Abstract
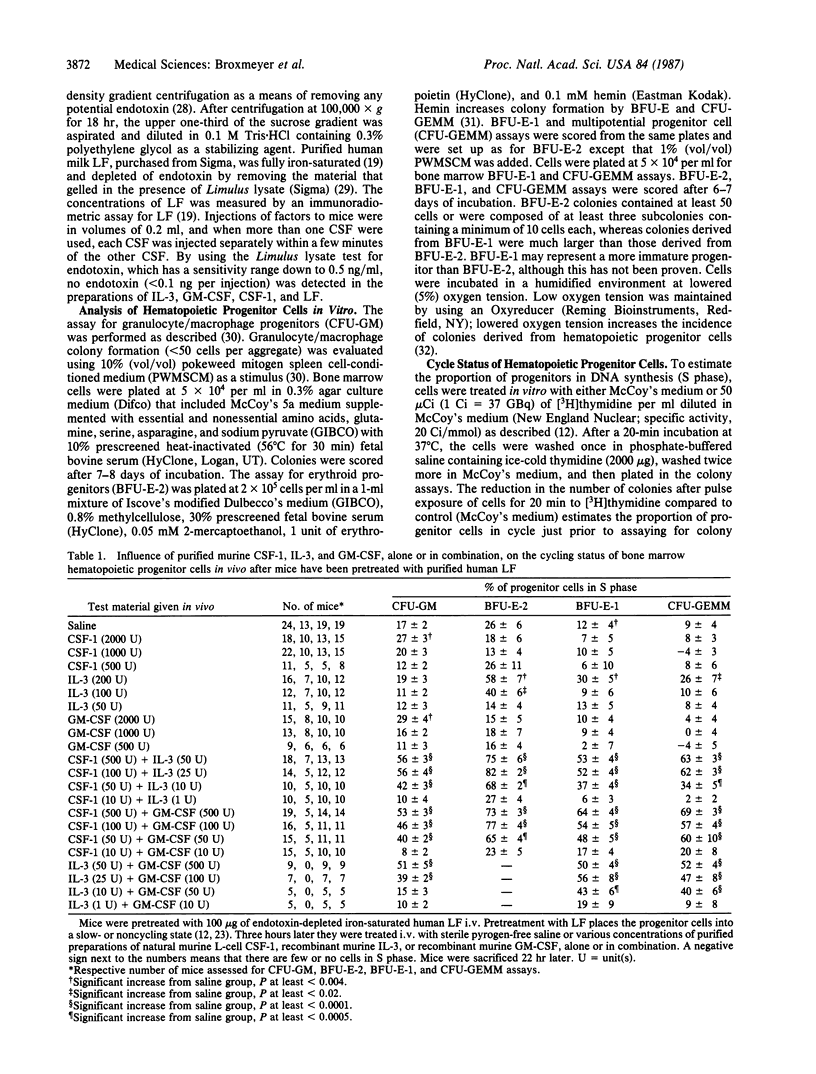
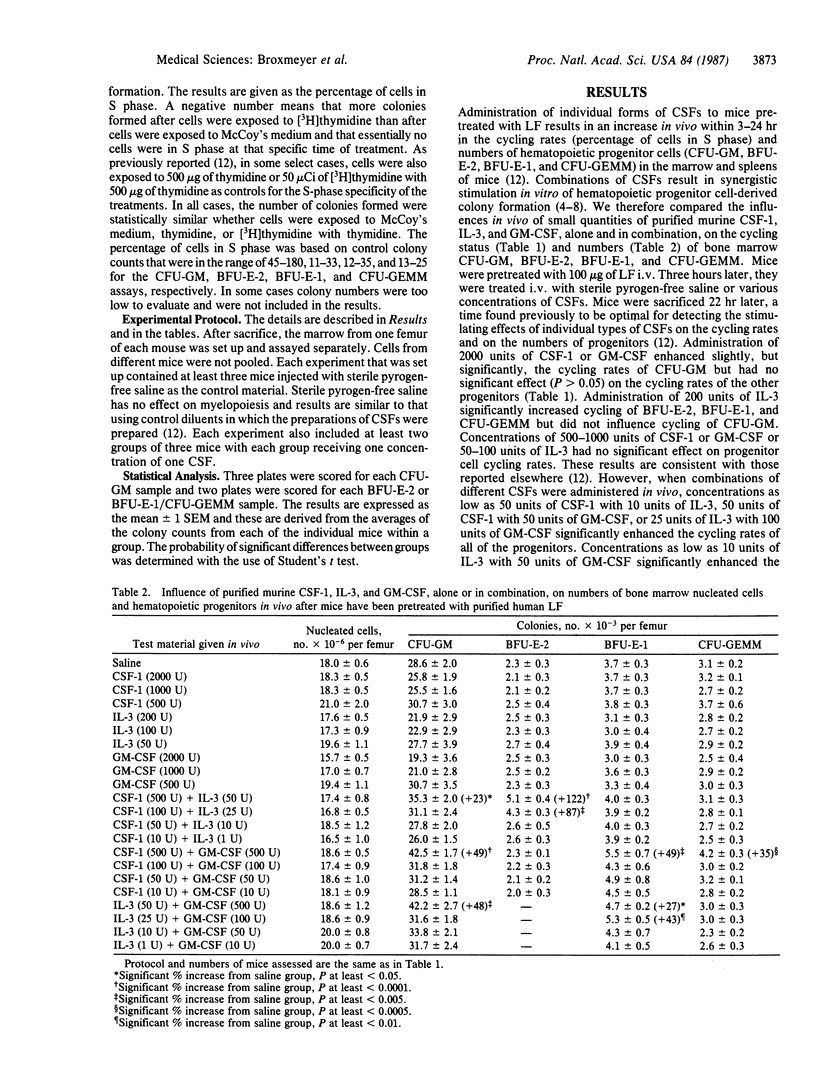
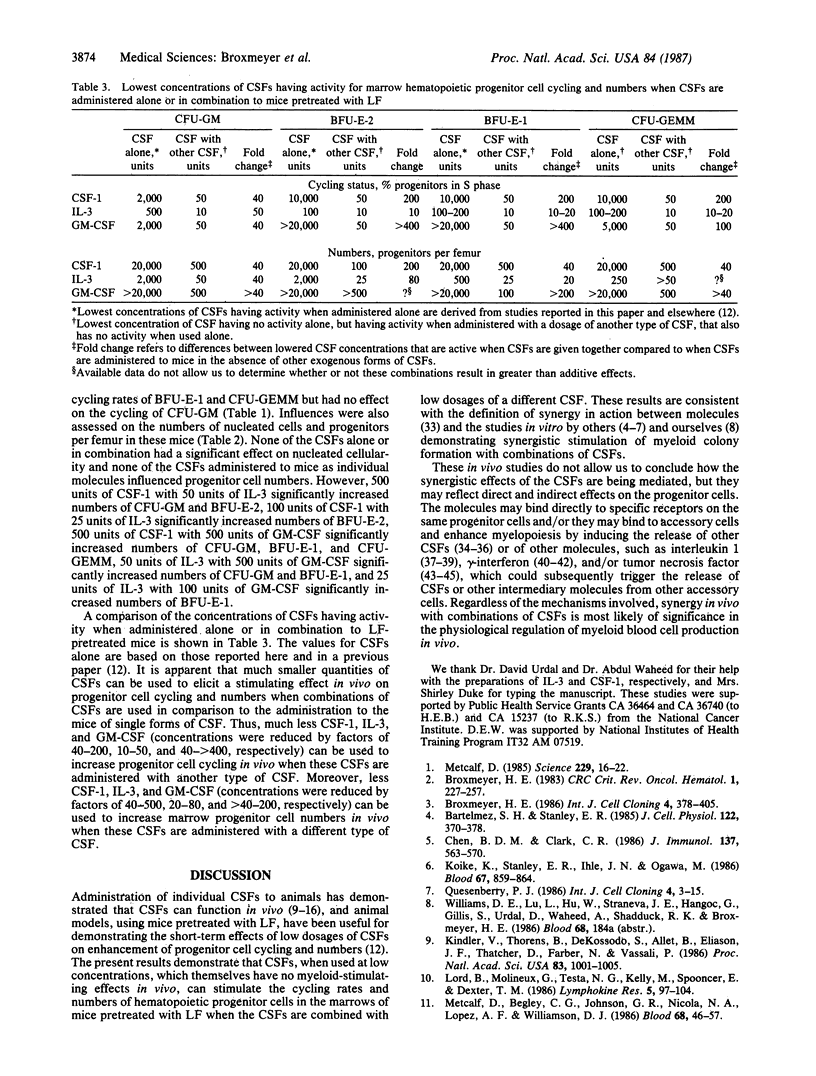
Combinations of low dosages of purified murine hematopoietic colony-stimulating factors (CSFs)--L-cell CSF type 1 (CSF-1), recombinant interleukin 3 (IL-3), and recombinant granulocyte/macrophage CSF (GM-CSF)--were compared with single CSFs for their influence on the cycling rates and numbers of bone marrow granulocyte/macrophage, erythroid, and multipotential progenitor cells in vivo in mice pretreated with human lactoferrin. Lactoferrin was used to enhance detection of the stimulating effects of exogenously administered CSFs. Concentrations of CSFs that were not active in vivo when given alone were active when administered together with other types of CSF. The concentrations of CSF-1, IL-3, and GM-CSF needed to increase progenitor cell cycling rates were reduced by factors of 40-200, 10-50, and 40- greater than 400, respectively; the concentrations needed to increase progenitor cell numbers were reduced by factors of 40-500 (CSF-1), 20-80 (IL-3), and greater than 40- greater than 200 (GM-CSF) when these forms of CSFs were administered in combination with low dosages of one of the other forms of CSFs. The results demonstrate that different CSFs can synergize when administered in vivo to increase the cycling rates and numbers of marrow hematopoietic progenitor cells. These findings may be of relevance physiologically to the regulation of myeloid blood cell production by CSFs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. C., Jr, Dinarello C. A., Wallace P., Wagner C., Hefeneider S., McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Bergstrom K. A., Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983 Sep;62(3):663–668. [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Layman D. L. Regulation of colony-stimulating activity production. Interactions of fibroblasts, mononuclear phagocytes, and lactoferrin. J Clin Invest. 1983 Feb;71(2):340–344. doi: 10.1172/JCI110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, Rigas V. D., Bennett R. M., Vandenbark A. A., Garewal H. S. Interaction of lactoferrin, monocytes, and T lymphocyte subsets in the regulation of steady-state granulopoiesis in vitro. J Clin Invest. 1981 Jul;68(1):56–63. doi: 10.1172/JCI110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelmez S. H., Stanley E. R. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: assay of hemopoietin-1. J Cell Physiol. 1985 Mar;122(3):370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Bicknell D. C., Gillis S., Harris E. L., Pelus L. M., Sledge G. W., Jr Lactoferrin: affinity purification from human milk and polymorphonuclear neutrophils using monoclonal antibody (II 2C) to human lactoferrin, development of an immunoradiometric assay using II 2C, and myelopoietic regulation and receptor-binding characteristics. Blood Cells. 1986;11(3):429–446. [PubMed] [Google Scholar]
- Broxmeyer H. E. Biomolecule-cell interactions and the regulation of myelopoiesis. Int J Cell Cloning. 1986 Nov;4(6):378–405. doi: 10.1002/stem.5530040601. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Bognacki J., Ralph P., Dörner M. H., Lu L., Castro-Malaspina H. Monocyte-macrophage-derived acidic isoferritins: normal feedback regulators of granulocyte-macrophage progenitor cells in vitro. Blood. 1982 Sep;60(3):595–607. [PubMed] [Google Scholar]
- Broxmeyer H. E. Colony assays of hematopoietic progenitor cells and correlations to clinical situations. Crit Rev Oncol Hematol. 1984;1(3):227–257. doi: 10.1016/s1040-8428(84)80013-x. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Platzer E. Lactoferrin acts on I-A and I-E/C antigen+ subpopulations of mouse peritoneal macrophages in the absence of T lymphocytes and other cell types to inhibit production of granulocyte-macrophage colony stimulatory factors in vitro. J Immunol. 1984 Jul;133(1):306–314. [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Cooper S., Shadduck R. K., Gillis S., Waheed A., Urdal D. L., Bicknell D. C. Comparative effects in vivo of recombinant murine interleukin 3, natural murine colony-stimulating factor-1, and recombinant murine granulocyte-macrophage colony-stimulating factor on myelopoiesis in mice. J Clin Invest. 1987 Mar;79(3):721–730. doi: 10.1172/JCI112877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Cooper S. The influence in vivo of natural murine interleukin-3 on the proliferation of myeloid progenitor cells in mice recovering from sublethal dosages of cyclophosphamide. Leuk Res. 1987;11(2):201–205. doi: 10.1016/0145-2126(87)90027-0. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Cooper S., Waheed A., Shadduck R. K. The influence in vivo of murine colony-stimulating factor-1 on myeloid progenitor cells in mice recovering from sublethal dosages of cyclophosphamide. Blood. 1987 Mar;69(3):913–918. [PubMed] [Google Scholar]
- Chen B. D., Clark C. R. Interleukin 3 (IL 3) regulates the in vitro proliferation of both blood monocytes and peritoneal exudate macrophages: synergism between a macrophage lineage-specific colony-stimulating factor (CSF-1) and IL 3. J Immunol. 1986 Jul 15;137(2):563–570. [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Gentile P., Broxmeyer H. E. Suppression of mouse myelopoiesis by administration of human lactoferrin in vivo and the comparative action of human transferrin. Blood. 1983 May;61(5):982–993. [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Griffin J. D. T cell-monocyte interactions in the production of humoral factors regulating human granulopoiesis in vitro. J Immunol. 1986 Apr 15;136(8):2856–2861. [PubMed] [Google Scholar]
- Ishizaka Y., Motoyoshi K., Hatake K., Saito M., Takaku F., Miura Y. Mode of action of human urinary colony-stimulating factor. Exp Hematol. 1986 Jan;14(1):1–8. [PubMed] [Google Scholar]
- Kindler V., Thorens B., de Kossodo S., Allet B., Eliason J. F., Thatcher D., Farber N., Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Stanley E. R., Ihle J. N., Ogawa M. Macrophage colony formation supported by purified CSF-1 and/or interleukin 3 in serum-free culture: evidence for hierarchical difference in macrophage colony-forming cells. Blood. 1986 Apr;67(4):859–864. [PubMed] [Google Scholar]
- Lord B. I., Molineux G., Testa N. G., Kelly M., Spooncer E., Dexter T. M. The kinetic response of haemopoietic precursor cells, in vivo, to highly purified, recombinant interleukin-3. Lymphokine Res. 1986 Spring;5(2):97–104. [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E. Comparative influences of phytohemagglutinin-stimulated leukocyte conditioned medium, hemin, prostaglandin E, and low oxygen tension on colony formation by erythroid progenitor cells in normal human bone marrow. Exp Hematol. 1985 Nov;13(10):989–993. [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E. The selective enhancing influence of hemin and products of human erythrocytes on colony formation by human multipotential (CFUGEMM) and erythroid (BFUE) progenitor cells in vitro. Exp Hematol. 1983 Sep;11(8):721–729. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Lopez A. F., Williamson D. J. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986 Jul;68(1):46–57. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Synthesis by mouse peritoneal cells of G-CSF, the differentiation inducer for myeloid leukemia cells: stimulation by endotoxin, M-CSF and multi-CSF. Leuk Res. 1985;9(1):35–50. doi: 10.1016/0145-2126(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Park L. S., Friend D., Gillis S., Urdal D. L. Characterization of the cell surface receptor for a multi-lineage colony-stimulating factor (CSF-2 alpha). J Biol Chem. 1986 Jan 5;261(1):205–210. [PubMed] [Google Scholar]
- Park L. S., Friend D., Gillis S., Urdal D. L. Characterization of the cell surface receptor for granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1986 Mar 25;261(9):4177–4183. [PubMed] [Google Scholar]
- Piacibello W., Lu L., Wachter M., Rubin B., Broxmeyer H. E. Release of granulocyte-macrophage colony stimulating factors from major histocompatibility complex class II antigen-positive monocytes is enhanced by human gamma interferon. Blood. 1985 Dec;66(6):1343–1351. [PubMed] [Google Scholar]
- Piacibello W., Lu L., Williams D., Aglietta M., Rubin B. Y., Cooper S., Wachter M., Gavosto F., Broxmeyer H. E. Human gamma interferon enhances release from phytohemagglutinin-stimulated T4+ lymphocytes of activities that stimulate colony formation by granulocyte-macrophage, erythroid, and multipotential progenitor cells. Blood. 1986 Dec;68(6):1339–1347. [PubMed] [Google Scholar]
- Quesenberry P., Levin J., Zuckerman K., Rencricca N., Sullivan R., Tyler W. Stem cell migration induced by erythropoietin or haemolytic anaemia: the effects of actinomycin and endotoxin contamination of erythropoietin preparations. Br J Haematol. 1979 Feb;41(2):253–269. doi: 10.1111/j.1365-2141.1979.tb05854.x. [DOI] [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Porcellini A., Rizzoli V., Levin J. A method for the removal of endotoxin from purified colony-stimulating factor. Proc Soc Exp Biol Med. 1980 May;164(1):40–44. doi: 10.3181/00379727-164-40821. [DOI] [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification and properties of L cell-derived colony-stimulating factor. J Lab Clin Med. 1979 Jul;94(1):180–193. [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification of colony-stimulating factor by affinity chromatography. Blood. 1982 Jul;60(1):238–244. [PubMed] [Google Scholar]
- Warren M. K., Ralph P. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986 Oct 1;137(7):2281–2285. [PubMed] [Google Scholar]
- Welte K., Bonilla M. A., Gillio A. P., Boone T. C., Potter G. K., Gabrilove J. L., Moore M. A., O'Reilly R. J., Souza L. M. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987 Apr 1;165(4):941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucali J. R., Broxmeyer H. E., Dinarello C. A., Gross M. A., Weiner R. S. Regulation of early human hematopoietic (BFU-E and CFU-GEMM) progenitor cells in vitro by interleukin 1-induced fibroblast-conditioned medium. Blood. 1987 Jan;69(1):33–37. [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]



