Abstract
Multiple death signals influence mitochondria during apoptosis, yet the critical initiating event for mitochondrial dysfunction in vivo has been unclear. tBID, the caspase-activated form of a “BH3-domain–only” BCL-2 family member, triggers the homooligomerization of “multidomain” conserved proapoptotic family members BAK or BAX, resulting in the release of cytochrome c from mitochondria. We find that cells lacking both Bax and Bak, but not cells lacking only one of these components, are completely resistant to tBID-induced cytochrome c release and apoptosis. Moreover, doubly deficient cells are resistant to multiple apoptotic stimuli that act through disruption of mitochondrial function: staurosporine, ultraviolet radiation, growth factor deprivation, etoposide, and the endoplasmic reticulum stress stimuli thapsigargin and tunicamycin. Thus, activation of a “multidomain” proapoptotic member, BAX or BAK, appears to be an essential gateway to mitochondrial dysfunction required for cell death in response to diverse stimuli.
Members of the “BH3-domain–only” subset of BCL-2 family proteins connect proximal death signals to the core apoptotic pathway (1–5). After activation of CD95 (Fas) or TNFR1 death receptors, BID is cleaved and activated to p15 tBID (6–8), which, in a model system using purified mitochondria, serves as a death ligand that induces the oligomerization of BAK (9) and BAX (10). tBID does not cause release of cytochrome c from purified Bak-deficient mitochondria, suggesting that interaction of tBID and BAK is a critical event at least in vitro (9).
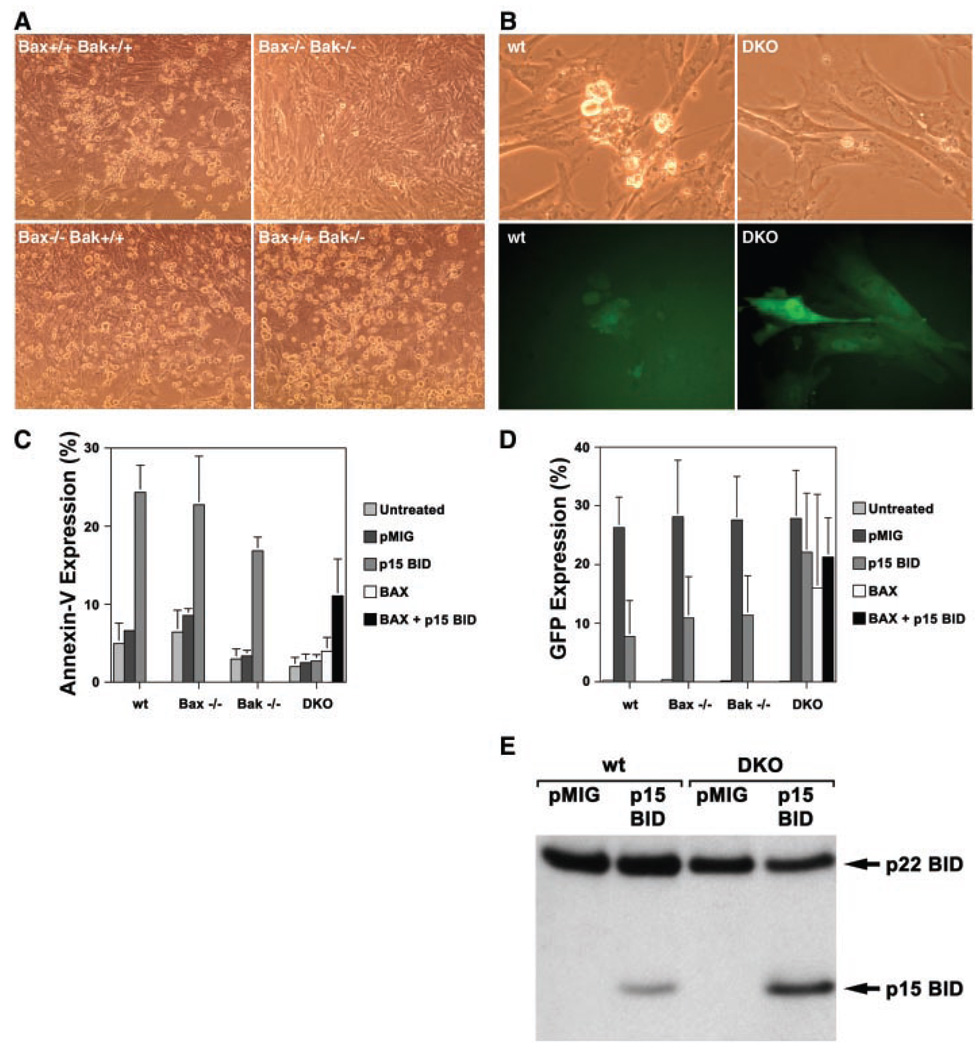
Here we assess whether BAK is required for tBID-induced apoptosis of intact, whole cells by using a retroviral vector to express tBID in murine embryonic fibroblasts (MEFs). Numerous cells with shrunken, apoptotic morphology were detected in Bak-deficient as well as wild-type MEFs 24 hours after infection with the tBID-expressing vector (Fig. 1A), but not with empty vector (pMIG) (11) expressing only green fluorescence protein (GFP). MEFs deficient for BAX, another “multidomain” proapoptotic BCL-2 member, were also killed. In contrast, Bax, Bak doubly deficient MEFs proved completely resistant to tBID-induced cell death as assessed by multiple markers including Annexin-V staining (Fig. 1C) and cell morphology (Fig. 1A). Infected cells could be identified by expression of GFP (Fig. 1B) and revealed a similar infection rate by control pMIG virus in all genetic backgrounds (Fig. 1D). Analysis of GFP expression in tBID-infected MEFs revealed small amounts of GFP in apoptotic cells of wild-type, Bax−/−, and Bak−/− MEFs (Fig. 1D). In contrast, introduction of the tBID vector into Bax, Bak doubly knocked out (DKO) MEFs revealed cells with normal morphology expressing high levels of GFP (Fig. 1, B and D). Simian virus 40 (SV40)–transformed MEF cell lines of wild-type and DKO genotypes also proved sensitive and resistant to tBID, respectively (12). Protein immunoanalysis confirmed expression of p15 BID in DKO MEFs and smaller amounts in wild-type MEFs undergoing apoptosis (Fig. 1E). p15 BID localized to the mitochondria as an integral membrane protein in both wild-type and DKO MEFs (12), indicating that although tBID was expressed and targeted to mitochondria, it did not kill DKO MEFs. Reexpression of BAX alone was not sufficient to kill DKO MEFs but did restore killing by tBID, confirming tBID’s requirement for a multidomain proapoptotic member to induce apoptosis (Fig. 1C).
Fig. 1.
Resistance of Bax, Bak doubly deficient murine embryonic fibroblasts (MEFs) to tBID-induced apoptosis. (A) Bright-field microscopy (×20 magnification) of wild-type, Bax−/− Bak−/−, Bax−/− Bak+/+, and Bax+/+ Bak−/− primary embryonic day 13.5 MEFs 24 hours after infection with a tBID-expressing vector. Murine p15 BID and BAX were cloned into the retroviral expression vector MSCV-IRES-GFP (pMIG) (11). Retroviruses were generated by transfecting 293GPG packaging cell line as described (33). Retrovirus-containing supernatant was collected 3 and 5 days after transfection and used to infect MEFs in the presence of polybrene (8 µg/ml). (B) Bright-field and GFP fluorescence of the same field (×40 magnification) of wild-type (wt) and DKO MEFs 24 hours after infection with the tBID vector. (C) Quantitation of apoptosis in MEFs shown at the 24-hour time point after infection with pMIG (empty vector control), tBID, and/or BAX vectors. Cell death was quantitated by flow cytometric detection of Annexin-V staining (Bio-vision). Values shown are mean ± 1 SD from three independent experiments. (D) Quantitation of GFP-positive cells 24 hours after infection with pMIG, tBID, and/or BAX vectors. GFP-positive cells were detected by flow cytometry. Values shown are mean ± 1 SD from three independent experiments. (E) Immunoblot with antibody to Bid of whole-cell lysates from SV40-transformed (34) wild-type and DKO MEFs at 17 hours after infection with pMIG or tBID vectors.
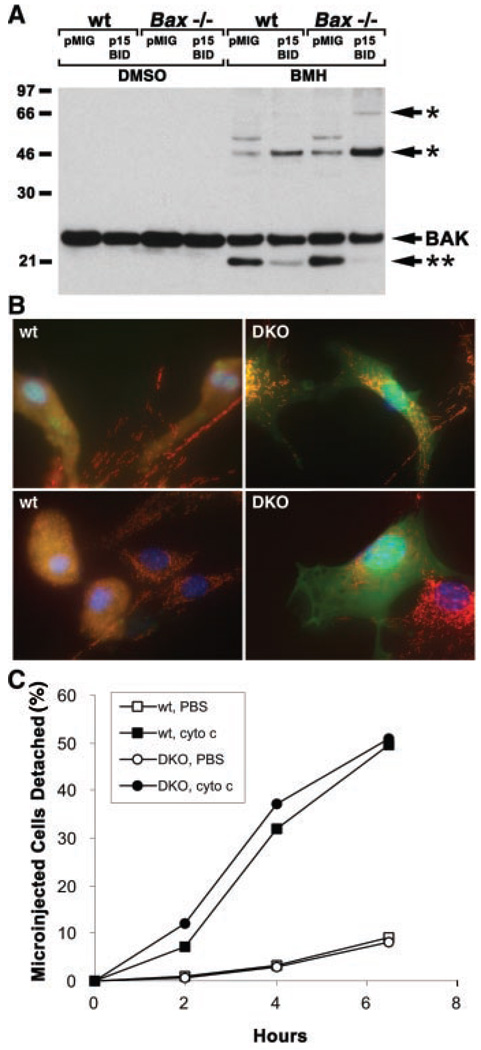
One mechanism to account for these observations would be if tBID induced the activation of BAX and BAK, resulting in mitochondrial dysfunction including the release of cytochrome c. We tested the activation status of BAX and BAK and the intracellular distribution of cytochrome c in primary MEFs expressing tBID. Mitochondria from control and p15 BID-expressing primary MEFs were treated with the chemical cross-linker bismaleimidohexane (BMH) to examine the status of BAK oligomerization. As we observed in hepatocytes (9), the faster migrating inactive BAK conformer in control cells disappeared in cells expressing p15 BID, and there was a corresponding increase in the amount of cross-linked BAK multimers consistent with formation of dimers and trimers (Fig. 2A). This process appeared to be independent of BAX, as p15 BID caused oligomerization of BAK in Bax−/− MEFs (Fig. 2A). Expression of p15 BID in wild-type MEFs caused cytosolic BAX to translocate and insert as an integral mitochondrial membrane protein (12), as BAX does in Fas-activated hepatocytes (Fig. 3B). In GFP-positive, tBID-expressing DKO MEFs, cytochrome c remained in a punctate mitochondrial pattern, but in tBID-expressing wild-type MEFs, cytochrome c redistributed to a diffuse cytosolic pattern (Fig. 2B). Thus, activation of proapoptotic BAX, BAK, or both is required for tBID-induced cytochrome c release in vivo. We tested whether Bax/Bak–deficient cells had an intact apoptotic pathway downstream of mitochondria by microinjecting cytochrome c into the cytoplasm of MEFs. DKO and wild-type MEFs showed comparable cell death after microinjection of cytochrome c (Fig. 2C), indicating that the apoptotic pathway downstream of cytochrome c release (13) does not depend on function of BAX or BAK.
Fig. 2.
Function of BAX and BAK downstream of tBID and upstream of cytochrome c release. (A) tBID-induced BAK oligomerization independent of BAX. Mitochondria-enriched fractions from pMIG or tBID vector-infected MEFs were prepared as described (14) and treated with dimethyl sulfoxide (DMSO) containing control buffer or 10 mM BMH cross linker (Pierce). Cross-linked BAK species were detected by antibody to BAK (Upstate Biotechnology) immunoblot. * indicates BAK complexes consistent with dimers or trimers; ** indicates inactive BAK conformer. (B) Cytochrome c release in DKO MEFs. Three-color fluorescence microscopy (35) of wild-type and DKO MEFs at 24 hours after infection with tBID and GFP-expressing vector. Red indicates cytochrome c immunostaining. Blue is Hoechst staining of DNA. Green is GFP expression in infected cells. Overlap of GFP and cytochrome c staining is denoted by yellow. Results are representative of three independent experiments. (C) Apoptosis of DKO MEFs after microinjection of cytochrome c. Primary MEFs were plated on 1% gelatin-coated gridded cover slips. Puri fied cytochrome c (1.6 mg/ml) dissolved in phosphate-buffered saline (PBS) or PBS control was mixed 1:1 with 0.5% fluorescein isothiocyanate–conjugated dextran and microinjected into cells with an Eppendorf 5246 Transjector at a pressure of 150 hPa and an injection time of 0.5 s. Apoptotic cells detach from the cover slip and were counted as a measure of apoptosis, enabling a time course of the mean percentages of microinjected cells that detach to be plotted from three independent experiments.
Fig. 3.
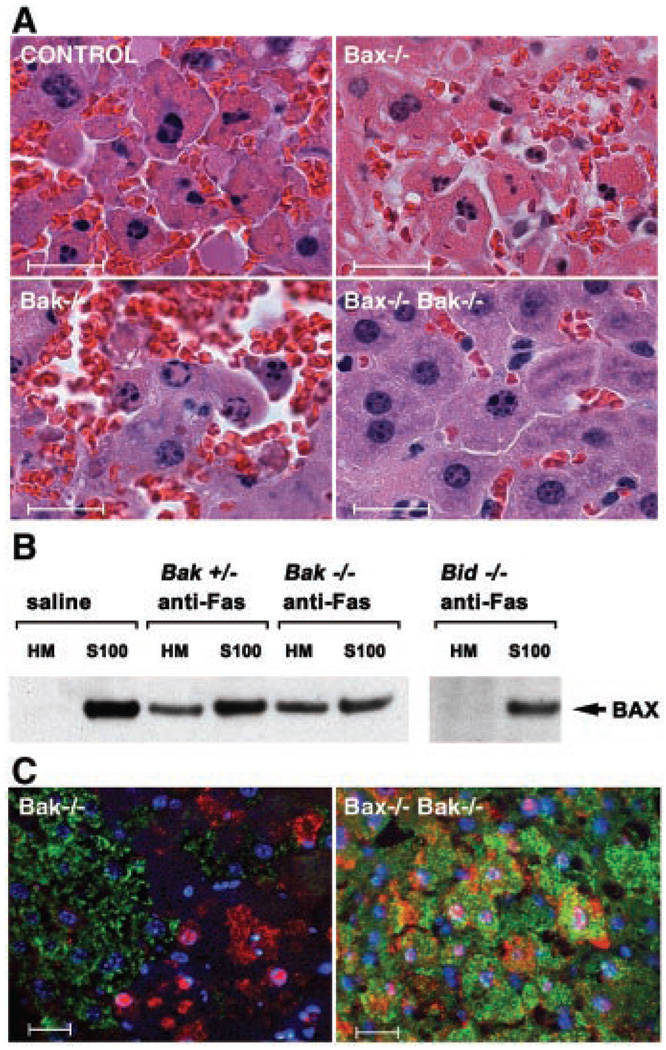
Function of BAX and BAK downstream of BID in Fas-induced hepatocellular apoptosis. (A) Hematoxylin and eosin-stained liver sections from Bak−/−, Bax−/−, and DKO mice treated with antibody to Fas (anti-Fas). Bar represents 20 µm. (B) Fas-induced BAX translocation from cytosol to mitochondria in hepatocytes is downstream of BID but independent of BAK. BAX immunoblot of mitochondrial (HM) and cytosolic (S100) fractions of hepatocytes prepared as previously described (8) from wild-type, Bid−/−, Bak+/−, and Bak−/− mice treated with saline or anti-Fas. (C) Three-color immunohistochemistry of livers from anti-Fas–treated Bak−/− and DKO mice. Cytochrome c staining is in green, activated Caspase-3/7 (CM1 antibody) in red, and nuclei stained with Hoechst in blue. Bar represents 20 µm. Histology and immunohistochemical staining of livers from anti-Fas–treated animals were done as described (14).
To examine whether BAK or BAX molecules are required for cell deaths in situ, we assessed apoptosis in the livers of mice injected with agonistic antibody to Fas. Bak-deficient mice were susceptible to Fas-induced hepatocellular apoptosis and hemorrhagic necrosis (Fig. 3A and Table 1), dying in a similar time course to that of wild-type or heterozygous littermate controls. This contrasts with the resistance to Fas of Bid-deficient mice (14). Subcellular fractionation of hepatocytes activated through Fas in vivo revealed that BAX translocates from cytosol to mitochondria in Bak-deficient, but not Bid-deficient mice (Fig. 3B). Bax-deficient mice also succumbed to Fas-induced hepatocellular apoptosis (Fig. 3A and Table 1). Thus, BID appears to function upstream of both BAX and BAK in hepatocytes as well as in MEFs.
Table 1.
Susceptibility of mice lacking Bax, Bak, or both to anti-Fas injection. Mice were injected intravenously with 0.5 µg of monoclonal antibody to Fas (Jo2, Pharmingen) per gram of body weight and killed when moribund or observed for up to 24 hours. Mean survival time in hours ± 1 SD is shown for mice that died.
| Genotype | Dead/ injected |
Mean survival (hours) |
|---|---|---|
| Bak−/− | 9/13 | 5.2 ± 1.7 |
| Bak+/− or +/+ | 9/14 | 6.0 ± 2.7 |
| Bax−/− | 7/10 | 3.8 ± 1.2 |
| Bax+/− or +/+ | 13/13 | 2.6 ± 1.9 |
| Bax−/−, Bak−/− | 0/3 | (killed after 9 hours) |
We next tested whether elimination of both BAX and BAK would confer resistance to Fas-induced hepatocellular apoptosis. The vast majority of Bax, Bak DKO mice die around the time of birth, and only a small percentage survive to adulthood (15). We injected the three available DKO mice with antibody to Fas, all of which survived 9 hours, at which time they were killed and their livers were examined (Table 1). The DKO mice displayed at most moderate apoptosis of hepatocytes, and some animals showed none (Fig. 3A). The immunohistochemistry profile of affected DKO hepatocytes was limited to focal areas of Caspase-3 activation without release of cytochrome c (Fig. 3C), similar to that seen in Bid−/− hepatocytes (14). These data on the limited number of DKO mice available contrasted with those from the singly deficient mice, indicating that BID-dependent apoptosis also used either BAX or BAK in hepatocytes. This illustrates that “extrinsic” (death receptor) paths, when activated in situ, may also depend on “multidomain” proapoptotic proteins in select cell types (14, 16).
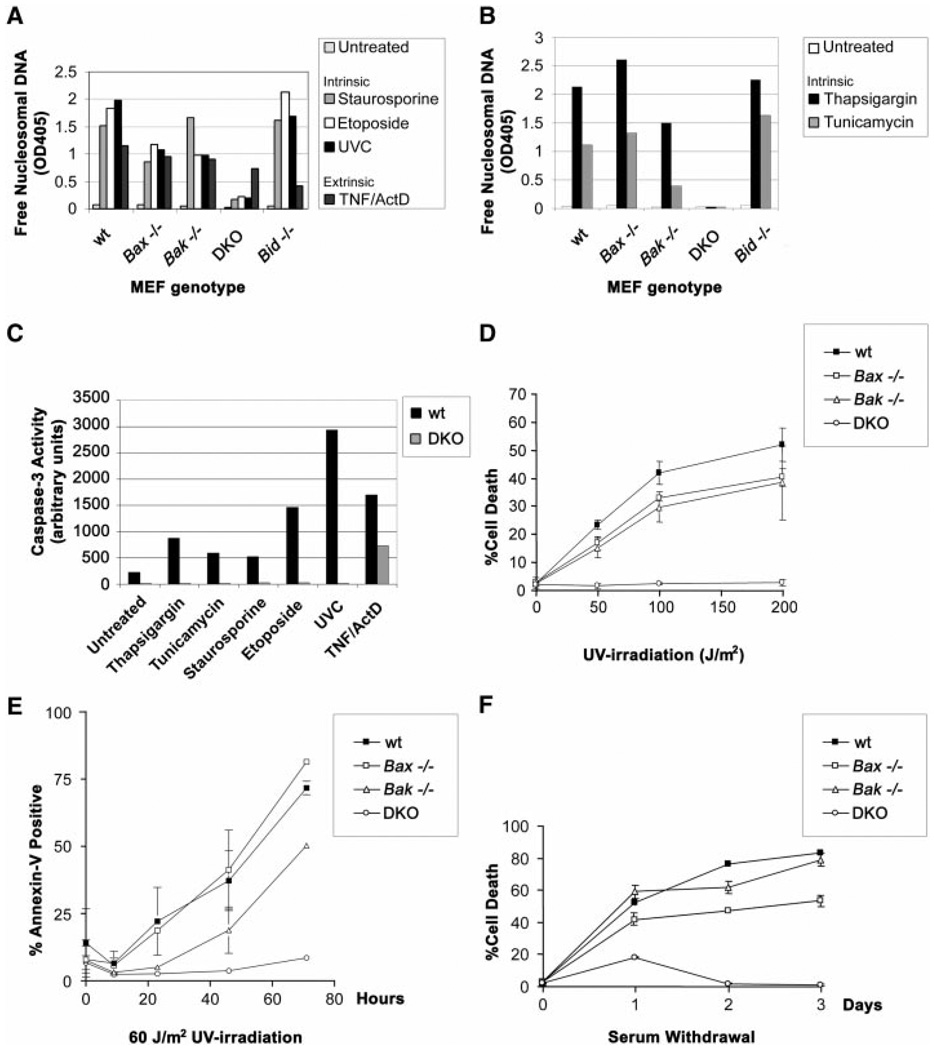
We next examined whether BAX, BAK, or both were required for death after other stimuli proposed to cause cytochrome c release from mitochondria (17–21). Wild-type, Bax−/−, and Bak−/− MEFs were susceptible, whereas DKO MEFs proved resistant to staurosporine (kinase inhibitor), etoposide (topoisomerase II inhibitor), and ultraviolet radiation (UVC), even at very high doses (Fig. 4, A to D). Time course studies as well as dose response evaluations indicated that DKO MEFs are markedly resistant to these stimuli, demonstrating long-term survival when assessed by nucleosomal DNA fragmentation (Fig. 4A), dye exclusion (Fig. 4D), or Annexin-V staining (Fig. 4E). Moreover, DKO MEFs, but not singly deficient MEFs, proved resistant to stress signaling from the endoplasmic reticulum (ER) (22–24) induced by thapsigargin (which inhibits the Ca2+ adenosine triphosphatase pump), tunicamycin (which inhibits N-linked glycosylation) (Fig. 4B), or brefeldin A (which inhibits ER-Golgi transport) (12). Further assessment indicated that postmitochondrial events were prevented in DKO cells, including the activation of effector caspase activity after all of these stimuli (Fig. 4C). DKO MEFs were also resistant to cell death caused by serum (growth factor) deprivation (Fig. 4F). In contrast, Bid−/− cells were susceptible to all of these signals (Fig. 4, A and B), indicating that they are not dependent on BID and that BID is not the sole activator of BAX or BAK. DKO MEFs were susceptible to the extrinsic signal of tumor necrosis factor α (TNF-α) plus actinomycin D, which in this cell type induces a mitochondria-independent caspase cascade (Fig. 4, A and C).
Fig. 4.
Resistance of Bax, Bak doubly deficient MEFs to multiple intrinsic death signals. (A) Susceptibility of MEFs to apoptotic death by mitochondria-dependent intrinsic signals. Wild-type, Bax−/−, Bak−/−, DKO, and Bid−/− primary MEFs were treated with 1 µM staurosporine, 100 µM etoposide, UVC (60 J/m2), or TNF-α (1 ng/ml) + actinomycin D (2 µg/ml), and a 48-hour time point is shown. Average values from duplicate samples of an enzyme-linked immunosorbent assay of apoptotic DNA fragmentation (Roche) are plotted as representative of three independent experiments. (B) Susceptibility of MEFs to apoptotic death by ER stimuli. As in (A), genotyped MEFs were treated with 2 µM thapsigargin or tunicamycin (1 µg/ml), and average values from duplicate samples at a 48-hour time point of apoptotic DNA fragmentation were plotted. DKO MEFs also demonstrated long-term survival when assessed by Annexin-V staining 4 days after the stimuli (12). (C) Quantitation of effector caspase activity (e.g., Caspase-3) using DEVD-AFC fluorogenic substrate (Clontech). Wild-type and DKO MEFs were treated with the following death signals and harvested at indicated time points: 2 µM thapsigargin (36 hours), tunicamycin (1 µg/ml; 36 hours), 1 µM staurosporine (16 hours), 100 µM etoposide (24 hours), UVC (60 J/m2; 24 hours), and TNF-α (1 ng/ml) + actinomycin D (2 µg/ml; 16 hours). Results are representative of three independent experiments. (D) Dose response of MEFs to UVC irradiation. Cell death was quantitated by trypan blue exclusion, and a 24-hour time point was plotted. (E) Time course of MEF apoptosis after exposure to UVC (60 J/m2). Cell death was quantitated by flow cytometric detection of Annexin-V staining. Long-term survival of DKO MEFs was also noted 4 days after staurosporine and etoposide, as well as UVC treatments (12). (F) Time course of MEF apoptosis in response to serum withdrawal. Cell death was quantitated by trypan blue exclusion.
Considerable uncertainty has existed as to whether anti- or proapoptotic BCL-2 members exert a dominant role. Our studies indicate that in vivo, intact cells require a multidomain proapoptotic member to respond to a diverse set of death signals. tBID must activate BAX or BAK to initiate mitochondrial dysfunction and cell death in hepatocytes and MEFs. Conceivably, in other tissues, this function may be served by other proapoptotic multidomain family members such as BOK (25). Activation and oligomerization of BAX or BAK have been proposed to result in formation of a homomultimeric pore (9, 26), formation of a voltage-dependent anion channel–containing pore (27), or permeabilization of mitochondrial membranes (28) to initiate cytochrome c release. Release of cytochrome c activates the Apaf-1–Caspase-9 apoptosome and downstream effector caspases (13), and after substantial loss of cytochrome c, progressive caspase-independent mitochondrial dysfunction can lead to cell death (29). Knockouts of cytochrome c or Caspase-9 and Apaf-1, which function downstream of mitochondria, indicate that damage from staurosporine, etoposide, and radiation depends on signals mediated by cytochrome c release from mitochondria (17–21). The Bax, Bak-deficient cells, which have a block immediately upstream of mitochondria, appear even better protected from these agents. Even ER stress-induced apoptosis requires BAX or BAK, which might reflect undefined roles of BAX or BAK at ER sites (30, 31) or an ultimate dependence of ER pathways on mitochondria (32). Other upstream activators of BAX and BAK clearly exist, as Bid-deficient cells are often susceptible to stimuli that fail to kill cells lacking both BAX and BAK. Our loss-of-function studies reveal that the absence of proapoptotic BAX and BAK molecules creates a profound block, preserving mitochondria and inhibiting apoptosis after seemingly unrelated signals initiated at multiple sites including plasma membrane, nucleus, and ER.
References and Notes
- 1.Green DR. Cell. 2000;102:1. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Reed JC. Nature Med. 2000;5:513. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 3.Adams JM, Cory S. Science. 1998;281:1322. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 4.Gross A, McDonnell JM, Korsmeyer SJ. Genes Dev. 1999;13:1899. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 5.Huang DC, Strasser A. Cell. 2000;103:839. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Zhu H, Xu CJ, Yuan J. Cell. 1998;94:491. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Cell. 1998;94:481. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 8.Gross A, et al. J. Biol. Chem. 1999;274:1156. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 9.Wei MC, et al. Genes Dev. 2000;14:2060. [PMC free article] [PubMed] [Google Scholar]
- 10.Eskes R, Desagher S, Antonsson B, Martinou JC. Mol. Cell. Biol. 2000;20:929. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Parijs L, et al. Immunity. 1999;11:281. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 12.Wei MC, Zong W-X. unpublished data. [Google Scholar]
- 13.Li P, et al. Cell. 1997;91:479. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 14.Yin XM, et al. Nature. 1999;400:886. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 15.Lindsten T, et al. Mol. Cell. 2000;6:1380. [Google Scholar]
- 16.Scaffidi C, et al. EMBO J. 1998;17:1675. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakem R, et al. Cell. 1998;94:339. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 18.Kuida K, et al. Cell. 1998;94:325. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, et al. Cell. 1998;94:739. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 20.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Cell. 1998;94:727. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 21.Li K, et al. Cell. 2000;101:389. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman RJ. Genes Dev. 1999;13:1211. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, et al. Nature. 2000;403:98. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 24.He H, Lam M, McCormick TS, Distelhorst CW. J. Cell Biol. 1997;138:1219. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12401. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito M, Korsmeyer SJ, Schlesinger PH. Nature Cell Biol. 2000;2:553. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu S, Narita M, Tsujimoto Y. Nature. 1999;399:483. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 28.Kluck RM, et al. J. Cell Biol. 1999;147:809. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mootha VK, et al. EMBO J. 2001;20:661. doi: 10.1093/emboj/20.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu W, et al. EMBO J. 1996;15:4130. [PMC free article] [PubMed] [Google Scholar]
- 31.Ng FW, et al. J. Cell Biol. 1997;139:327. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacki J, et al. Oncogene. 2000;19:2286. doi: 10.1038/sj.onc.1203592. [DOI] [PubMed] [Google Scholar]
- 33.Ory DS, Neugeboren BA, Mulligan RC. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11400. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Primary murine embryonic fibroblasts were generated from Bax+/−;Bak+/− or Bax+/−;Bak−/− timed matings at 13.5 days after conception. Primary MEFs were immortalized by transfection with a plasmid containing SV40 genomic DNA. Primary MEFs were plated in six-well plates and were transfected with 1 µg of total DNA using Fugene (Roche) according to the manufacturer’s instructions. Stable immortalized clones were generated through serial dilution.
- 35.Cells were fixed in 3% paraformaldehyde and permeabilized in 1% bovine serum albumin and 0.1% Triton X-100. Cells were sequentially incubated with primary antibody to cytochrome c (clone 6H2.B4, Pharmingen) and Cy3-conjugated goat secondary antibody to mouse (Jackson Immunoresearch Laboratories) and Hoechst 33258 (Molecular Probes) and then mounted under a cover slip with N-propyl gallate. Images were acquired with a SPOT camera (Diagnostics) mounted on Nikon Eclipse E600 with PlanFluor objectives or Olympus IX50 microscopes.
- 36.We thank E. Smith for figure and manuscript preparation, B. Avery and S. Wade for animal husbandry, C. Gramm for technical assistance, C. Beard for assistance with retrovirus, P. Tegtmeyer for SV40 expression vector, and W. Sellers for use of the microinjector. M.C.W. is supported in part by NIH training grant 5T32AT09361. W.-X.Z. is supported by a postdoctoral grant from the Cancer Research Institute. This work is supported in part by NIH grants R01CA50239 and R37CA48023.