Abstract
Young women aged 15–24 years have the highest rates of sexually transmitted infections (STIs). The vulnerability of adolescents is often attributed to risky sexual behaviors, whereas biological factors affecting mucosal immunity are poorly understood. The objective of this cross-sectional study was to examine associations between the type of cervical epithelium and protein levels of 11 cervicovaginal cytokines and chemokines in non-pregnant healthy young women. Cervical epithelial types were viewed on colpophotography and measured quantitatively using computerized planimetry. We selected 16 women with immature epithelium (predominantly columnar and early/mid squamous metaplasia), and 16 women with mature epithelium (predominantly squamous epithelium). Cytokine levels were measured in cervicovaginal lavage samples by MILLIPLEX™ MAP Human Cytokine/Chemokine multiplex immunoassay. Bivariate Box-Cox regression models compared cytokine levels between immature and mature groups. Multivariate Box-Cox models adjusted separately for age, years since menarche, days since last menses, years of sexual activity, number of lifetime sexual partners, HPV infection, hormonal contraceptive use, smoking, bacterial vaginosis by Nugent’s criteria, and polymorphonuclear cells on wet prep. The mean age was 19.2 years. Women with immature epithelium demonstrated significantly higher levels of IL-1α, IL-1β, IL-6, IL-8, MIP-1α, RANTES, TNFα, IL-10, IL-12 and IFNγ (each p<0.01), compared to women with mature epithelium. Results remained highly significant in the multivariate models. Cytokine profiles in the healthy state may foreshadow differential responses to pathogens. Cervical epithelial type should be measured in clinical studies involving cervicovaginal immune markers.
Keywords: adolescent women, cervical epithelial maturation, mucosal immunity, sexually transmitted infections, uterine cervix
1. Introduction
Young women aged 15–24 years experience the highest rates of sexually transmitted infections (STIs) including Chlamydia trachomatis, Neisseria gonorhoeae, and human papillomavirus (HPV) (CDC 2009). The increased vulnerability of adolescents compared to adults is often attributed to risky behaviors, but biological differences in mucosal immune function at the cervix may also contribute. Adolescent and adult cervices differ strikingly in their epithelial composition (Moscicki and Singer 2006). The term “immature epithelium” refers to the pattern of abundant columnar and metaplastic epithelia typically found in adolescents, whereas “mature epithelium” refers to a predominantly squamous epithelium most commonly found in adults. Throughout adolescence, the on-going physiologic process of squamous metaplasia transforms columnar cells into squamous cells, such that adolescents exhibit varying distributions of cell types at any given time.
Cervical epithelial cells are proposed to be central orchestrators of the mucosal immune response. In vitro and ex vivo studies have shown cervical cells to secrete cytokines, express toll-like receptors, and maintain differential apical and basolateral compartments (Stephens 2003, Fahey et al. 2005, Wira et al. 2005). Cell line studies have demonstrated differential immune capabilities by columnar and squamous epithelial cells (Fichorova and Anderson 1999). However, little is known about the in vivo state. Ex vivo immunohistochemical analyses of tissue samples have rarely addressed factors affecting in vivo immunity, such as hormonal contraception, smoking, sexual behaviors, infections, and menstrual cycle (Scott et al. 2006). The cervicovaginal compartment is complex as epithelial cells not only secrete cytokines/chemokines but also are exposed to cervical mucous containing cytokines/chemokines from other cellular sources.
The current study examined in vivo associations between the type of cervical epithelium, as defined by clinical colpophotography, and the levels of eleven inflammatory and regulatory cytokines and chemokines [interleukin (IL)-1α, IL-1β, IL-6, IL-8, macrophage inflammatory protein (MIP)-1α, RANTES (regulated upon activation, normal T cell expressed and secreted), TNFα, IFNα2, IL-10, IL-12, and IFNγ]. This study of healthy young women collected detailed information about sexual and substance use behaviors, menstrual cycles, and infection status.
2. Materials and Methods
2.1 Participants
Study participants were selected from a larger prospective cohort of healthy young women enrolled (October 2000-September 2002) in a study of the natural history of HPV infection that has been described elsewhere (Moscicki et al. 2004, Scott et al. 2006). Briefly, women 13–21 years of age with recent onset of sexual activity (maximum 5 years history) were enrolled from a family planning clinic and a college student health center. Women were excluded for pregnancy, history of surgical treatment of the cervix, immunosuppression, or histology-proven cervical intra-epithelial neoplasia grade 2 or 3. The Committees on Human Subject Research at the University of California, San Francisco and San Francisco State University approved the study.
2.2 Data collection
As part of the larger cohort study, women were evaluated at baseline and every 4 months. At each visit, the following data were collected. Questionnaires were administered to collect information about sociodemographic characteristics (including age, race/ethnicity, age at first sex, age at menarche), behaviors (including contraceptive use, condom use, sexual partners, substance use), and reproductive health history (including pregnancies and menstrual cycle). Physical examinations were performed to collect cervical samples for HPV detection for 37 types by Roche Reverse Line Blot assay (Gravitt et al. 1998); and vaginal samples for diagnosis of bacterial vaginosis, Trichomonas vaginalis, and yeast vaginitis by routine wet prep and KOH microscopy (Amsel et al. 1983). Cervicovaginal lavage (CVL) samples were obtained as follows. A plastic pipette was used to wash 10cc of sterile normal saline directly over the cervical os; then the accumulated normal saline in the vaginal fornix was washed 2 more times over the cervix, drawn up with the pipette, and placed into a sterile 10cc collection tube for storage at −80°C until testing of cytokine/chemokine levels. Colpophotographs (10X, 16X magnifications) were taken to document the appearance of the cervical epithelium after applying 3% acetic acid. In addition, genital C. trachomatis and N. gonorrhoeae were tested by commercial assays using cervical samples obtained at annual visits and also when participants were symptomatic.
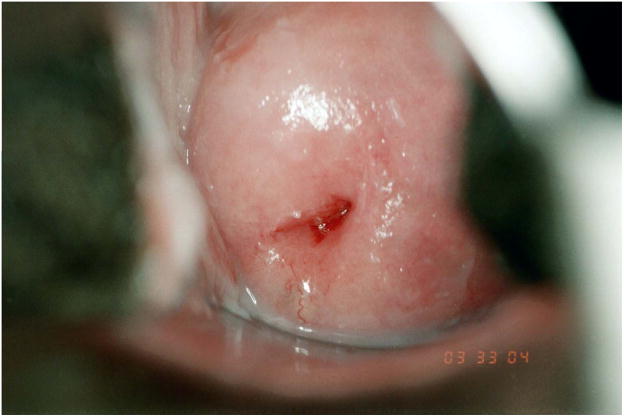
Quantitative measures of cervical immaturity were performed on digitized colpophotographs using computerized planimetry techniques in Photoshop CS2 (Adobe Systems Incorporated; San Jose, California) as described previously (Hwang et al. 2009). Briefly, cervical immaturity was defined as areas of columnar and early-mid squamous metaplasia. These areas were outlined and measured as pixel counts and expressed as a percentage of the total cervical face (Figure 1).
Figure 1.
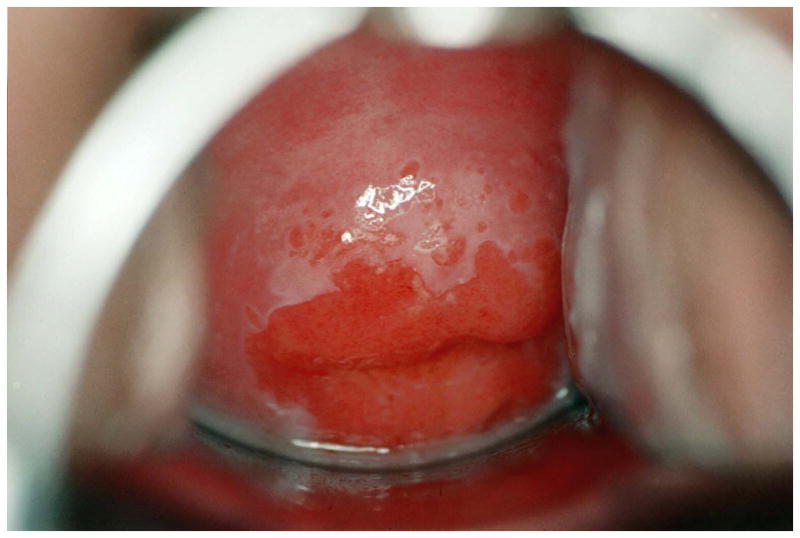
Quantitative measurements of the cervix performed by colpophotography and computerized planimetry. Cervical immaturity was defined as areas of columnar and early-mid squamous metaplasia and calculated as a percentage of the total cervical face. (A) Example of cervical immaturity measured as 67% of the total cervical face. (B) Example of cervical immaturity measured as <5% of the total cervical face.
2.3 Study design and selection of participants
This cross-sectional study selected 32 women for cytokine/chemokine testing, with each woman contributing 1 study visit. Selection was retrospective and based on the cervical immaturity measurements. To avoid misclassification, we selected women who differed substantially. The “immature group” comprised 16 women with cervical immaturity of greater than 50% of the total cervical face. The “mature group” comprised 16 women with cervical immaturity less than 5% of the total cervical face. Since this study focused on the constitutive healthy state, the participants also satisfied these additional inclusion criteria: non-pregnant; asymptomatic for genital complaints; negative for T. vaginalis, yeast, and bacterial vaginosis (by Amsel’s criteria); and negative for C. trachomatis and N. gonorrhoeae if available. However, to increase sensitivity for bacterial vaginosis, all 32 samples were also tested using gram stain according to Nugent’s criteria, and 2 cases were identified (Nugent et al. 1991). HPV infection is extremely common in adolescents and was not an exclusion criterion. Thus the analyses adjusted for bacterial vaginosis diagnosed by Nugent’s criteria and for HPV infection.
2.4 Cytokine/chemokine measurements
The CVL samples were thawed and centrifuged for measurements of protein levels of cytokine/chemokines by the MILLIPLEX™ MAP Human Cytokine/Chemokine Kit (Millipore Corp., Billerica, Massachusetts). Samples were measured in duplicate in the cell-free supernatants per manufacturer’s instructions. Assay plates were run on a Luminex 100 instrument (Luminex, Austin, Texas). Five-parameter logistic standard curves were fit using MiraiBio MasterPlex QT software (Hitachi Software, South San Francisco, California). This study took a conservative approach by considering the lower limit of the assay sensitivity to be the “minimum detectable concentration plus 2 standard deviations” (MinDC+2SD), as provided by the manufacturer. These MinDC+2SD values were as follows: IL-1α, 6.4 pg/mL; IL-1β, 0.7 pg/mL; IL-6, 0.7 pg/mL; IL-8, 0.3 pg/mL; MIP-1α, 6.4 pg/mL; RANTES, 2.0 pg/ml; TNFα, 0.1 pg/mL; IFNα2, 40.6 pg/mL; IL-10, 0.5 pg/mL; IL-12, 0.8 pg/mL; and IFNγ, 0.3 pg/mL. Of note, the IFNα2 levels were below this sensitivity limit in all 32 samples and could not be further studied. For this multiplex assay, the manufacturer tested for internal cross-reactivity in each individual kit as part of its validation protocol and allowed no more than 1% internal cross-reactivity in the final product. Within-woman reproducibility of the duplicate measurements was assessed by the intraclass coefficient [ICC = (msbetween − mswithin)/(msbetween + mswithin)] (Shrout and Fleiss 1979). The ICCs (95% confidence interval) for each cytokine were as follows: IL-1α, 0.93 (0.89, 0.96); IL-1β, 0.84 (0.75, 0.90); IL-6, 0.85 (0.77, 0.91); IL-8, 0.85 (0.77, 0.91); MIP-1α, 0.91 (0.86, 0.94); RANTES, 0.53 (0.33, 0.68); TNFα, 0.86 (0.78, 0.91); IL-10, 0.80 (0.69, 0.87); IL-12, 0.99 (0.98, 0.99); and IFNγ, 0.98 (0.97, 0.99).
2.5 Data Analyses
The predictor variable of interest was the type of cervical epithelium, immature or mature. The 10 outcome variables were each of the cytokine/chemokine protein levels, except for IFNα2 which could not be analyzed. Cytokine/chemokine levels that fell below the MinDC+2SD (as listed above in section 2.4) were imputed using the exact value of the MinDC+2SD. This imputation approach was the most conservative because the highest possible value was assigned to the sample, which minimized the opportunity to detect significant differences between samples. Separate analyses were conducted for each cytokine/chemokine, and the imputed values were included in all analyses. Bivariate analyses were performed by Box Cox regression models that consisted of power transformation of the outcome variable to alleviate heteroscedasticity followed by linear regression (Box and Cox 1964). Since the levels of several cytokines were low and imprecise, bivariate analyses were also conducted by non-parametric Wilcoxon rank-sum tests (Wilcoxon 1945). The results were unchanged (data not shown). Multivariate Box Cox models adjusted for each of ten covariates that may influence cytokine/chemokine expression: age, years since menarche, days since last menses, years of sexual activity, number of lifetime sexual partners, HPV infection, hormonal contraceptive use, smoking, bacterial vaginosis by Nugent’s criteria, and polymorphonuclear cells on wet prep. All analyses were performed using SAS software (v9.2, Cary, NC). Summary statistics were reported using the term “<LLD” to represent any value that was below the MinDC+2SD.
3. Results
This young sample had an overall mean age of 19.2 years (SD 2.1) and was diverse in race/ethnicity, self-reporting as 16% African-American, 28% Asian, 6% Latina, 28% Mixed/Other, and 22% White. Women reported a mean of 6.7 years since menarche, and a median of 18 days (IQR, 12, 23) since the last menses. We observed relatively low rates of hormonal contraceptive use by 7 (23%) women, and smoking by 3 (10%) women. Women in the immature and mature groups did not differ in their sociodemographic characteristics, behaviors, or test results (Table 1). Among the immature group, the median cervical immaturity was 66% (IQR, 59, 79) of the total cervical face. By definition, the median cervical immaturity of the mature group was <5%.
Table 1.
Sociodemographic characteristics, behaviors, and test results of healthy young women with immature and mature cervical epithelium
| Immature epithelium (n=16) | Mature epithelium (n=16) | p-valuea | |
|---|---|---|---|
| mean (SD) | mean (SD) | ||
| Age | 19.5 (2.5) | 18.8 (1.8) | 0.39 |
| Age of menarche | 12.3 (1.8) | 12.5 (1.8) | 0.72 |
| Years of sexual activity | 3.5 (2.7) | 3.3 (1.8) | 0.78 |
| median (interquartiles) | median (interquartiles) | ||
| Days since last menses | 19 (17, 35) | 17 (10, 23) | 0.26 |
| Number of lifetime sexual partners | 4 (2,9) | 3 (3,4) | 0.70 |
| Polymorphonuclear cells on wet prep | 2 (0, 14) | 0 (0, 13) | 0.39 |
| n (%) | n (%) | ||
| Race/Ethnicity, | 0.63 | ||
| African-American | 2 (12) | 3 (19) | |
| Asian | 6 (37) | 3 (19) | |
| Latina | 0 (0) | 2 (12) | |
| Mixed/other | 4 (25) | 5 (31) | |
| White | 4 (25) | 3 (19) | |
| Past pregnancy | 3 (19) | 3 (19) | 1.0 |
| HPV infection | 4 (25) | 3 (19) | 1.0 |
| Current oral contraception use | 2 (13) | 5(31) | 0.20 |
| Current Depo-Provera use | 1 (6) | 0 (0) | 1.0 |
| Current contraceptive ring or patch use | 0 (0) | 0 (0) | 1.0 |
| Current smoking | 1 (6) | 2(13) | 1.0 |
| Nugent score of wet prep | 0.57 | ||
| 0–3 | 8 (50) | 12 (75) | |
| 4–6 | 8 (50) | 2 (12) | |
| 7–10 | 0 (0) | 2 (12) |
Statistical comparisons were conducted by t-test, Wilcoxon rank-sum, fisher’s exact test, or Cochrane Mantel Haenszel test as appropriate.
Protein levels of 10 cytokines/chemokines were significantly higher in the immature group compared to the mature group (Table 2). IFNα2 levels were below the lower limit of detection (<LLD) for all 32 women and could not be further analyzed. Of note, although TNFα levels were significantly higher in the immature group, the overall variability was limited as 30 (94%) women had levels <1 pg/ml (range 0.59–0.74 pg/ml). The significant associations between immaturity and each cytokine/chemokine were unchanged in all multivariate Box-Cox models adjusting for each of the ten covariates (data not shown).
Table 2.
Protein levels of cervicovaginal cytokine/chemokines in healthy young women with immature and mature cervical epithelium
| Cytokinea | Immature epithelium (n=16) | Mature epithelium (n=16) | Box Cox regression | ||||
|---|---|---|---|---|---|---|---|
| Median (pg/ml) | Interquartiles (pg/ml) | Median (pg/ml) | Interquartiles (pg/ml) | p-value | |||
| IL-1α | 395.89 | 89.65 | 894.96 | 51.10 | 18.88 | 94.56 | 0.005 |
| IL-1β | 54.11 | 11.7 | 261.43 | <LLD | <LLD | 1.24 | <0.001 |
| IL-6 | 50.01 | 21.07 | 94.01 | 2.97 | 1.41 | 8.77 | <0.001 |
| IL-8 | 3139.1 | 401.8 | 7557.2 | 115.5 | 58.5 | 307.3 | <0.001 |
| MIP-1α | 24.39 | 10.02 | 109.18 | <LLD | <LLD | <LLD | <0.001 |
| RANTES | 2.55 | 2.00 | 15.42 | <LLD | <LLD | <LLD | 0.006 |
| TNF | 0.67 | 0.63 | 0.73 | 0.62 | 0.60 | 0.63 | <0.001 |
| IL-10 | 0.69 | 0.50 | 1.54 | <LLD | <LLD | <LLD | <0.001 |
| IL-12 | 0.9 | 0.81 | 1.01 | <LLD | <LLD | <LLD | <0.001 |
| IFNγ | 0.30 | 0.30 | 0.66 | <LLD | <LLD | <LLD | 0.002 |
<LLD, below the lower limit of detection; IL, interkeukin; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T cell expressed and secreted; TNF, tumor necrosis factor; IFN, interferon
IFNα2 levels were <LLD in all 32 women and thus are not reported.
Results were unchanged in multivariate Box Cox regression models adjusting for each covariate separately: age, years since menarche, days since last menses, years of prior sexual activity, number of lifetime sexual partners, HPV infection, hormonal contraceptive use, smoking, bacterial vaginosis diagnosed by Nugent’s criteria, polymorphonuclear cells seen on wet prep
4. Discussion
To our knowledge, this is the first study to examine in vivo cervicovaginal cytokine/chemokine profiles according to the predominant epithelial cell type of the ectocervix as clinically visualized. Remarkably, healthy young women with predominantly columnar and metaplastic epithelium exhibited higher levels of 10 of the 11 cytokines/chemokines tested, compared to those with predominantly squamous epithelium. In this clinical setting, the CVL samples would include immune factors derived from cervical and vaginal sources. However, vaginal sources would not be expected to differ between the immature and mature groups as the CVL technique was applied consistently across groups. Thus the focus was on the type of cervical epithelium as the main difference between groups. The colpophotography and computerized planimetry techniques enabled objective clinical definitions and the application of quantitative measures, which minimized misclassification of cervical immaturity.
Our results were consistent with prior in vitro work that found a columnar-derived cervical epithelial cell line to produce higher levels of IL-8 compared to a squamous-derived cell line in the basal non-stimulated state (Fichorova and Anderson 1999). IL-6, IL-7, and RANTES were detectable from the columnar cell line, but undetectable from the squamous cell line. Similarly, most of the low cytokine/chemokine levels in our study were found among the mature group. Another study of cervical and vaginal tissues demonstrated higher concentrations of immune cells, specifically macrophages, CD4+ T cells and CD8+ T1A1+ T cells, in areas of metaplasia (Pudney et al. 2005). Although these prior studies characterized immune parameters according to epithelial cell type, they were unable to examine the overall in vivo milieu which includes cytokines secreted into the cervical mucous from multiple sources, the presence of immune cells in the epithelium, and other potentially influential factors such as smoking, hormones, and menstrual cycle (Scott et al. 2006). Our study highlights the importance of identifying the cervical epithelial composition when interpreting data from clinical microbicide trials and epidemiological studies of STI risk. The type of cervical epithelium may act as a confounder, especially in younger populations who exhibit varying extents of cervical immaturity.
The difference between our immature and mature groups was highly significant, but the implications are unclear. A teleological hypothesis is that a higher cytokine profile at the columnar epithelium could reflect a greater “readiness” to mount a prompt immune response, since the columnar epithelium is only single-cell-layered and perhaps more prone to injury. However, this hypothesis assumes that a stronger or quicker inflammatory response is more protective. Alternatively, a higher cytokine profile could foreshadow a tendency towards a more severe inflammatory response that leads to harmful tissue sequelae. For example in C. trachomatis infection, the biological basis for inflammatory tissue damage is a major area of study. The cellular paradigm proposes that inflammation is initiated and sustained by the infected epithelial cells via cytokine and chemokine secretion (Stephens 2003). Conversely, the immunological paradigm proposes that Chlamydial-specific adaptive T cell responses are largely responsible for collateral tissue damage (Brunham and Peeling 1994). Our study examined only healthy non-infected women and the implications for infection can only be speculative without further study. Future prospective studies will be critical for understanding the role of cervical epithelial type in acquisition of infection and the immune response during active infection.
The main limitation of our study is the small sample size that does not support a more extensive analysis of possible covariates. However, the results were striking and remained significant in the multivariate models. In addition, the absolute levels of many of the cytokine/chemokines were quite low and difficult to interpret. In the data analyses, we conservatively estimated the very low values to be at the highest possible cut-off for the sensitivity limit thereby maximizing a type II error; yet the differences remained highly significant. The challenge is that there are no established normative values for cervicovaginal cytokine/chemokine levels. Normative values are difficult to define given the dynamic nature of cytokines and the immune response. A focus on the comparisons rather than the absolute values may be most relevant until cytokine profiles and their measurement are better understood. Although we tested the commonly known genital infections, there may also be unknown microbes associated with immature epithelium that influence cytokine profiles. Our initial selection of cytokines could not address the certainly complex array of immune factors comprising the host defense. Investigating a wider repertoire of immune factors and identifying specific cell sources were outside our study scope. Finally, the women in our sample represented a sexually mature group well beyond puberty. This homogeneity supported our focus on cervical immaturity as the main variable, but future studies to characterize the biology of the immune response in younger adolescents are warranted. STI risk is greatest in younger adolescents, but this population is the most difficult to study clinically because of the inherent difficulty in conducting pelvic examinations and collecting biological samples in very young adolescents, especially those who are not sexually active.
In conclusion, this study found that higher levels of cervicovaginal cytokines are associated with a more immature columnar and metaplastic cervical epithelium compared to a more mature squamous epithelium. Future clinical studies of cervicovaginal immune markers and therapeutic trials of cervicovaginal agents should incorporate quantitative measures of cervical epithelial composition. A better understanding of mucosal immunity specifically in adolescent women will inform efforts to prevent and treat STIs in this high-risk population.
Acknowledgments
This research was supported by the National Institutes of Health: the National Cancer Institute (#R37CA51323-17); National Institute of Allergy and Infectious Disease (#K23AI076670); National Institute of Child Health and Development (#T32HD044331), and the National Center for Research Resources (UCSF-CTSI #UL1 RR024131). The funders had no role in the conduct of the research. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
We thank Susanna Miller Benningfield BA, Evelyn Hanson RNP MS, Julie Jay RNC WHNP, Janet Jonte BSN NP, and Cheryl Godwin de Medina BA for their hard work at the clinical sites and assistance in data collection and management. We thank Sepideh Nozzari MSc, Dien Vo BS, and Yanhong Lu BA for their meticulous care in managing and processing the laboratory samples.
Abbreviations
- bacterial vaginosis
bacterial vaginosis
- HPV
human papillomavirus
- IL
interleukin
- IFN
interferon
- IQR
interquartile range
- MIP
macrophage inflammatory protein
- RANTES
regulated upon activation, normal T cell expressed and secreted
- STIs
sexually transmitted infections
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Homes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Ser B (Methodological) 1964;26:211–46. [Google Scholar]
- Brunham RC, Peeling RW. Chlamydia trachomatis antigens: Role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–33. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2008. U.S. Department of Health and Human Services; Atlanta, Georgia: 2009. [Google Scholar]
- Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–14. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus pcr products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LY, Ma Y, Benningfield SM, Clayton L, Hanson EN, Jay J. Factors that influence the rate of epithelial maturation in the cervix in healthy young women. J Adolesc Health. 2009;44:103–10. doi: 10.1016/j.jadohealth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–83. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- Moscicki AB, Singer A. The cervical epithelium during puberty and adolescence. In: Jordan J, et al., editors. The cervix. 2. Blackwell Publishing Professional; Massachusetts: 2006. pp. 81–101. [Google Scholar]
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–63. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mrna expression by real-time pcr in adolescents and young women: Effects of chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: A central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]