Abstract
Neuropathologic change underlying primary progressive aphasia (PPA) most commonly includes one of the frontotemporal lobar degenerations, such as FTLD-tau or FTLD-ubiquitin. The next most frequent etiology of PPA is Alzheimer’s disease (AD). We describe 5 subjects with clinical diagnoses of semantic dementia, who underwent longitudinal clinical evaluation and postmortem neuropathology examination of the central nervous system. This case series examines retrospectively which clinical parameters might have pointed to the neuropathological diagnosis of AD.
Conclusion
Family history of late onset dementia, APOEε4 status, combined features of semantic dementia and progressive non-fluent aphasia present early in illness, or generalized seizures, may indicate AD as the underlying pathology of semantic dementia.
Dementia of the Alzheimer type (DAT) is frequently in the differential diagnosis for the syndrome of semantic dementia (SD). There is significant overlap in clinical presentations of SD due to frontotemporal lobar degeneration (FTLD) or neuropathologically proven Alzheimer’s disease (AD).(Varma, Snowden, Lloyd et al.) Semantic difficulties of SD are concomitant with early episodic memory impairment,(Hodges & Patterson, 1995) and ages at onset can be similarly late in life.(Chow, Boone, Mishkin, & Miller, 2001) Patients in later decades of life have an increased risk of showing AD pathologic change either along with or instead of frontotemporal lobar degeneration with either tau- or ubiquitin-immunoreactive inclusions (FTLD-tau, FTLD-U).
At the time of diagnosis of the subjects in this study, clinical criteria for primary progressive aphasia (PPA) were classified as two types: progressive non-fluent aphasia (PNFA) and semantic dementia (SD). (McKhann et al., 2001; Neary & al., 1998) Since then, a third type of aphasic syndrome has been described by consensus; logopenic progressive aphasia (LPA).(Gorno-Tempini, Brambati, Ginex et al., 2008; Gorno-Tempini, Dronkers, Rankin et al., 2004; Kertesz & Munoz, 1997). LPA may resemble semantic dementia in that patients with LPA remain fluent with severe anomia. However, this type of FTD may be more frequently attributable to regional AD pathologic change than to FTLD-tau or FTLD-U. (Gorno-Tempini et al., 2004) We conducted a retrospective chart review to explore clinical details that might have distinguished those patients with semantic dementia due to neuropathological AD.
METHODS
This retrospective chart review was approved by the University of Southern California Health Sciences Campus Institutional Review Board. We selected subjects who had completed autopsy by January, 2008, and who had been diagnosed with semantic dementia (SD). Subjects were clinically diagnosed with SD by a single clinician (TWC) using consensus criteria (McKhann et al., 2001; Neary & al., 1998) at the Rancho Los Amigos Alzheimer’s Center of California and underwent autopsy at the Neuropathology Cores of the USC Alzheimer’s Disease Research Center or the UCLA Alzheimer’s Disease Center.
We accessed clinical records, including clinician notes and prior correspondence with caregivers describing subject symptomatology, as well as results of neuroimaging and clinical laboratory tests. Data extracted from the charts onto coded data collection sheets included demographics, timing of onset of illness, and features of the aphasia described by Neary, (Neary & al., 1998) McKhann, (McKhann et al., 2001) and Gorno-Tempini.(Gorno-Tempini et al., 2004) Included were diagnostic criteria for PNFA, SD, and LPA, if available, from the first 5 years of illness see; and also neuropsychiatric profiles; neuroimaging findings; neuropsychological evaluations; and neuropathologic findings.
We then re-assessed whether, over the course of illness, the cases met criteria for PPA and correlated those retrospective clinical diagnoses with the neuropathologic diagnoses. The number of cases was too small (n =5 SD) to conduct meaningful statistical analyses.
RESULTS AND CASE SUMMARIES
All 5 subjects were men. None of the cases met criteria for apraxia of speech (AOS) as described by Josephs et al.: slow speaking rate, abnormal prosody and distorted sound substitutions, additions, repetitions and prolongations, sometimes accompanied by groping and trial and error articulatory movements (Josephs, Duffy, Strand et al., 2006). Patients with SD were treated with cholinesterase inhibitors, considering the likelihood that they had atypical AD, but language did not improve with this intervention.
CASE SUMMARIES
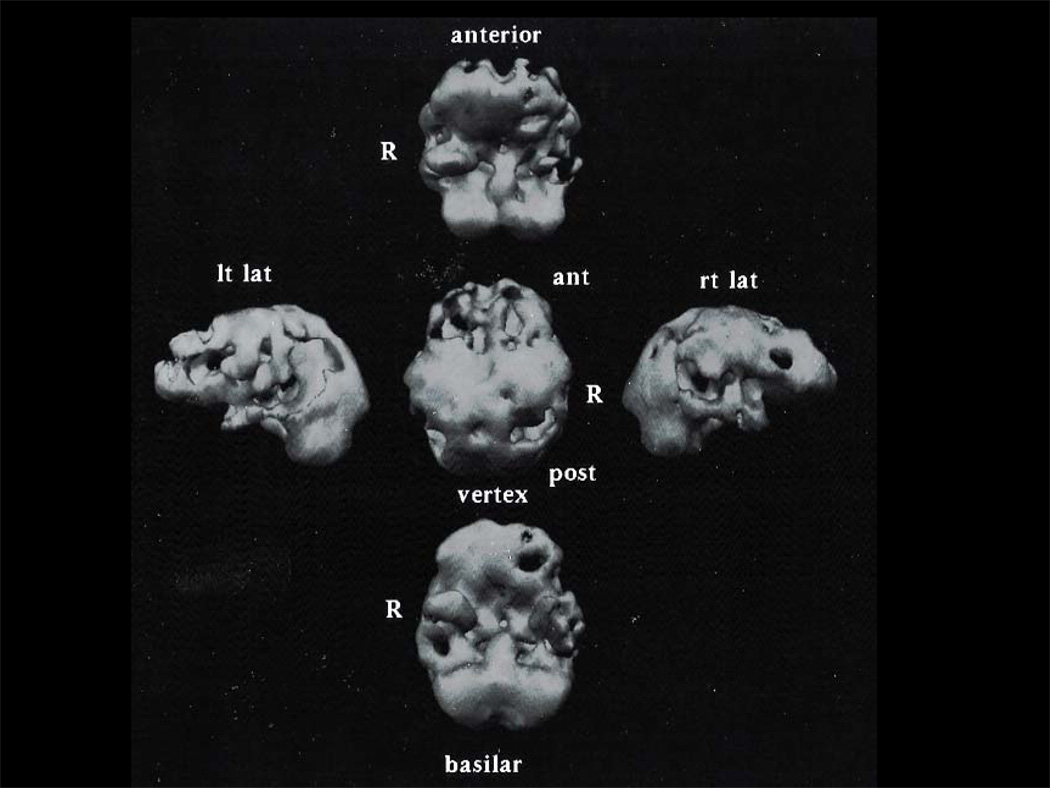
FM presented to the Behavioral Neurology Clinic at age 56, 10 years after new onset of stuttering. He made phonemic and semantic errors during fluent, empty speech with anomia and eventually was not able to follow commands. It is possible that he had apraxia but, by the time of presentation, he was too impaired for neuropsychological testing. Even in retrospect, the patient’s wife reports that he didn’t lose much memory, citing as examples retained wayfinding, even in another city, long after the patient was unable to speak or participate in simple chores. At ten years into his illness, he displayed aggressive behavior and sexual disinhibition. Later into the illness, he had 2 generalized tonic-clonic seizures. SPECT scan showed left-sided hypoperfusion that included frontal, temporal and parietal regions (see Figure 1). Although the SPECT scan showed parietal involvement which may have been indicative of AD, the patient’s early onset age, long history of primary aphasia follwed by major disinhibition (e.g., urinating into the sink in the clinical exam room during a follow-up appointment) seemed to fit SD more so than AD. The patient died after 13 years of illness.
Figure 1.
Three-dimensional reconstruction of HMPAO-SPECT for subject FM, who had clinical diagnosis of semantic dementia and AD at pathology. Scan shows predominantly left-sided hypoperfusion involving temporal lobe with extension to frontal and parietal regions.
The neuropathologic diagnosis was AD with rarefaction of temporal white matter. The distribution of neuropathologic changes was more often symmetrical than asymmetrical. Atrophy was moderate throughout the brain, including the hippocampal regions. Diffuse plaques were frequent in bilateral mid frontal, bilateral superior and middle temporal, left inferior parietal, right primary visual, right visual association cortices; sparse in the right hippocampus. Neuritic plaques were frequent in both frontal, both temporal, left inferior parietal, and right visual association cortices. Neurofibrillary tangles were frequent in left middle frontal and both superior and middle temporal cortices, and right hippocampus. The right hippocampus also featured moderal granulovacuolar degeneration and Hirano bodies. Although he had manifested fasciculations in his lower extremities during life, he did not have motor neuron disease (MND) pathology, or ubiquitinated neuronal cytoplasmic inclusions by immunohistochemistry.
DO presented to clinic at age 66, 3 years after initial onset with difficulty reading aloud. Symptoms of depression were apparent before his language declined. The depression may have been related to diagnosis of lung cancer, but the tumor was resected successfully. His family noted a decline in language shortly after the surgery. His speech was fluent and empty, with phonemic and semantic paraphasias and impaired naming and understanding. Formal neuropsychological testing was attempted but could not be completed due to the severity of his language deficits. Also because of this aphasia, it was difficult for the family to tell whether he had significant memory loss. Four years into the illness, he was engaging in bathing rituals, had increased appetite, and developed bowel and bladder incontinence. One year after that, he had a generalized tonic-clonic seizure. Over the course of the illness, he developed cogwheeling, rigidity, and resting tremor, as well as displaying frontal release signs. Brain imaging showed predominantly temporal lobe atrophy. Family history included a father with late onset dementia. Similar to FM, he had fasciculations in his lower extremities clinically, but did not have MND pathology. The early onset age, predominance of progressive aphasia, early incontinence, and onset of obsessive-compulsive behaviors midway into illness led to the diagnosis of SD over AD.
After 10 years of illness, DO came to autopsy. Neuropathologic diagnosis was AD with severe atrophy of the frontal and temporal lobes and moderate atrophy elsewhere, including the hippocampus. Diffuse plaques were more frequent in the left than right middle frontal, right more than left superior and middle temporal, right more than left inferior parietal, and right more than left primary visual cortex. Neuritic plaques were frequent in both superior and middle temporal regions, the left inferior parietal and the left visual association cortex.
Neurofibrillary tangles were frequent in all cortical sections except for absence from left primary visual and visual association cortex. Neurofibrillary tangles were frequent in all cortical sections except for absence from left primary visual and visual association cortex. TDP-43 immunostain of frontal, temporal, parietal and occipital neocortices revealed no inclusions. The hippocampus, including dentate granule neurons and pyramidal neurons, was immunoreactive. No positive inclusions were present in the basal ganglia (putamen, globus pallidus); mesencephalon; pons; cerebellum; or spinal cord (cervical, thoracic, and lumbar). There was moderate, diffuse, nonspecific immunostain of neurons of the basal pontis and anterior horn cells. The neuropathologic findings, plus the clinical dementia fulfilled the NIA/Reagan criteria for high probability Alzheimer’s disease using NIA/Reagan criteria.
LA presented at age 58, 5 years after onset with problems with expressive language and word finding. Single word repetition was intact, but he had impaired comprehension of word meaning, prosopagnosia and associative agnosia, and later developed hypophonia. He and his wife denied memory loss as a symptom long into the course of illness, citing aphasia as the main problem. He was unable to execute learned purposeful movements (ideomotor apraxia). Serial MMSE scores declined 2–3 points per year over the next 4 years. He developed a quick temper and was also inappropriately affectionate with female acquaintances. He had symptoms of depression and anxiety related to retained insight signaling his aphasia and the poor prognosis for recovery. Brain MRI showed atrophy of the left frontal lobe. He had a sister who suffered from depression and a maternal uncle with ALS. This family history, along with early onset of predominant progressive aphasia for which the patient had good insight, and development of disinhibition highly uncharacteristic of his baseline indicated SD more than AD.
The patient died after 13 years of illness and the neuropathologic diagnosis was AD with amyloid angiopathy. Atrophy was rated as mild, with no specific asymmetry. Diffuse plaques were frequent in both superior and middle temporal, and the left inferior parietal regions, while neuritic plaques were more lateralized to the left superior and middle temporal, left inferior parietal and left lateral geniculate body. Neurofibrillary tangles were frequent in left middle frontal, bilateral superior and middle temporal, and bilateral hippocampal regions. TDP-43 immunohistochemistry showed no reactivity of any neocortical regions (frontal, parietal, temporal, occipital). The basal ganglia, brainstem (mesencephalon, pons, and medulla) and spinal cord neurons revealed TDP-43 positive inclusions. The neuropathologic findings, combined with a clinical history of dementia were consistent with a high probability of Alzheimer’s disease.
MC presented at age 70, 1 year after onset of anomia. Repetition was intact, but he had impaired comprehension of word meaning and low average scores on tests for associative agnosia. MMSE scores declined precipitously from 20 to 0 out of 30 in only 2 years due to profound impairment in comprehension. He developed separation anxiety, irritiability, and amotivational syndrome within the first year of illness. Memory loss was only apparent more than halfway into the course of illness. The patient used to recount stories of his days which were difficult to comprehend due to the absence of key nouns. His wife, however, was able to understand what he meant and corroborated the relative accuracy of the stories. Brain MRI showed mild atrophy at presentation. SPECT scans revealed left parietal hypoperfusion extending to the ipsilateral temporal lobe and perisylvian fissure. He had a fall with subdural hematoma 3 years into illness. A generalized seizure late that same year was attributed to subtherapeutic levels of phenytoin prescribed after the evacuation of a subdural hematoma. MC’s mother had died with a late onset dementia. Despite the later onset age and parietal hypoperfusion indicating AD in this patient, the length of predominantly progressive aphasia as the sole symptom, his persistent euphoria through much of the illness, and the relative retention of memory function were interpreted as SD, not AD.
After 10 years of illness, the neuropathologic diagnosis was AD. There was severe atrophy in the frontal and temporal lobes. Diffuse plaques were frequent in frontal, temporal, parietal regions, with neuritic plaques more frequent in the superior and middle temporal and hippocampal than in frontal or parietal regions. Neurofibrillary tangles were frequent in superior and middle temporal, hippocampus and entorhinal cortex. There was no comment to support an asymmetry of neuropathologic changes. TDP-43 immunostaining of frontal, temporal, parietal and occipital cortices revealed moderate to strong but diffuse staining of pyramidal neurons, particularly the nuclei. Hippocampal neurons, including dentate-granule cells and pyramidal neurons were negative. No neuronal inclusions were noted, here or in the brainstem and spinal cord. The clinical history of dementia plus neuropathologic changes was consistent with high probability Alzheimer’s disease according to NIA/Reagan criteria.
LR presented at age 69, 3 years after the onset of anomia. Single word repetition was intact, but he had impaired naming and comprehension without prosopagnosia or associative agnosia. He developed alexia rather quickly, yet remained able to compose music (his vocation) until year 6 of his illness. Serial MMSE scores fluctuated from 29 to 22 to 25 to 19 over years 1–5 of illness. He became very self-absorbed, arrogant and distractible over time. He retained insight about his aphasia and poor prognosis for recovery far into the illness. The aphasia made it difficult for his wife to recognize memory loss, but memory loss and disorientation were clear after the 8th year of illness. The example given was that he could no longer play music he had composed on the piano, even the one tune on which he had come to perseverate earlier in the illness. Inability to recognize his own son, even with cuing may have represented visuoperceptive difficulties. His wife had the impression that he would sometimes pretend to remember a person or story after being reminded, yet it was clear he really did not. Specific recognition of his wife was impaired by year 11 of illness; he indicated that she was familiar and someone he was happy to see. At that point, he had lost interest in dogs, otherwise a long time passion but sought cigarettes to the end of his life.
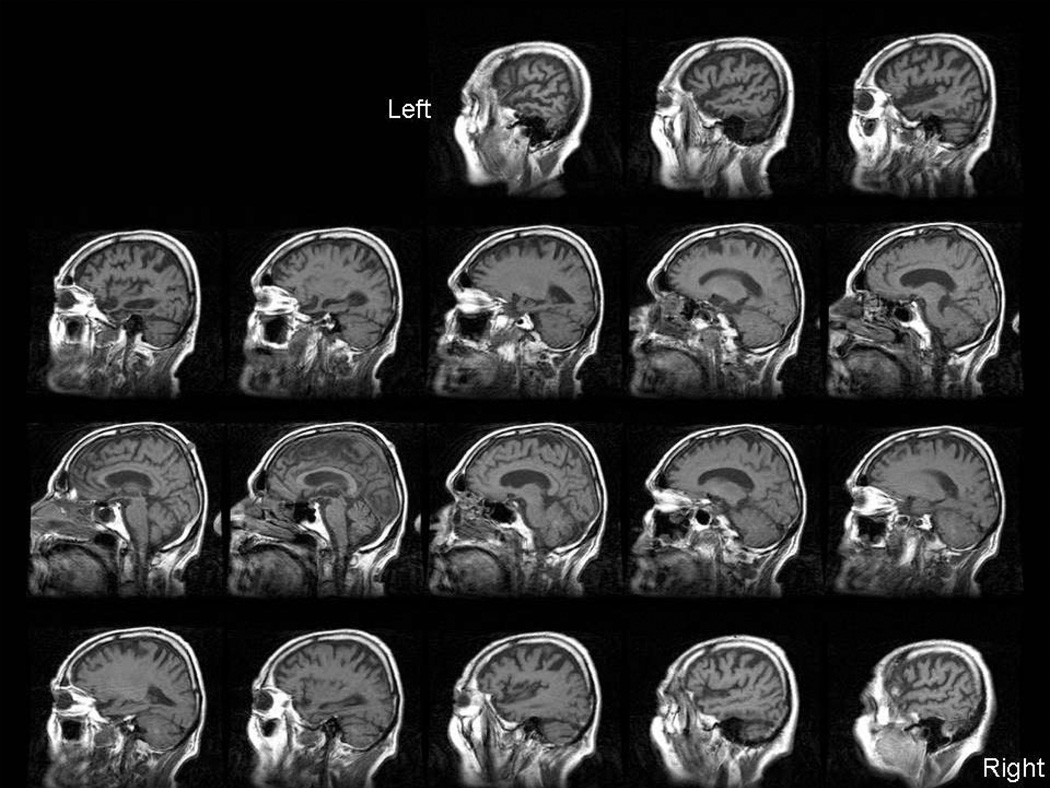
Brain MRI showed atrophy of the left temporal lobe (see Figure 2). He had a brother who suffered from depression. This patient ranged to the older end of early onset but his ability to compose music far into the progressive aphasia course and frontal behavioral features led to the diagnosis of SD more than AD. His course lasted long enough that a potential diagnosis of AD was discussed with his family after the first 10 years of illness.
Figure 2.
T1-weighted sagittal MRI for subject LR, who had clinical diagnosis of semantic dementia and FTLD-U at pathology. Marked atrophy in left temporal lobe with extension to frontal and parietal regions.
After 12 years of illness, the neuropathologic diagnosis was FTLD-U. Anti-TDP-43 and ubiquitin immunostains detected 1) abundant intracytoplasmic inclusions in temporal and frontal cortices and 2) both intranuclear and neuritic inclusions in hippocampal regions.
Of five subjects clinically diagnosed with SD, four were pathologically diagnosed with AD and one had ubiquitinated inclusions (FTLD-U). All APOEε4-positive SD cases (3/5) had AD. The FTLD-U case had no APOEε4 allele. Despite the presence of APOEε4, family histories did not consistently raise suspicion for AD. Two of the four AD subjects had a parent with late onset dementia. One subject with AD and the one with FTLD-U each had a sibling with depression. A subject with AD also had a maternal uncle with ALS.
None of cases met more criteria for LPA than for SD. Those SD cases with AD pathology were impaired in comprehension and repetition, while the subject with FTLD-U retained repetition ability longer into course of illness. In two AD subjects and the one with FTLD-U, agraphia and alexia did not develop for most of the illness. Characteristic for AD was the rate of progression of decline in the MMSE scores. Two with AD for whom MMSE progression could be tracked lost 6–7 points over a 3 year period. A third with AD was unable to respond to MMSE questions due to rapid progression of aphasia by year 3 or 4 into illness. The subject with FTLD-U had fluctuations in MMSE scores over a 5-year period.
Behavioural disturbances were seen in some of the AD subjects: one subject with AD developed sexual disinhibition; another engaged in bathing rituals and had increased appetite. Subjects with AD had depression or mixed depression and anxiety preceding their aphasia. This was erroneously interpreted as a pertinent negative finding of the belle indifference associated with AD. The subject with FTLD-U demonstrated new selfishness and arrogance.
The five subjects clinically diagnosed with SD had more features atypical of SD rather than features characteristic of AD. None reported any developmental delay or learning disorder. 3 of 4 with pathological AD made both phonemic (PNFA) and semantic (SD) paraphasic errors on early examination. None of the five (including FTLD-U) met all Neary criteria for SD (2 did not have perceptual disorder testing). Each AD subject met 2 or all 5 Neary criteria for PNFA, in addition to aspects of SD. Also atypical for SD, 3 of the 4 subjects with AD had generalized seizures. The one with FTLD-U did not have seizures. Atrophy on neuroimaging was symmetrical in 2 of 4 subjects with AD, which is uncharacteristic of SD. One of four subjects with AD lived longer than typically reported for SD (13 years instead of 6–10), but the one with FTLD-U pathology also survived 12 years with illness.
There were also characteristics atypical of AD in the subjects with pathological diagnosis of AD. Average age of onset of 62.5 years (range 56–70) in the 4 AD subjects was young. Age of onset of the one with FTLD-U was age 69. The referring physician in only 1 of 4 AD cases cited memory loss as a reason for referral, but this was in addition to anomia and impairment in expressive language. Each subject retained insight into his aphasia which is unusual for patients with AD.
In addition, the four SD subjects diagnosed with AD at autopsy had certain pathological features which were atypical of AD. Neuritic plaques were more dense in frontotemporal than temporoparietal regions in one of three (FM); no neurofibrillary tangles were seen in the parietal lobe of the same subject. Neuritic plaques and NFTs were moderate to severe in hippocampus (CA1), frontal, temporal, and parietal cortices. Left vs. right hemisphere comparisons for plaque burden were not available for all subjects, but two of three AD subjects had asymmetric plaque burden and not both concentrated on the left. Parietal atrophy was absent in one subject (LA).
DISCUSSION
Twelve previous studies address patients diagnosed clinically with PNFA or SD and who were neuropathologically confirmed (see Table 1). Tallying all of the reported clinicopathologic confirmations of FTLD shows more concordance in diagnosing SD (79%) than PNFA (65%). Alzheimer’s disease pathology was the most frequent non-FTLD finding, but AD with concomitant dementia with Lewy bodies has also been reported.
Table 1.
Review of prior clinicopathological correlation studies.
| Authors Names (reference) | Number of SD Subjects in Sample, % Confirmed as FTLD |
Number of PNFA Subjects in Sample, % of Confirmed as FTLD |
Non-FTD Dx’s at Autopsy |
|---|---|---|---|
| Davies, RR et al.(Davies et al., 2005) | 18, 89% | n/a | AD |
| Godbolt, AK et al.(Godbolt, Josephs, Revesz et al., 2005) | 7, 100% | n/a | n/a |
| Josephs, KA et al. (Josephs, Whitwell, Duffy et al., 2008) | 3, 100% | n/a | AD |
| Mandell, AM et al. (Mandell, Alexander, & Carpenter, 1989) | 1, 100% | n/a | n/a |
| Knibb, JA et al. (Knibb et al., 2006) | 15, 66% | 23, 65% | AD |
| Kertesz, A et al.* (Kertesz et al., 2007) | 2, 100% | 20, 100% | n/a |
| Mesulam, M et al. (Mesulam et al., 2008) | 1, 0% | 17, 58.8% | AD |
| Takao, M et al. (Takao, Tsuchiya, Mimura et al., 2006) | n/a | 1, 100% | n/a |
| Mochizuki, A et al. (Mochizuki, Ueda, Komatsuzaki et al., 2003) | n/a | 1, 100% | n/a |
| Caselli, RJ et al. (Caselli, Beach, Sue, Connor, & Sabbagh, 2002) | n/a | 1, 0% | AD with DLB |
| Li, F et al. (Li, Iseki, Kato et al., 2000) | n/a | 1, 0% | AD with asymmetrical brain atrophy |
| Scheltens, P et al. (Scheltens, Ravid, & Kamphorst, 1994) | n/a | 1, 100% | n/a |
All cases in this paper described as beginning as behavioral variant FTD.
We present 4 subjects with clinical diagnoses of SD and neuropathologic diagnoses of AD. As previously reported for SD due to FTLD (Davies, Hodges, Kril et al., 2005; McMonagle & Hodges, 2007), all 5 subjects with SD manifested behaviors typical of behavioral variant frontotemporal dementia (bvFTD) by 5–6 years after the onset of illness, yet 4 of the 5 SD subjects ultimately had AD. Behavioral disturbance therefore did not point accurately away from a diagnosis of atypical DAT.
Retrospective diagnosis did not change from SD to LPA in the subjects with AD at autopsy, and it remains unclear that this subtyping revels underlying pathology. Mesulam et al. found AD pathology in many but not all (7 of 11) subjects with LPA.(Mesulam, Wicklund, Johnson et al., 2008)
Although superior letter and category fluency may distinguish between AD and FTD (whether tau negative or tau positive),(Grossman, Xie, Libon et al., 2008) this indicator did not help anticipate the neuropathologic changes of AD in our study. The Repeat and Point test has recently shown differentiation between SD and PNFA; further study may reveal whether impairment on this test also betrays SD due to FTLD vs. AD.(Hodges, Martinos, Woollams, Patterson, & Adlam, 2008)
Lack of asymmetry on neuroimaging was inconsistently seen in the AD group, unlike previously reported autopsy-confirmed SD cases with FTLD-U or Pick’s disease who show asymmetry.(Davies et al., 2005)
Three of our 4 SD cases with AD pathology showed sparing of neuritic plaques in hippocampus and entorhinal cortex relative to AD cases with typical presentations. We did observe predominance of neurofibrillary tangle burden in the entorhinal cortex as documented in typical AD elsewhere.(Galton, Patterson, Xuereb, & Hodges, 2000; Mesulam et al., 2008) We report high AD neuropathologic burden in the left inferior parietal area, not focused only in temporal regions. If this had been more apparent on imaging (e.g., SPECT imaging early in course of illness), perhaps AD would have figured larger on the clinical differential diagnosis. APOEε4 positive genotype seemed indicative in our small series of AD pathology, but Mesulam et al. report FTLD pathology in a larger sample of PPA cases despite APOEε4 positive genotype and mixed aphasia characteristics at presentation.(Mesulam et al., 2008) Other indicators for AD risk were found in our series: family history of late onset dementia and/or development of generalized seizures were present in some but not all of the 4 subjects who had AD pathology. Further studies with larger numbers of cases may show which of these characteristics are reliably predictive of AD as the underlying pathology of semantic dementia.
Certain limitations of this case series may account for the lower diagnostic accuracy rate than reported in previous studies. Included are the variable phases of illness at presentation and the resulting inconsistency of data available. Some subjects presented much later into the course of illness, too late to evaluate initial aspects of their aphasia, with the illness itself limiting evaluation. Formal neuropsychological and speech and language pathology evaluations would have afforded a consistently full set of language descriptors and clinical criteria. The systematic evaluation of patients with SD by Davies et al., for instance, undoubtedly assisted in their higher clinical diagnostic accuracy of 89% in 18 cases.(Davies et al., 2005) Evaluations for the presence of apraxia in the current study were uncertain late in the courses of illness. It was mostly difficult to know how much impairment was secondary to comprehension deficit, especially among the patients with SD. Other investigators have formalized a battery for the evaluation of limb apraxia in PPA that would have been helpful in follow-up of our sample.(Joshi, Roy, Black, & Barbour, 2003)
Rogalski et al. have recorded 14.6% prevalence of learning disability in patients, with double that of family members of index cases. (Rogalski, Johnson, Weintraub, & Mesulam, 2008) We did not find histories of learning disability in any of the subjects in this study, but their family histories of learning disability were not collected. Either small sample size or missing data may account for this difference from the other study. It remains unclear whether this aspect of clinical characterization might assist in differentiating between SD due to FTLD vs. AD pathology.
Subjects in this study enjoyed prolonged survival after diagnosis, compared with the reported 6–7 year survival rate in FTD (Roberson, Hesse, Rose et al., 2005). The survival of our subjects exceeded even the prolonged survival reported for SD vs. bvFTD (Davies et al., 2005; Roberson et al., 2005). Since AD generally has a longer course of illness, longer survival might have been a clue to SD with AD pathology in this sample, but long survival has been reported among a subgroup of patients with FTD.(Johnson, Diehl, Mendez et al., 2005) The 2 subjects with SD due to AD described by Davies et al. had relatively young onset ages, perhaps confirming that age at onset and survival are not likely to predict which SD cases have underlying AD (Davies et al., 2005).
CONCLUSION
The current diagnostic criteria for semantic dementia have been shown in previous studies and the current one to be non-specific for FTLD-U, corticobasal degeneration or Pick’s disease. Given that AD belongs high on the differential diagnosis for semantic dementia, risk factors for AD such as family history of late onset dementia and APOE genotype may help determine whether interventions for AD should be part of the management plan for a patient with semantic dementia.
ACKNOWLEDGMENTS
We are grateful to the patients and families who generously participated in this study, expressly to help others affected with frontotemporal dementias. We also thank Dr. Daniel Geschwind and the Genetics Core of the UCLA Alzheimer’s Disease Center for APOE genotyping. This work was funded by NIA Alzheimer’s Disease Research Center Grant Numbers P50 AG05142, P50 AG16570, and P30 AG08017; and Department of Health Services, Alzheimer’s Research Center of California Grant No. 94-20356 (TWC, AV, CM), the University of Toronto Dean's Fund for New Faculty (#457494 TWC), and an endowment to the Sam and Ida Ross Memory Clinic (TWC), and the Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine (HVV).
REFERENCES
- Caselli RJ, Beach TG, Sue LI, Connor DJ, Sabbagh MN. Progressive aphasia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(2):55–58. doi: 10.1159/000064925. [DOI] [PubMed] [Google Scholar]
- Chow TW, Boone K, Mishkin F, Miller BL. Behavioral changes in subtypes of primary progressive aphasia and other frontotemporal dementias. Brain and Cognition. 2001;47:270–272. [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128(Pt 9):1984–1995. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(3):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- Godbolt AK, Josephs KA, Revesz T, Warrington EK, Lantos P, King A, Fox NC, Al Sarraj S, Holton J, Cipolotti L, Khan MN, Rossor MN. Sporadic and familial dementia with ubiquitin-positive tau-negative inclusions: clinical features of one histopathological abnormality underlying frontotemporal lobar degeneration. Arch Neurol. 2005;62(7):1097–1101. doi: 10.1001/archneur.62.7.1097. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Xie SX, Libon DJ, Wang X, Massimo L, Moore P, Vesely L, Berkowitz R, Chatterjee A, Coslett HB, H.I. H, Forman MS, Lee VM, Trojanowski JQ. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology. 2008;70(22):2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Martinos M, Woollams AM, Patterson K, Adlam AL. Repeat and Point: differentiating semantic dementia from progressive non-fluent aphasia. Cortex. 2008;44(9):1265–1270. doi: 10.1016/j.cortex.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33(4):441–459. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Johnson JKP, Diehl JMD, Mendez MFMDP, Neuhaus JP, Shapira JSRNP, Forman MMDP, Chute DJMD, Roberson EDMDP, Pace-Savitsky CMA, Neumann MMD, Chow TWMD, Rosen HJMD, Forstl HMD, Kurz AMD, Miller BLMD. Frontotemporal Lobar Degeneration: Demographic Characteristics of 353 Patients. Archives of Neurology. 2005;62(6):925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, Boeve BF, Graff-Radford NR, Parisi JE, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70(1):25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Roy EA, Black SE, Barbour K. Patterns of limb apraxia in primary progressive aphasia. Brain Cogn. 2003;53(2):403–407. doi: 10.1016/s0278-2626(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Blair M, McMonagle P, Munoz DG. The diagnosis and course of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2007;21(2):155–163. doi: 10.1097/WAD.0b013e31806547eb. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Munoz DG. Primary progressive aphasia. Clinical Neuroscience. 1997;4(2):95–102. [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Annals of Neurology. 2006;59(1):156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Li F, Iseki E, Kato M, Adachi Y, Akagi M, Kosaka K. An autopsy case of Alzheimer's disease presenting with primary progressive aphasia: a clinicopathological and immunohistochemical study. Neuropathology. 2000;20(3):239–245. doi: 10.1046/j.1440-1789.2000.00343.x. [DOI] [PubMed] [Google Scholar]
- Mandell AM, Alexander MP, Carpenter S. Creutzfeldt-Jakob disease presenting as isolated aphasia. Neurology. 1989;39(1):55–58. doi: 10.1212/wnl.39.1.55. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Archives of Neurology. 2001;58(11):1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McMonagle P, Hodges JR. A longitudinal study of semantic dementia. Neurology. 2007;68 Suppl 1:A351. [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63(6):709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki A, Ueda Y, Komatsuzaki Y, Tsuchiya K, Arai T, Shoji S. Progressive supranuclear palsy presenting with primary progressive aphasia--clinicopathological report of an autopsy case. Acta Neuropathol. 2003;105(6):610–614. doi: 10.1007/s00401-003-0682-5. [DOI] [PubMed] [Google Scholar]
- Neary D, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Paper presented at the Neurology. 1998 doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neary D, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Paper presented at the Neurology. doi: 10.1212/wnl.51.6.1546. (1998%V 51) [DOI] [PubMed] [Google Scholar]
- Roberson ED, Hesse JH, Rose KD, Slama H, Johnson JK, Yaffe K, Forman MS, Miller CA, Trojanowski JQ, Kramer JH, Miller BL. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65(5):719–725. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol. 2008;65(2):244–248. doi: 10.1001/archneurol.2007.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Ravid R, Kamphorst W. Pathologic findings in a case of primary progressive aphasia. Neurology. 1994;44(2):279–282. doi: 10.1212/wnl.44.2.279. [DOI] [PubMed] [Google Scholar]
- Takao M, Tsuchiya K, Mimura M, Momoshima S, Kondo H, Akiyama H, Suzuki N, Mihara B, Takagi Y, Koto A. Corticobasal degeneration as cause of progressive non-fluent aphasia: clinical, radiological and pathological study of an autopsy case. Neuropathology. 2006;26(6):569–578. doi: 10.1111/j.1440-1789.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DM, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1999;66(2):184–188. doi: 10.1136/jnnp.66.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]