Abstract
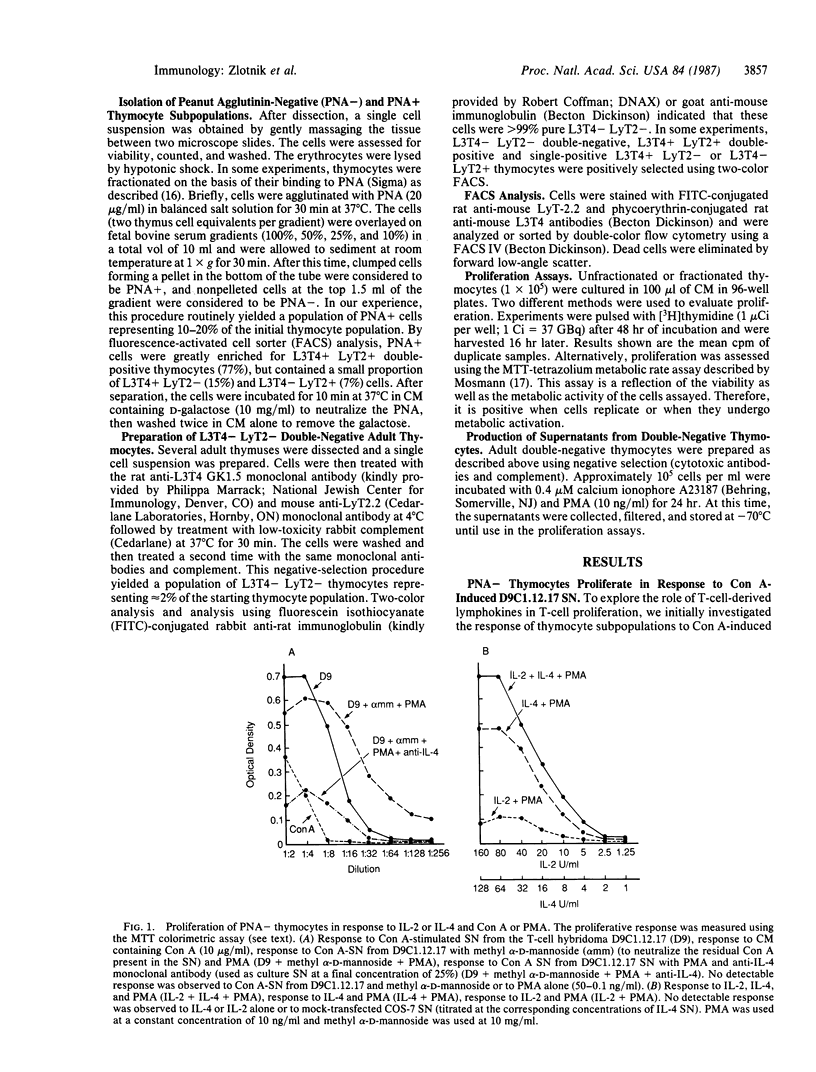
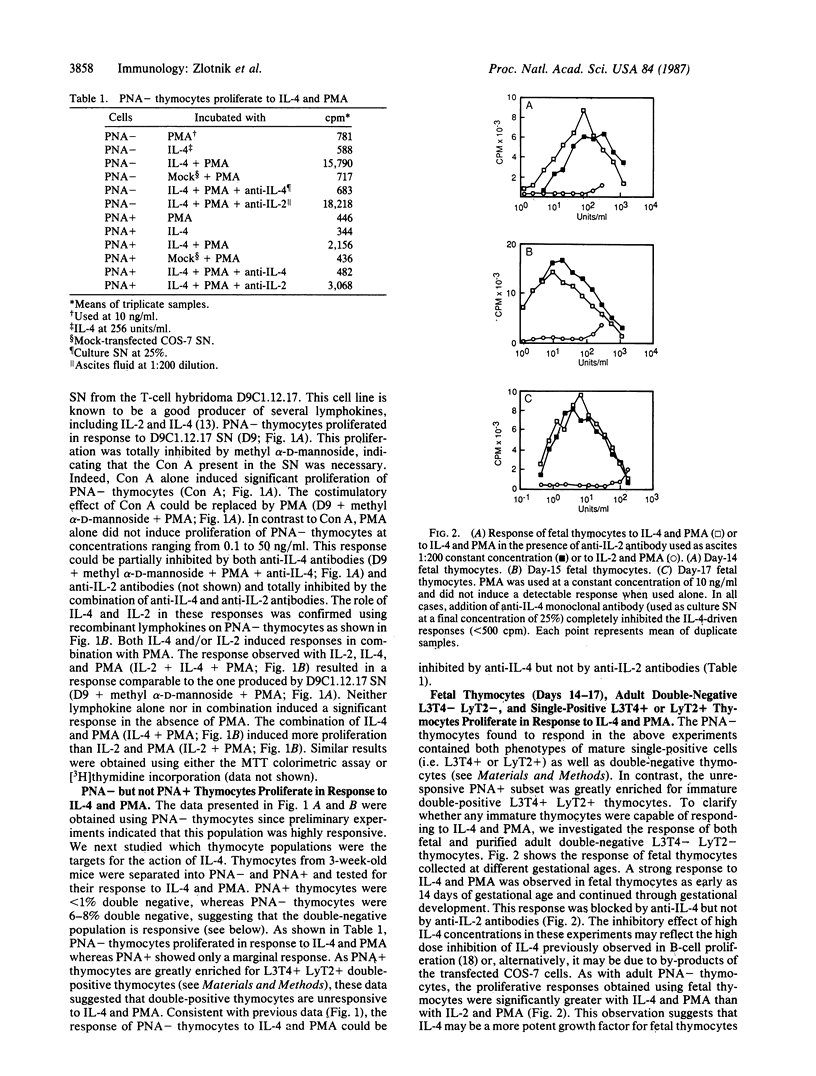
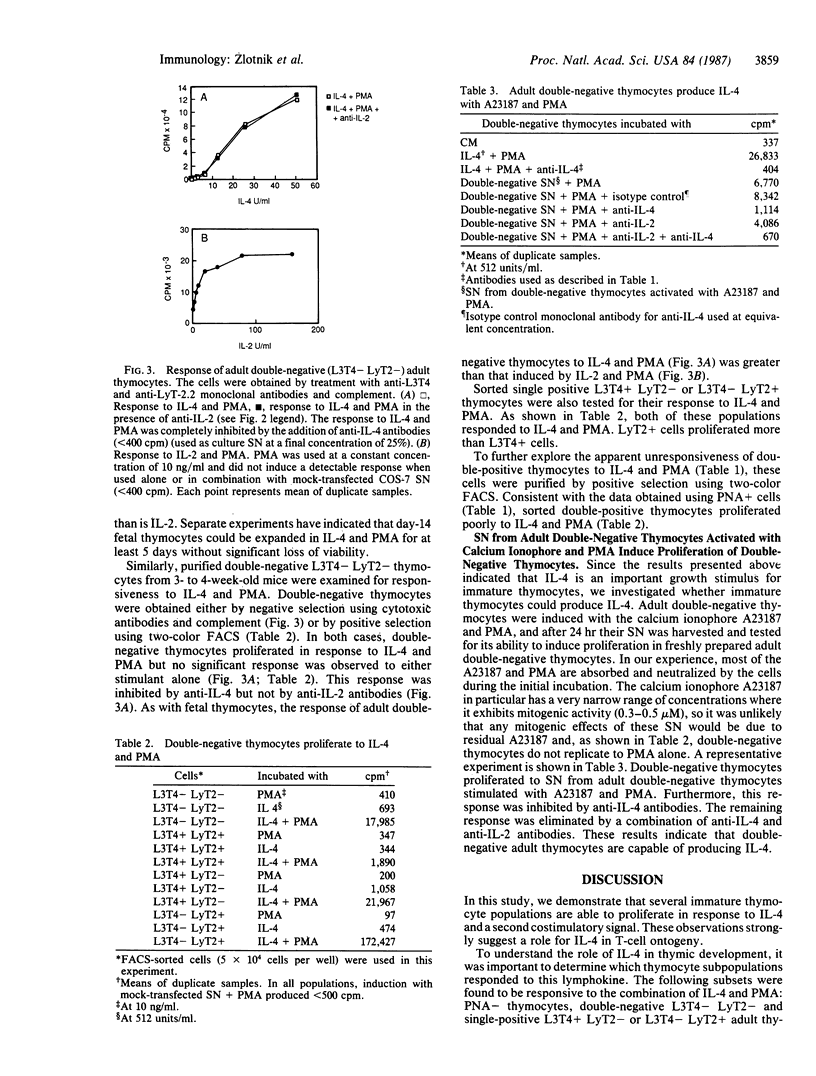
We have shown that recombinant or natural interleukin 4 (IL-4) (formerly called B-cell stimulatory factor 1) induces proliferation of activated adult or fetal thymocytes. In the case of adult thymocytes, IL-4 in combination with Con A or phorbol 12-myristate 13-acetate (PMA) stimulated the proliferation of peanut agglutinin (PNA)-negative (-) thymocytes, while PNA-positive (+) thymocytes showed only marginal responses. Further investigation revealed that day 14-17 fetal thymocytes, purified L3T4- LyT2- double-negative adult thymocytes, and single positive L3T4+ LyT2- or L3T4- LyT2+ thymocytes failed to respond to IL-4 or PMA alone but proliferated strongly with both IL-4 and PMA. In contrast, purified double-positive L3T4+ LyT2+ adult thymocytes showed only a marginal proliferative response to these stimuli. Responsiveness of thymic subpopulations to PMA and IL-4 could be inhibited with anti-IL-4 but not with anti-IL-2 monoclonal antibodies, indicating that they were IL-2 independent. Finally, we have observed that supernatants from calcium ionophore and PMA-stimulated adult double-negative L3T4- LyT2- thymocytes induce proliferation of double-negative adult thymocytes. This latter response is inhibited by anti-IL-4 monoclonal antibodies, suggesting that under appropriate stimulation conditions, these immature thymocytes are able to produce IL-4. These observations suggest a role for IL-4 in T-cell ontogeny.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardsley T. R., Pierschbacher M., Wetzel G. D., Hays E. F. Induction of T-cell maturation by a cloned line of thymic epithelium (TEPI). Proc Natl Acad Sci U S A. 1983 Oct;80(19):6005–6009. doi: 10.1073/pnas.80.19.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R. Proliferation in vitro and interleukin production by 14 day fetal and adult Lyt-2-/L3T4- mouse thymocytes. J Immunol. 1986 Oct 1;137(7):2260–2267. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Dupuy d'Angeac A., Monis M., Rème T. Mitogenic effect of PMA + IL 2 on subpopulations of corticoresistant thymocytes. J Immunol. 1986 Dec 1;137(11):3501–3508. [PubMed] [Google Scholar]
- Fowlkes B. J. Intrathymic differentiation: introductory remarks on problems and approaches. Surv Immunol Res. 1985;4(2):81–86. doi: 10.1007/BF02918804. [DOI] [PubMed] [Google Scholar]
- Herron L. R., Abel C. A., VanderWall J., Campbell P. A. Immature thymocytes isolated using a sialic acid-specific lectin are unresponsive to concanavalin A. Eur J Immunol. 1983 Jan;13(1):73–78. doi: 10.1002/eji.1830130115. [DOI] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Howe R. C., Ceredig R., MacDonald H. R. Functional status of interleukin 2 receptors expressed by immature (Lyt-2-/L3T4-) thymocytes. J Immunol. 1986 Oct 15;137(8):2579–2584. [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Koen P., Malek T., Rothenberg E. Proliferation of thymic stem cells with and without receptors for interleukin 2. Implications for intrathymic antigen recognition. J Exp Med. 1985 May 1;161(5):1048–1062. doi: 10.1084/jem.161.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Rothenberg E. V. Early precursor thymocytes can produce interleukin 2 upon stimulation with calcium ionophore and phorbol ester. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1862–1866. doi: 10.1073/pnas.83.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Lahet S., Inman J., Paul W. E. Partial purification of murine B cell stimulatory factor (BSF)-1. J Immunol. 1985 Oct;135(4):2518–2523. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Sideras P., von Boehmer H. Recombinant interleukin 4/BSF-1 promotes growth and differentiation of intrathymic T cell precursors from fetal mice in vitro. EMBO J. 1987 Jan;6(1):91–95. doi: 10.1002/j.1460-2075.1987.tb04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Von Boehmer H. Requirements for growth of immature thymocytes from fetal and adult mice in vitro. Eur J Immunol. 1986 Jan;16(1):12–19. doi: 10.1002/eji.1830160104. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N., Herron L., Cambier J., DiGuisto D., Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984 Sep;38(2):577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Shimonkevitz R. P., Gefter M. L., Kappler J., Marrack P. Characterization of the gamma-interferon-mediated induction of antigen-presenting ability in P388D1 cells. J Immunol. 1983 Dec;131(6):2814–2820. [PubMed] [Google Scholar]



