeris J., Alt F. W., Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986 Jul 10;322(6075):179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borst J., Alexander S., Elder J., Terhorst C. The T3 complex on human T lymphocytes involves four structurally distinct glycoproteins. J Biol Chem. 1983 Apr 25;258(8):5135–5141. [PubMed] [Google Scholar]
- Borst J., Prendiville M. A., Terhorst C. Complexity of the human T lymphocyte-specific cell surface antigen T3. J Immunol. 1982 Apr;128(4):1560–1565. [PubMed] [Google Scholar]
- Borst J., van de Griend R. J., van Oostveen J. W., Ang S. L., Melief C. J., Seidman J. G., Bolhuis R. L. A T-cell receptor gamma/CD3 complex found on cloned functional lymphocytes. Nature. 1987 Feb 19;325(6106):683–688. doi: 10.1038/325683a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Davies A. A., Crumpton M. J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan E. A., Wang C. Y., Wang L. C., Evans R. L. Noncovalently bonded subunits of 22 and 28 kd are rapidly internalized by T cells reacted with anti-Leu-4 antibody. J Immunol. 1983 Aug;131(2):536–539. [PubMed] [Google Scholar]
- Kanellopoulos J. M., Wigglesworth N. M., Owen M. J., Crumpton M. J. Biosynthesis and molecular nature of the T3 antigen of human T lymphocytes. EMBO J. 1983;2(10):1807–1814. doi: 10.1002/j.1460-2075.1983.tb01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Krangel M. S. Secretion of HLA-A and -B antigens via an alternative RNA splicing pathway. J Exp Med. 1986 May 1;163(5):1173–1190. doi: 10.1084/jem.163.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. Two tandemly organized human genes encoding the T-cell gamma constant-region sequences show multiple rearrangement in different T-cell types. Nature. 1985 Aug 1;316(6027):464–466. doi: 10.1038/316464a0. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Smith B. R., Barbosa J. A., Crimmins M. A., Herrmann S. H., Burakoff S. J. CTL adhesion and antigen recognition are discrete steps in the human CTL-target cell interaction. J Immunol. 1987 Mar 1;138(5):1325–1330. [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moingeon P., Jitsukawa S., Faure F., Troalen F., Triebel F., Graziani M., Forestier F., Bellet D., Bohuon C., Hercend T. A gamma-chain complex forms a functional receptor on cloned human lymphocytes with natural killer-like activity. Nature. 1987 Feb 19;325(6106):723–726. doi: 10.1038/325723a0. [DOI] [PubMed] [Google Scholar]
- Murre C., Waldmann R. A., Morton C. C., Bongiovanni K. F., Waldmann T. A., Shows T. B., Seidman J. G. Human gamma-chain genes are rearranged in leukaemic T cells and map to the short arm of chromosome 7. Nature. 1985 Aug 8;316(6028):549–552. doi: 10.1038/316549a0. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Zanders E. D., Lamb J. R., Beverley P. C., Wallace D. L., Tatham P. E., Tax W. J., Linch D. C. Investigation of early T cell activation: analysis of the effect of specific antigen, interleukin 2 and monoclonal antibodies on intracellular free calcium concentration. Eur J Immunol. 1985 Jan;15(1):7–11. doi: 10.1002/eji.1830150103. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C., Cantley L. C., Rosoff P. M. Stimulation of the T3-T cell receptor complex induces a membrane-potential-sensitive calcium influx. Cell. 1985 Mar;40(3):583–590. doi: 10.1016/0092-8674(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Harford J. B., Klausner R. D. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985 Nov;43(1):223–231. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Newton M., Crommie D. Expression of T3 in association with a molecule distinct from the T-cell antigen receptor heterodimer. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6998–7002. doi: 10.1073/pnas.83.18.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Daley J. F., Hodgdon J. C., Reinherz E. L. Calcium dependency of antigen-specific (T3-Ti) and alternative (T11) pathways of human T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6836–6840. doi: 10.1073/pnas.81.21.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M., Samelson L. E., Klausner R. D. A new subunit of the human T-cell antigen receptor complex. Nature. 1986 Dec 4;324(6096):480–482. doi: 10.1038/324480a0. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- Zanders E. D., Lamb J. R., Feldmann M., Green N., Beverley P. C. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 1983 Jun 16;303(5918):625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Borst J., Coligan J. E., Markham A. F., Orkin S., Terhorst C. Isolation of cDNA clones encoding the 20K T3 glycoprotein of human T-cell receptor complex. 1984 Nov 29-Dec 5Nature. 312(5993):413–418. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]