Abstract
The field of tissue engineering is severely limited by a lack of microvascularization in tissue engineered constructs. Biomimetic poly(ethylene glycol) hydrogels containing covalently immobilized platelet-derived growth factor BB (PDGF-BB) were developed to promote angiogenesis. Poly(ethylene glycol) hydrogels resist protein absorption and subsequent non-specific cell adhesion, thus providing a “blank slate”, which can be modified through the incorporation of cell adhesive ligands and growth factors. PDGF-BB is a key angiogenic protein able to support neovessel stabilization by inducing functional anastomoses and recruiting pericytes. Due to the widespread effects of PDGF in the body and a half-life of only 30 min in circulating blood, immobilization of PDGF-BB may be necessary. In this work bioactive, covalently immobilized PDGF-BB was shown to induce tubulogenesis on two-dimensional modified surfaces, migration in three-dimensional (3D) degradable hydrogels and angiogenesis in a mouse cornea micro-pocket angiogenesis assay. Covalently immobilized PDGF-BB was also used in combination with covalently immobilized fibroblast growth factor-2, which led to significantly increased endothelial cell migration in 3D degradable hydrogels compared with the presentation of each factor alone. When a co-culture of endothelial cells and mouse pericyte precursor 10T1/2 cells was seeded onto modified surfaces tubule formation was independent of surface modifications with covalently immobilized growth factors. Furthermore, the combination of soluble PDGF-BB and immobilized PDGF-BB induced a more robust vascular response compared with soluble PDGF-BB alone when implanted into an in vivo mouse cornea micropocket angiogenesis assay. Based on these results, we believe bioactive hydrogels can be tailored to improve the formation of functional microvasculature for tissue engineering.
Keywords: Angiogenesis, Hydrogel, Poly(ethylene glycol), Platelet-derived growth factor, Biofunctional materials
1. Introduction
While organ transplantation saves thousands of lives each year, in the USA over 100,000 people are currently waiting on the organ transplant list and 16 patients die every day awaiting an organ (United Network for Organ Sharing, www.unos.org). The increasing demand for donated organs to replace damaged or diseased tissues cannot be met by the current supply from cadaveric and living donors. The field of tissue engineering aims to meet this demand by replacing injured and diseased tissues with functional engineered counterparts. However, these efforts are severely limited by a lack of microvascularization within engineered constructs, as diffusion-based transport of nutrients within the timeframe and at the concentrations necessary to support cell survival is limited to a few hundred microns [1].
Platelet-derived growth factor (PDGF) is a molecule important in the establishment of the vasculature that also has roles in the development of the kidneys, lungs and central nervous system. Four iso-forms of PDGF are known, PDGF-A–PDGF-D, with PDGF-A and PDGF-B forming homo- and heterodimers. The homodimer PDGF-BB, often involved in angiogenesis, is released in high concentrations by endothelial cells at the sprouting tip of forming capillaries in response to hypoxia, growth factors and shear stress, and serves to mediate pericyte recruitment [2,3]. Although not involved in initial vessel formation, PDGF-BB is involved in neovessel stabilization and functionalization by inducing anastomoses and recruiting pericytes. Vessel stabilization has been shown to be dependent upon expression of PDGF β-receptors, which are expressed by fibroblasts, endothelial cells and smooth muscle cells [4,5]. PDGF-BB also stimulates pericyte production of extracellular matrix proteins, including fibronectin, collagen and proteoglycans, necessary for the basement membrane of capillaries. In addition, PDGF-BB increases expression levels of vascular endothelial growth factor (VEGF) in mural cells and stimulates fibroblasts to produce and secrete collagenases, key for cell migration in angiogenesis [6].
Growth factors with widespread effects, such as PDGF-BB, have seen relatively few clinical successes despite documented in vitro efficacy [7]. This is possibly due to a short circulating half-life [8] or the potential for unintended action due to the ubiquity of PDGF-BB targets. Covalent immobilization enables controlled, spatial presentation of potent growth factors to stimulate a desired response and mediate potential drawbacks. Immobilization of bio-molecules can be accomplished by attaching them to a polymer, such as poly(ethylene glycol) (PEG), which is then incorporated into a larger scaffold network. The conjugation of growth factors to PEG has been shown to improve solubility, decrease immunogenicity and increase stability [9,10], while at the same time retaining bioactivity of the original molecule. For example, PEG-conjugated epidermal growth factor (EGF), which does not diffuse or become endocytosed, has been shown to be bioactive and capable of inducing DNA synthesis in a manner comparable with soluble EGF [7]. In another study, PEG–VEGF incorporated into a biodegradable gel not only increased endothelial cell tubulogenesis, but also increased endothelial cell motility 14-fold and cell–cell connections 3-fold [11].
Synthetic polymer matrices facilitate the design of scaffold materials with reproducible and modifiable characteristics, such as drug delivery rates, degradation rates and mechanical properties. Poly(ethylene glycol) diacrylate (PEGDA) is a hydrophilic and bio-compatible polymer which can be designed to mimic the mechanical properties of soft tissue [12]. A PEGDA hydrogel acts as a “blank slate” by resisting protein absorption and cell adhesion, enabling precise modification with bioactive ligands and growth factors to induce desired responses, such as cell adhesion, migration and proliferation [13]. In addition, matrix metalloproteinase (MMP)-sensitive sequences can be incorporated into the monomer backbone to enable biodegradation of the synthetic matrix [14]. PEGDA can be photocrosslinked by introducing a photosensitive chemical catalyst into the prepolymer solution, and mild crosslinking conditions permit cellular encapsulation [11]. Upon stimulation with growth factors that induce migration, encapsulated cells will secrete MMPs, which enable degradation of the specifically tailored matrix.
The current studies used bioactive, PEG-based hydrogels modified with covalently immobilized PDGF-BB to promote in vitro tubule formation and stabilization, as well as in vivo angiogenesis. Bioactive, immobilized PDGF-BB was shown to enhance the angiogenic activities of tubule formation on modified surfaces in two-dimensional (2D) and promote cell migration into three-dimensional (3D) degradable hydrogels. Since angiogenesis and vessel stabilization in vivo require precise coordination between multiple growth factors, the combination of PDGF-BB with fibroblast growth factor-2 (FGF-2) was also investigated. PDGF-BB and FGF-2 have previously been found to induce a synergistic vascular response in both the mouse cornea and ischemic hindlimb models [15]. In the current work the combination of covalently immobilized PDGF-BB and FGF-2 showed enhanced cell migration in 3D degradable hydrogels.
Previous research has shown that a co-culture of human umbilical vein endothelial cells (HUVECs) and 10T1/2 pericyte precursor cells formed long-term stable vessels in vivo on a fibronectin–type I collagen matrix [16]. Additionally, 10T1/2 cells, when cultured with HUVECS, displayed a smooth muscle cell morphology and began expressing pericyte markers, such as smooth muscle α-actin, smooth muscle myosin and calponin via the transforming growth factor β1 (TGF-β1) pathway [17]. However, FGF-2 has been shown to act as an antagonist of TGF-β1-induced smooth muscle cell gene expression in 10T1/2 cells [18]. In the current studies a co-culture of cells resulted in tubule formation independent of surface modifications with covalently immobilized growth factors, and a FGF-free medium enhanced tubule formation. Finally, bioactive hydrogels containing the combination of both soluble PDGF-BB to initiate angiogenesis and immobilized PEG–PDGF-BB exhibited a significant increase in vessel density when assessed in the mouse cornea micropocket angiogenesis assay. These studies reaffirm that PEG-based hydrogels can be designed with covalently immobilized growth factors to stimulate a desired cellular response.
2. Materials and methods
2.1. Cell maintenance
HUVECs (Lonza, Walkersville, MD) were cultured in endothelial growth medium EGM-2 (Lonza), supplemented with ascorbic acid, epidermal growth factor, fibroblast growth factor (hFGF-2), heparin, hydrocortisone, insulin-like growth factor, GA-1000 (gentamicin and amphotericin-B), 2% fetal bovine serum (Bulletkit, Lonza), 2 mM L-glutamine, 1 U ml−1 penicillin and 1 μg ml−1 streptomycin (GPS) (Sigma, St. Louis, MO). 10T1/2 cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium with high glucose (DMEM) (Gibco, North Andover, MA) supplemented with 10% fetal bovine serum and GPS. HUVECs were used from passages 4 to 6, and 10T1/2 cells were used from passages 15 to 19. Cells were maintained in an incubator at 37 °C and 5% CO2 with medium replenished every 2 days and subculturing as necessary.
2.2. Synthesis and purification of poly(ethylene glycol) diacrylate (PEGDA)
PEG (molecular weight 6000 Da; Fluka, Milwaukee, WI) was acrylated by reacting dry PEG with acryloyl chloride (Sigma, St. Louis, MO) and triethyl amine (TEA) (Sigma) in anhydrous dichloromethane (DCM) (Sigma) under argon gas overnight at 1:4 PEG:acryloyl chloride and 1:2 PEG:TEA molar ratios. The resulting solution was washed with 2 M K2CO3 and allowed to separate into aqueous and organic phases overnight. PEGDA, in the organic phase, was dried using anhydrous MgSO4 followed by filtration. The polymer was precipitated in diethyl ether, filtered and dried overnight under vacuum. PEGDA powder was stored at −20 °C under argon gas.
2.3. Synthesis and purification of PEG–RGDS and degradable PEG–PQ–PEG
PEG–RGDS was synthesized by dissolving the cell-adhesive peptide Arg–Gly–Asp–Ser (RGDS) (American Peptide, Sunnyvale, CA) in anhydrous dimethyl sulfoxide and adding diisopropylethylamine. Dissolved RGDS was added to dry acryloyl-PEG succinimidyl carboxymethyl (PEG-SCM) (Laysan, Arab, AL) at a 1.1:1 PEG-SCM:RGDS molar ratio. This mixture was placed on a rocker overnight, dialyzed against water in a regenerated cellulose membrane to remove unwanted products and lyophilized.
Hydrogels were rendered degradable by the incorporation of a collagenase-sensitive peptide, GGGPQGIWGQGK (abbreviated to PQ), into the backbone of the PEGDA base polymer. The PQ peptide was first synthesized using Fmoc chemistry on an APEX 396 solid phase peptide synthesizer (Aapptec) and characterized using MAL-DI-TOF. The synthesized peptide was conjugated to PEG by following a similar procedure as above with a 2.1 M excess of PEG-SCM. Conjugation of PEG–RGDS and PEG–PQ–PEG were confirmed using a gel permeation chromatography (GPC) system equipped with a PLgel column (5 μm, 500 Å, Polymer Laboratories, Amherst, MA) and an evaporative light scattering (ELS) detector (Polymer Laboratories). The PEG–RGDS was dissolved in 0.1% ammonium acetate in dimethylformamide (DMF) solvent and tested against a PEG-SCM standard.
2.4. Synthesis and purification of acryloyl-PEG-succinimidyl carbonate (PEG-SMC)
The organic solvents needed to accommodate the short half-life of the succinimidyl carboxymethyl reactive group on the PEG-SCM, which was purchased and used for conjugation of the peptides, were not compatible with the proteins used in this study. It was therefore necessary to synthesize heterobifunctional acryloyl-PEG succinimidyl carbonate (PEG-SMC) in-house. PEG-SMC is functionally similar to the commercially available PEG-SCM, but has a longer reaction half-life and thus can be used under aqueous conditions.
PEG (molecular weight 3400 Da; Fluka/Sigma) was reacted with Ag2O (Sigma, St. Louis, MO), acryloyl chloride (Sigma) and KI (Sigma) in anhydrous dichloromethane (DCM) (Sigma) at 4 °C overnight, at molar excess ratios of 1.5, 1.1 and 0.3, respectively. Silver was removed by filtering the solution through Celite 521 (Spectrum Chemical Mfg Corp., Gardena, CA). A Rotovap was used to dry the solution prior to dissolution in deionized H2O. The pH was adjusted to 3 with HCl and the solution was heated to 35 °C for 1 h. Iodine was removed by adding activated charcoal (Fisher, Pittsburg, PA) and the solution was filtered through Celite 521. NaCl and DCM were added, followed by DCM extraction. Phase separation with 2 M K2CO3 was used to remove acid and chloride ions. Monoacrylated PEG was dried with sodium sulfate (Fisher), and a Rotovap was used to concentrate the solution, followed by ethyl ether precipitation and vacuum filtration. A four molar excess of disuccinimidyl carbonate (Sigma) was reacted with the monoacrylated PEG in anhydrous acetonitrile (Sigma) and pyridine (Sigma) under argon overnight. The product was dried using a Rotovap prior to dissolution in anhydrous DCM. The solution was filtered, and PEG-SMC was purified in acetate buffer (0.1 M, pH 4.5, 15% NaCl) via phase separation. The purified PEG-SMC was dried with anhydrous MgSO4. PEG-SMC was precipitated into ethyl ether, filtered and dried overnight under vacuum. PEG-SMC was characterized by proton nuclear magnetic resonance spectroscopy (1H NMR) (Avance 400 Hz, Bruker, Billerica, MA) and matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF) (Bruker Daltonics, Billerica, MA). The final PEG-SMC product was stored at −80 °C under argon.
2.5. Synthesis of PEG–PDGF-BB and PEG–FGF-2
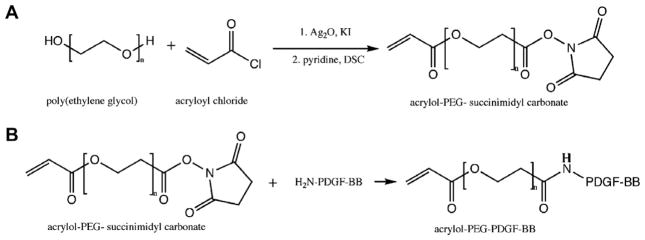
In-house synthesized acryloyl-PEG-SMC was dissolved in 50 mM sodium bicarbonate buffer (pH 8.5) and sterilized via filtration (0.2 μm). PDGF-BB (ProSpec Bio, Israel) was conjugated to PEG-SMC using a 400:1 PEG-SMC:PDGF-BB molar ratio in 200 mM sodium bicarbonate buffer (pH 8.5) at 4 °C for 4 days (Fig. 1). The resulting PEG–PDGF-BB solution was lyophilized under sterile conditions. PEG–PDGF-BB powder was reconstituted and stored in HEPES-buffered saline (HBS) (100 mM NaCl, 10 mM HEPES in deionized water, pH 7.4) with 0.1% BSA at 4 °C for up to 3 months. A similar procedure was followed for PEG–FGF-2 (ProSpec Bio) at a 100:1 PEG-SMC:FGF-2 M ratio. All growth factor conjugations were confirmed via Western blot analysis on a 15% Tris–HCl precast polyacrylamide gel (BioRad, Hercules, CA). Primary antibodies included rabbit poly-clonal anti-PDGF-B antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-FGF-basic antibody (Millipore, Billerica, MA). Secondary horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Santa Cruz Biotechnology) and an ECL™ chemiluminescent Western blotting analysis system (GE Health-care, Chalfont St. Giles, UK) were used. The Western blot membrane was exposed to film (Kodak, Rochester, NY) for 15 s and developed using Micromax Developer (Hope, Seattle, WA) with T2 developer and T2 fixer (White Mountain Imaging, Salisbury, NY). The presence of PEG was confirmed using a PEG stain adapted from Zhang et al. [19], which allows barium iodine to react with PEG to form a yellow color.
Fig. 1.
(A) Acryloyl-PEG–succinimidyl carbonate (PEG-SMC) was synthesized by reacting PEG with acryloyl chloride in the presence of silver oxide and potassium iodide followed by disuccinimidyl carbonate. (B) Acryloyl-PEG–PDGF-BB was synthesized by reacting PEG-SMC with the primary amines of PDGF-BB.
Unconjugated PDGF-BB was quantified using a PDGF-BB ELISA (R&D Systems, Minneapolis, MN). PEG chains mask antibody binding to PEG–PDGF-BB, so the amount of unconjugated PDGF-BB was quantified after running a sample of the reacted solution on an ELI-SA compared with a soluble PDGF-BB standard curve. With the amount of soluble PDGF-BB quantified, appropriate concentrations of PEG–PDGF-BB were calculated.
2.6. Formation and surface modification of PEGDA hydrogels
6 kDa PEGDA was dissolved in HBS to a 10 wt.% polymer solution. A stock solution of the photoinitiator 2-dimethoxy-2-phenylacetophenone (acetophenone) (Sigma) was prepared by dissolving 300 mg acetophenone in 1 ml of N-vinylpyrrolidone (NVP) and then added to the polymer solution at a concentration of 10 μl ml−1. The solution was vortexed and sterile filtered. Hydrogels were polymerized between two glass slides separated by a 0.75 mm thick poly(tetrafluoroethylene) (PTFE) spacer. The glass slides and spacer were secured using clips. The polymer solution was exposed to UV light (B-200SP UV lamp, 365 nm, 10 mW cm−2) for 30 s and stored in PBS with 0.1% sodium azide.
The effects of PEGylated proteins in 2D were studied by modifying the surface of bulk PEGDA hydrogels as previously described [20]. Briefly, 5 mm diameter circles were punched from the hydrogel slabs prepared above. A solution containing 10 μl ml−1 acetophenone along with PEG–RGDS and PEGylated growth factors was then added in sufficient volume to completely cover the surface of the hydrogel. The exact combination and concentrations of each factor are given in the individual experiments below. Hydrogels were then exposed to UV light for 2 min, followed by soaking in sterile PBS to allow swelling and removal of the photo-initiator solution.
Patterned hydrogel surfaces were also made to emphasize the utility of spatial control of PEG–PDGF-BB. Hydrogels were patterned by pipetting a solution containing 10 μl ml−1 acetophenone along with PEGylated growth factors onto the surface of a 5 mm diameter punch of a bulk hydrogel. A black transparency was used to block UV light from part of the gel. Hydrogels were exposed to UV light for 2 min, followed by extensive rinsing. PEG–RGDS was then immobilized on the entire surface by adding a solution containing 10 μl ml−1 acetophenone with PEG–RGDS to completely cover the surface of the hydrogel followed by UV light exposure for 2 min.
For all studies unconjugated PDGF-BB and PEG-SMC were removed via diffusion before the hydrogels were used. Alternatively, soluble PDGF-BB could also be left in place to act as a diffusible growth factor to encourage cell migration in the surrounding tissue for other applications.
To quantify the surface concentration of immobilized PEG–PDGF-BB hydrogels were synthesized as described above and then degraded in 0.1 N sodium hydroxide for 3 days. After complete hydrogel degradation the protein concentration was read on a Nanodrop 2000 (Thermo Scientific, Wilmington, DE).
2.7. Bioactivity of conjugated PDGF-BB via 10T1/2 proliferation
In order to evaluate the bioactivity of covalently attached PEG–PDGF-BB, 10T1/2 cells (8.5 × 104 cells cm−2) were seeded onto gels modified with 5 mg ml−1 PEG–RGDS alone or in combination with 0.70 nmol ml−1 PEG–PDGF-BB. As a positive control, 0.70 nmol ml−1 soluble PDGF-BB was added to the medium of gels modified with PEG–RGDS alone. Cells were imaged using an Axiovert 135 (Zeiss) inverted fluorescence microscope after 48 h culture. Cell nuclei were visualized via incubation with Hoechst 33342 dye (Sigma) at a concentration of 5 μg ml−1 in DMEM without serum for 1 h at 37 °C. Four fluorescent images were taken per gel at excitation = 350 nm and emission = 460 nm. Cell nuclei were quantified using ImageJ. Cell counts in the presence of PDGF-BB were normalized to counts on surfaces modified with PEG–RGDS alone. Data from three separate experiments using gels with PEG–RGDS (n = 31), PEG–PDGF-BB and PEG–RGDS (n = 28) and PEG–RGDS and soluble PDGF-BB (n = 18) were pooled for statistical analysis as described below.
2.8. Quantification of tubule formation
To investigate the impact of covalently incorporated growth factors on vascular tubulogenesis, surface-modified PEGDA hydrogels were used. Bulk hydrogels were prepared from 10% 6 kDa PEGDA and surfaces were modified as described above. Four modified surfaces were investigated: (1) 7.5 μmol ml−1 PEG–RGDS, (2) PEG–RGDS and 0.27 nmol ml−1 PEG–FGF-2, (3) PEG–RGDS and 0.70 nmol ml−1 PEG–PDGF-BB and (4) PEG–RGDS, PEG–PDGF-BB and PEG–FGF-2. HUVECs (8.5 × 105 cells cm−2) or a co-culture of HUVECs and 10T1/2 cells at a ratio of 4:1 were seeded onto the modified gels and cultured in EGM-2 medium either with or without FGF-2 and imaged using an Axiovert 135 (Zeiss) inverted fluorescence microscope. Hydrogels were observed over a 30 day period and images of the entire surface of the gels were merged in Adobe Photoshop Elements. Tubules and modified surface areas were traced in Adobe Illustrator and quantified using ImageJ. For gels seeded with HUVECs alone data from three separate experiments using gels with PEG–RGDS (n = 10), PEG–FGF-2 and PEG–RGDS (n = 3), PEG–PDGF-BB and PEG–RGDS (n = 10) and PEG–FGF-2, PEG–PDGF-BB (n = 4) were pooled. For the co-culture of HUVEC and 10T1/2 hydrogels data from three separate experiments using gels in medium with FGF-2 with PEG–RGDS (n = 10), PEG–FGF-2 and PEG–RGDS (n = 3), PEG–PDGF-BB and PEG–RGDS (n = 12) and PEG–FGF-2, PEG–PDGF-BB and PEG–RGDS (n = 12) were pooled. Similarly, data from three separate experiments using gels in medium without FGF-2 with PEG–RGDS (n = 7), PEG–FGF-2 and PEG–RGDS (n = 6), PEG–PDGF-BB and PEG–RGDS (n = 8) and PEG–FGF-2, PEG–PDGF-BB and PEG–RGDS (n = 7) was pooled.
2.9. Immunohistochemistry
To examine tubule morphology, immunohistochemistry was performed to identify endothelial pericyte cell markers. After 30 days in culture the gels were fixed in 4% paraformaldehyde for 30 min and washed with phosphate-buffered saline (PBS). Cells were permeabilized with 0.5% Triton X-100 for 10 min followed by a second PBS wash. 3% normal donkey serum (Sigma) was used as a blocking agent prior to application of the primary antibodies. Gels were incubated overnight at 4 °C in a 1:200 dilution of mouse anti-smooth muscle α-actin (R&D systems, Minneapolis, MN) and a 1:100 dilution of goat anti-PE-CAM-1 (Santa Cruz Biotechnology) in 3% BSA solution in PBS. Following incubation, gels were rinsed five times in PBS for 1 h each time. A 1:400 dilution of Alexafluor 488 donkey anti-goat IgG (Invitrogen) and Alexafluor 555 donkey anti-mouse IgG (Invitrogen, Carlsbad, CA) was applied overnight at 4 °C in order to visualize the primary antibodies. After washing the gels were incubated in 2 μM DAPI solution (Invitrogen) for 45 min. Images were taken using a confocal microscope (Zeiss5 LIVE, Plan-Apochromat 20× objective with 0.8 numerical aperture and Plan-Apochromat oil immersion 63× with 1.4 numerical aperture: for Alexafluor 488 excitation = 489 nm, emission BP filter = 500–525 nm; for Alexafluor 555 excitation = 532 nm, emission BP filter = 560–675 nm; for DAPI excitation = 405 nm, emission BP filter = 415–480 nm).
2.10. Cellular encapsulation into hydrogels
To further understand the effects of covalently immobilized growth factors oncells in 3D HUVECs were encapsulated in MMP-sensitive hydrogels containing covalently immobilized growth factors. HUVECs were fluorescently labeled the day before encapsulation by incubation with 10 μg green CMFDA Cell Tracker® (Invitrogen, Eugene, OR) in the culture medium for 1 h. Following incubation, the cells were rinsed with PBS and fresh medium was added.
Polymer solution was prepared in HBS (10 mM, pH 7.4) with a final formulation of 10% PEG–PQ–PEG, 3.5 μmol ml−1 PEG–RGDS and 0.3% (w/v) Irgacure 2959 (Ciba Corp., Basel, Switzerland). Four treatment groups were observed, including hydrogels containing (1) PEG–RGDS alone, (2) PEG–RGDS and PEG–FGF, (3) PEG–RGDS and PEG–PDGF-BB and (4) PEG–RGDS, PEG–FGF-2 and PEG–PDGF-BB. 0.03 nmol l−1 PEG–FGF and 0.08 nmol l−1 PEG–PDGF-BB were used for both the individual factor groups and the combination of factors. Fluorescently labeled HUVECs were harvested using trypsin–EDTA and counted using a Coulter counter to determine the cell concentration. After counting the cells were pelleted by centrifugation at 2700 rpm for 4 min and resuspended in polymer solution to a concentration of 30,000 cells μl−1. 5 μl droplets of cell-laden polymer were formed and exposed to UV light for 7 min and the resulting cell-laden hydrogels were immediately immersed in EGM-2 medium for in vitro analysis.
Activity of the cells in the hydrogels was monitored for 60 h using a Zeiss LSM 5 LIVE confocal microscope. Images were captured every hour and analyzed using Logger Pro Software to track cell movement. Data from three separate experiments were pooled (n = 30) for data analysis.
2.11. Zymography
To determine MMP activity the medium was collected from gels with encapsulated HUVECs 60 h after encapsulation. Standard zymography was performed on a 10% precast polyacrylamide gel with gelatin (BioRad, Hercules, CA) following a Millipore protocol. Briefly, after running electrophoresis the gels were immersed in a 25% Triton X-100 solution in water with gentle mixing for 30 min. After decanting the solution a developing buffer (50 mM Tris base, 50 mM Tris–HCl, 0.2 M NaCl, 5 mM CaCl2, 5 mM Brij 35) was applied overnight at 37 °C. Gels were then stained with Coomassie brilliant blue and destained with methanol:acetic acid:water (50:10:40). Gel images were obtained using a Fujifilm LAS 4000 and analyzed for the presence of MMP bands.
2.12. Hydrogel implantation into the mouse cornea
Hydrogels were prepared following the protocol outlined in Poche et al. [21]. Briefly, the prepolymer solution was prepared as a 10 wt.% (100 mg ml) of PEG–PQ–PEG, 3.5 μmol ml−1 PEG–RGDS, 10 μl ml−1 acetophenone stock solution and 160 ng soluble PDGF-BB per gel, with or without 1.6 ng PEG–PDGF-BB per gel (n = 8). Hydrogels were prepared by injecting 0.12 μl of polymer solution in between glass slides spaced by a 0.005 in thick PTFE spacer secured with binder clips and exposed to UV light for 2 min.
Bioactive hydrogels were implanted into the cornea following a modified mouse cornea micropocket angiogenesis assay [22]. All animals were used under an approved protocol of the Institutional Animal Care and Use Committee at Baylor College of Medicine. Briefly, mice were anesthetized and a partial thickness incision of approximately 50 μm in depth was made. A micropocket in the cornea stroma was made using a Von Graff knife with immediate hydrogel implantation after crosslinking. Flk1-myr::mCherry transgenic mice were utilized, which enabled visualization of vessel invasion via endothelial cell-specific florescence [23]. Mice were euthanized and the corneas collected and fixed in 4% paraformaldehyde 14 days after implantation. Flat mounts of tissue were made and imaged in a Zeiss LSM 510 META confocal microscope using a 40×/1.2NA C-Apochromat water immersion objective lens corrected for UV/visible/infrared with a working distance of 0.28 mm. A 543 nm laser was used to excite the mCherry fluorophore. Images of vessels on the hydrogel were compiled from projections of z-stacks exactly 22 μm in thickness, spaced 1.1 μm apart.
2.13. Statistics
One-way ANOVA and subsequent Tukey’s least significant difference (LSD) tests were used to statistically analyze the bioactivity of PEG–PDGF-BB and HUVEC tubulogenesis on surface-modified hydrogels and the movement of cells inside the hydrogels. In the tubulogenesis study a generalized linear model in Minitab was used to analyze statistical differences in tubule formation of the co-culture of HUVEC and 10T1/2 cells. For each analysis P < 0.05 was considered significant.
3. Results
3.1. Polymer characterization
Conjugation of acryloyl-PEG-SMC to the primary amines of PDGF-BB was confirmed using a Western blot. Conjugated PDGF-BB was compared with unmodified PDGF-BB, with an increase in molecular weight representing conjugation of PEG chains to the protein (Fig. 2A). The presence of an intense PEG band after PEG staining (arrow, Fig. 2B) at the same molecular weight as the conjugated PDGF-BB band on the Western blot further verified PEG conjugation.
Fig. 2.
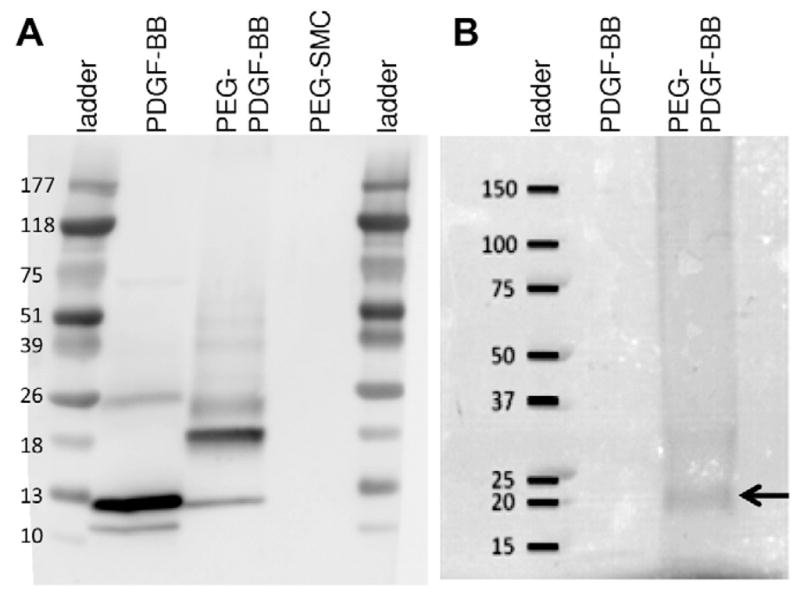
(A) Successfully conjugated PDGF-BB was confirmed on a Western blot by the increase in molecular weight after conjugation. (B) A PEG stain confirmed the presence of a PEG band at the same molecular weight (arrow) as PEG–PDGF-BB in the Western blot, further validating PEG–PDGF-BB conjugation.
3.2. PEG–PDGF-BB bioactivity
Bioactivity of the conjugated PDGF-BB was confirmed by quantifying 10T1/2 cell proliferation on surfaces modified with PEG–RGDS and PEG–PDGF-BB compared with surfaces modified with PEG–RGDS alone. Soluble PDGF-BB was added to the medium of some gels modified with PEG–RGDS to serve as a positive control. As shown in (Fig. 3A), 10T1/2 cell proliferation increased significantly in the presence of either soluble or bound PDGF-BB at 48 h (ANOVA, P < 0.05). There was no significant difference in proliferation in the presence of soluble PDGF-BB compared with PEG–PDGF-BB, suggesting that bioactivity of this growth factor wais not adversely affected by conjugation to PEG. The capability to spatially control immobilized PDGF-BB was demonstrated with hydrogels patterned with immobilized PDGF-BB (Fig. 3B and C). 10T1/2 cells were evenly seeded on surfaces with and without PEG–PDGF-BB at 4 h (Fig 3B). By 48 h 10T1/2 cells seeded onto these patterned hydrogels appeared to have proliferated more on areas with immobilized PDGF-BB (Fig 3C).
Fig. 3.
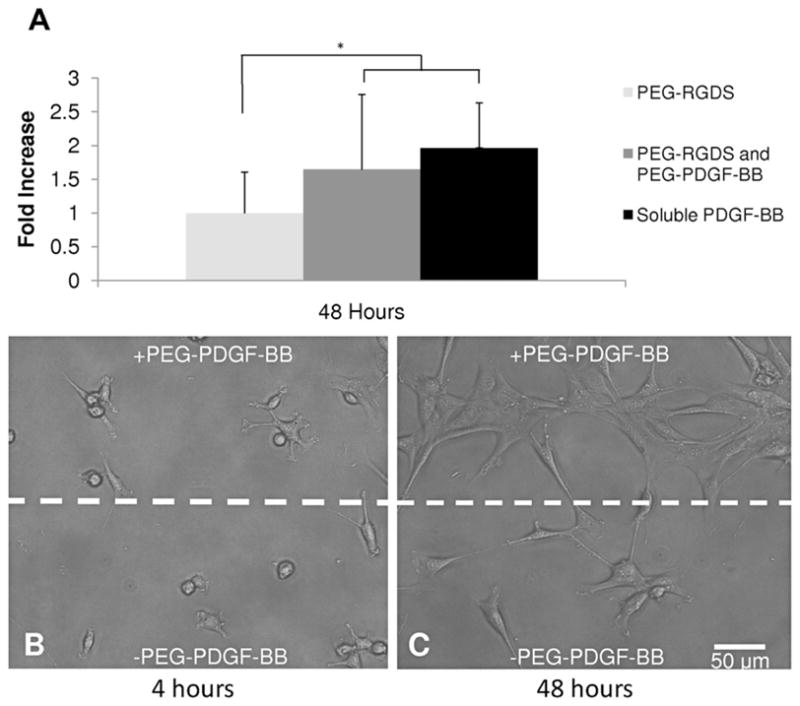
Bioactivity of PEG–PDGF was confirmed by seeding 10T1/2 cells onto modified surfaces. (A) By 48 h 10T1/2 cell proliferation had increased significantly in the presence of PDGF-BB (ANOVA, #P < 0.05). No significant difference between the soluble and bound forms of PDGF-BB indicate that covalently immobilized PEG–PDGF-BB retained its bioactivity. (B, C) Hydrogels with patterned regions of PEG–PDGF-BB demonstrate the utility of spatial control of PDGF-BB. 10T1/2 cells seeded onto these patterned gels were evenly dispersed at 4 h (B), but exhibited increased proliferation on regions patterned with PDGF-BB by 48 h (C).
3.3. Covalently immobilized PDGF-BB promotes tubulogenesis
30 days after seeding HUVECs on surfaces modified with PEG–RGDS and PEG–PDGF-BB endothelial cell tubules and branching networks were visible (Fig. 4A). HUVECs on surfaces modified with PEG–RGDS alone exhibited the normal cobblestone morphology of the cell type (Fig. 4B). Early tubulogenesis was apparent after 18 days, with robust tubulogenesis at day 30. The presence of tubulogenesis after 30 days suggests that the bioactivity of covalently immobilized PEG–PDGF-BB was maintained. Statistical analyses indicated significantly higher tubule formation on surfaces modified with PEG–RGDS and PEG–PDGF-BB, as well as surfaces modified with the combination of PEG–RGDS, PEG–PDGF-BB and PEG–FGF-2, when compared with surfaces modified with PEG–RGDS alone (ANOVA with Tukey’s LSD, P < 0.05, Fig. 4C).
Fig. 4.
Modified surfaces significantly enhanced endothelial cell tubule formation. (A) HUVECs seeded onto modified surfaces exhibited extensive tubule formation (arrows) as early as 18 days, compared with the cobblestone appearance of HUVECs seeded on surfaces with only PEG–RGDS (B). (C) A significant increase in tubule formation on surfaces with covalently immobilized PDGF-BB (*P < 0.01) and surfaces with both covalently immobilized PDGF-BB and FGF-2 (#P < 0.05) can be seen compared with surfaces modified with PEG–RGDS alone.
3.4. Tubule formation by co-cultures of HUVEC and 10T1/2 cells is independent of surface modification
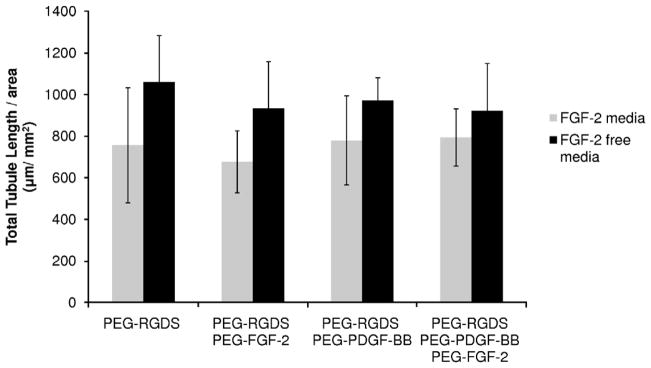
The co-culture of HUVECs and 10T1/2 cells in a 4:1 ratio was seeded onto PEGDA hydrogels modified with cell-adhesive peptides and growth factors. Cells in these studies formed tubules within 6 days, with some tubule formation visible by 2 days. Tubulogenesis was independent of surface modification, indicating that covalently immobilized FGF-2 and PDGF-BB are not necessary to stimulate tubule formation. Additionally, because FGF-2 is known to antagonize the TGF-β1-dependent differentiation of 10T1/2 cells into pericytes, cultures were maintained in medium both with and without this additive. A generalized linear model in Minitab showed a significant difference in tubule formation between these two experimental groups (Fig. 5, P < 0.0005), with FGF-2-free medium resulting in the greatest degree of tubulogenesis.
Fig. 5.
Analysis of total tubule length using a generalized linear model confirmed a significant difference between cells cultured in medium with and without FGF-2 (P < 0.0005). The co-culture of HUVECs and 10T1/2 cells exhibited robust tubule formation independent of surface modifications with covalently immobilized growth factors.
3.5. Endothelial and smooth muscle cell marker expression induced by immobilized PEG–FGF-2 and PEG–PDGF-BB
Immunohistochemistry was performed to confirm the expression of endothelial and smooth muscle cell markers in tubules formed by co-cultures of HUVEC and 10T1/2 cells on modified surfaces. 10T1/2 cells were found to express smooth muscle α-actin, suggesting a pericyte phenotype (Fig. 6). PECAM-1 labeled HUVECs formed extensive and branching tubule networks throughout the surface. No visible difference in tubule formation was observed between cells seeded on surfaces modified with PEG–RGDS alone or with the cell-adhesive peptide in combination with PEG–PDGF-BB and/or PEG–FGF-2. This suggests that tubule formation in the co-culture of HUVECs and 10T1/2 cells was independent of surface modification (Fig. 6AA and B). Upon closer examination single 10T1/2 cells were found to associate with multiple HUVECs in a typical pericyte “umbrella-like” morphology (Fig. 6C). In addition, the appearance of large vacuoles within HUVECs in these networks (arrows, Fig. 6D) was suggestive of the early stages of capillary lumen formation.
Fig. 6.

Immunofluorescent staining of PECAM-1 (green), smooth muscle α-actin (red) and cell nuclei (blue). No significant difference in tubule formation was visible between surfaces modified with RGDS (A) and surfaces modified with covalently immobilized PDGF-BB (B). 10T1/2 cells exhibiting a pericyte morphology can be seen encompassing an endothelial cell and associating with multiple endothelial cells on surfaces modified with RGDS (C). Vacuole formation is visible as an early step towards lumen formation on surfaces modified with covalently immobilized PDGF-BB (D, arrows).
3.6. Covalently immobilized PEG–PDGF-BB promotes cell migration in 3D degradable hydrogels
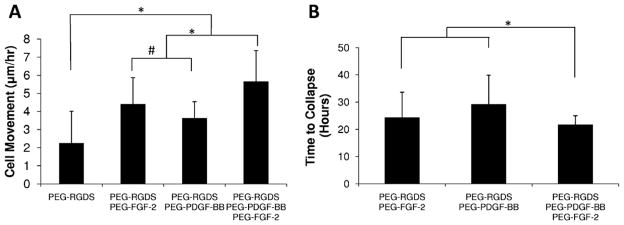
Time lapse confocal microscopy of fluorescently labeled HUVECs encapsulated in 3D degradable hydrogels (Supplementary videos) exhibited a significant increase in migration distance per hour in the presence of covalently immobilized growth factors. Cell movement was quantified for the first 14 h, at which time extensive cell clumping and eventual gel collapse hindered measurement. Endothelial cells encapsulated in 3D degradable hydrogels exhibited significantly higher migration in the presence of covalently immobilized PEG–PDGF-BB and PEG–FGF-2 compared with hydrogels containing only PEG–RGDS (Fig. 7A, *P < 0.01). Also, PEG–FGF-2 significantly increased migration relative to PEG–PDGF-BB (Fig. 7A, #P < 0.05), most likely due to the role of FGF-2 as a chemoattractant [24]. The combination of covalently immobilized PEG–PDGF-BB and PEG–FGF-2 was found to significantly increase migration (Fig. 7A, *P < 0.01) and decrease the time to gel collapse (Fig. 7B, *P < 0.01) compared with presentation of either of the factors alone.
Fig. 7.
(A) HUVECs encapsulated in 3D degradable hydrogels exhibited greater cell movement in gels containing covalently immobilized growth factors (*P < 0.01). PEG–FGF-2 significantly increased migration compared with PDGF-BB (#P < 0.05), and the combination of PEG–PDGF-BB and PEG–FGF-2 induced significantly increased cell migration compared with PDGF-BB and FGF-2 alone (*P < 0.01). (B) Hydrogels containing covalently immobilized growth factors collapsed after 20–40 h. The combination of PDGF-BB and FGF-2 led to earlier gel collapse compared with each growth factor alone (*P < 0.01).
3.7. MMP activation in response to covalently immobilized growth factors
Gelatin zymography was performed to analyze the presence of MMP-2 in hydrogels containing encapsulated HUVECs with covalently immobilized growth factors (Fig. 8). Interestingly, the 72 kDa proform of MMP-2 was present in all samples, but only samples with covalently immobilized PEG–PDGF-BB and/or PEG–FGF-2 contained active MMP-2 (68 kDa), which complements the increase in cell movement in the same materials.
Fig. 8.

Gelatin zymography confirmed the presence of pro-MMP-2 (molecular weight 72 kDa) in all samples, but active MMP-2 (molecular weight 68 kDa, arrow) was only seen in the presence of covalently immobilized growth factors.
3.8. PEG–PDGF-BB enhances in vivo vascular response
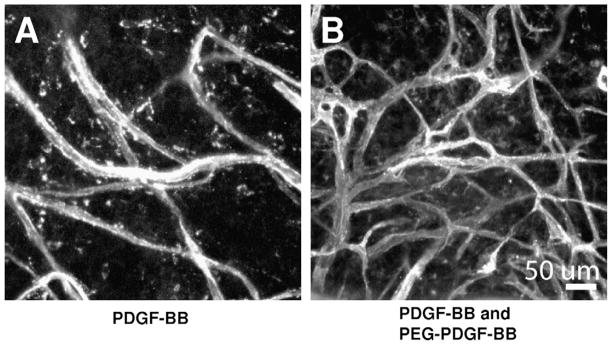
To confirm the bioactivity of PEG–PDGF-BB during angiogenesis in vivo hydrogels containing PEG–PDGF-BB were implanted into the mouse cornea, a well-defined method to assess angiogenesis [25]. Hydrogels containing soluble PDGF-BB initiated an angiogenic response by stimulating vessels from the surrounding limbus to invade the normally avascular cornea. Once vessels reached the bioactive hydrogel the immobilized PEG–PDGF-BB (Fig. 9B) induced a more robust vascular response than hydrogels with soluble PDGF-BB alone (Fig. 9A).
Fig. 9.
Bioactive hydrogels incorporating both releasable and covalently immobilized PDGF-BB (B) were implanted into the mouse cornea micropocket and resulted in a more robust vascular response than hydrogels with releasable PDGF-BB alone (A).
4. Discussion
Controlled delivery of PDGF has been investigated in several forms. Primarily, biomaterials have been employed as slow release delivery mechanisms [26]. Rather than relying on growth factor release, fibrin matrices using heparin binding to mimic the body’s delivery system have also been designed to deliver PDGF-BB and FGF-2, which increased fibroblast proliferation in tendon tissue [27]. However, heparin binding does not provide as controlled an environment, as other proteins can bind to heparin, and proteins can release and rebind. Recently, two groups have immobilized PDGF, enabling more prolonged delivery than controlled release from biomaterials. Aizawa et al. immobilized PDGF-AA, which induces PDGF receptor α α homodimers, as opposed to PDGF-BB, which binds all three dimeric combinations of α- and β-receptors. Maleimide functionalized PDGF-AA was immobilized to a natural material, agarose, containing sulfhydryl groups and used for the known effect of PDGF-AA in regulating the differentiation of neural stem cells [28]. Chen et al. introduced sulfhydryl groups into collagen scaffolds and used them to immobilize PDGF-BB, which stimulated fibroblast proliferation and increased blood vessel density after subcutaneous implantation [29].
The current work focused on PDGF-BB for its involvement in angiogenesis. PDGF-BB was conjugated to PEG and immobilized in synthetic PEG-based scaffolds, which allowed precise control over incorporated biological molecules, as well as the mechanical properties. PEG–PDGF-BB was shown to maintain bioactivity in a cell proliferation assay. In addition, PEG–PDGF-BB and PEG–FGF-2 demonstrated usefulness in stimulating angiogenesis in engineered PEG-based hydrogels.
Angiogenesis requires the precise coordination of various signaling molecules to form stable and functional vessels. While combinations of growth factors have been used in releasable form, they have never been presented in an immobilized form. This work demonstrates the feasibility of using immobilized PDGF-BB with FGF-2, a previously established synergistic combination [15], to promote endothelial migration in 3D. HUVECs seeded onto modified surfaces exhibited significantly higher tubule formation on surfaces modified with PEG–RGDS and PEG–PDGF-BB and the combination of PEG–RGDS, PEG–PDGF-BB and PEG–FGF-2 compared with surfaces modified with PEG–RGDS alone. Here we have shown that growth factors can be used to assist endothelial cells in forming tubes, with robust tubule formation in 30 days.
Capillaries are naturally composed of two cell types, with stabilizing pericytes needed to promote endothelial cell survival [30] and form the basement membrane required for vessel stabilization and function [31]. Furthermore, vessels lacking pericytes exhibit undesirable features, such as leakage and impaired perfusion due to abnormal endothelial junctions, endothelial hyperplasia and hypervariable diameter [2]. In order to more rapidly form stable tubule networks, this work made use of a co-culture of HUVEC and 10T1/2 cells, which has previously been shown to form stable vessels in vivo [16]. Mouse pericyte precursor 10T1/2 cells are known to differentiate into pericytes in the presence of HUVECs via the TGF-β1 pathway [17]. The co-culture of HUVEC and 10T1/2 cells confirmed the role of stabilizing pericytes, as the presence of 10T1/2 cells was sufficient to induce tubule formation independent of surface modification as early as 2 days after cell seeding. While robust tubule formation was visible 6 days after cell seeding, regression of tubules was observed at later time points.
FGF-2 is a known antagonist of the TGF-β1-dependent differentiation of 10T1/2 cells into pericytes, and thereby is an important factor in the stabilization of new vessels. In this work the removal of FGF-2 from the culture medium resulted in increased tubule formation by HUVECs and 10T1/2 cells seeded on modified surfaces, suggesting that the critical pericyte differentiation had occurred. Confirmation of the presence of endothelial pericyte markers via immunohistochemistry further supported this reasoning. As a demonstration, tubule-like networks were visible on all surfaces, with smooth muscle α-actin-positive cells seen extending over several endothelial cells in a pericyte-like fashion. Furthermore, the presence of multiple large vacuoles within endothelial cells suggested lumen formation and capillary development [32] on these functionalized hydrogels.
Reduced tubule formation in the presence of FGF-2 in vitro may affect the design of the hydrogels for in vivo use. Koike et al. [16] have shown that a co-culture of HUVEC and 10T1/2 cells is capable of forming functional tubule networks in vivo, which is supported by the tubule formation seen in this in vitro work. However, to enhance tubule formation local FGF-2 may need to be reduced, possibly through the design of hydrogels with FGF-2 antibodies. As an alternative, hydrogels could be designed with only HUVECs with the addition of immobilized PDGF-BB, as the immobilized PDGF-BB induced HUVEC tubule formation.
It has been well established that growth factors, and in particular FGF-2 and PDGF-BB, effectively increase the migratory behavior of cultured endothelial cells. Soluble factors have been shown to induce a migratory phenotype, characterized by cytoskeletal rearrangement and pseudopodia formation, and to increase random movement in wound healing models of endothelial cells cultured in monolayer [33,34]. In the present study we observed a similar effect in degradable hydrogels containing immobilized PEG–FGF-2 and PEG–PDFG-BB. HUVECs encapsulated in these MMP-sensitive hydrogels exhibited a significant increase in random migration distance per hour compared with materials without the growth factors. In addition, the combination of PEG–FGF-2 and PEG–PDGF-BB resulted in a decreased time to gel collapse, likely due to FGF-2-induced PDGF receptor expression in vascular endothelial cells [35]. Synergism may result from PDGF-BB inducing a positive feedback signal to amplify FGF-2 expression in vascular mural cells, which in turn enhances mural PDGF β-receptor expression [35,36].
Growth factors stimulate a cellular response, including release of MMP-2 and MMP-9. The degradable hydrogels used in this work were comprised of crosslinked polymer chains that contained a protease-sensitive, PQ peptide, segment. As such, when MMPs were secreted near or within the hydrogel material the PQ peptide was degraded, causing the polymer backbone to fragment. As more peptides were cleaved polymer fragments were released into solution and the hydrogel lost its integrity. In the current study the increase in cell motility in the presence of growth factors appeared to have hastened the collapse of the collagenase-sensitive hydrogel, presumably due to the secretion of MMPs by migrating cells. This reasoning is supported by results from gelatin zymography, which confirmed the presence of MMP-2 in hydrogels containing encapsulated HUVECs. MMPs play an active role in angiogenesis, particularly MMP-2, by enabling migration by sprouting endothelial cells and by regulating growth factor release and activation [37]. In addition, MMP activity is often controlled by proteolytic cleavage, in which a propeptide is cleaved to generate an active form of the enzyme. Interestingly, only samples with covalently immobilized growth factors contained active MMP-2 (68 kDa), which complements the increase in cell movement and earlier hydrogel collapse in the same materials.
Controlled delivery of PDGF-BB is particularly useful for in vivo applications. Soluble PDGF-BB was necessary to stimulate the initial angiogenic response of the surrounding limbic vessels into the cornea. Since soluble PDGF-BB degrades very rapidly in vivo, the presence of PEG–PDGF-BB enables prolonged angiogenic signaling on the hydrogel, leading to an increase in vessel density and diameter. This manuscript presents the first work with a PEGy-lated growth factor in vivo.
5. Conclusion
Biomimetic hydrogels can be designed to incorporate cell-adhesive sequences and covalently immobilized growth factors to stimulate a desired cellular response in tissue engineered constructs. This work has demonstrated that PEG–PDGF-BB is bioactive and can be successfully incorporated into PEG-based hydrogels alone and in combination with PEG–FGF-2. HUVECs seeded onto hydrogel surfaces modified with covalently immobilized growth factors formed extensive and branching networks of tubules. Additionally, a co-culture of HUVECs and mouse pericyte precursor 10T1/2 cells seeded onto modified surfaces induced tubule formation independent of growth factor modification as early as 2 days after cell seeding. Endothelial cells encapsulated in MMP-sensitive hydrogels with covalently immobilized growth factors exhibited the key steps in angiogenesis, such as a significantly higher migration rate and MMP-2 activation. Finally, bioactive hydrogels containing both soluble PDGF-BB and immobilized PEG–PDGF-BB led to a significant increase in vessel density when assessed in the mouse cornea angiogenesis assay compared with hydrogels with soluble PDGF-BB alone. Further work is necessary to determine the optimal combinations of cell types as well as both bound and soluble growth factors to form stable and functional vascular networks. The results presented here demonstrate the potential of biomimetic, PEG-based hydrogels containing immobilized growth factors as a promising methodology to engineer a microvascular system within tissue engineered constructs for regenerative medical applications.
Supplementary Material
Acknowledgments
This work was supported by NIH grant P20 EB007076 and a National Science Foundation Graduate Research Fellowship.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.actbio.2010.08.018.
Appendix B. Figures with essential colour discrimination
Certain figures in this article, particularly Figs. 4 and 6, are difficult to interpret in black and white. The full colour images can be found in the on-line version, at doi:10.1016/j.actbio.2010.08.018.
References
- 1.Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J Biomater Sci Polym Ed. 2004;15(6):701–26. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 2.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;94:115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 3.Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96(2):1169–75. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009;23(1):153–63. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]
- 5.Bar RS, Boes M, Booth BA, Dake BL, Henley S, Hart MN. The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology. 1989;124(4):1841–8. doi: 10.1210/endo-124-4-1841. [DOI] [PubMed] [Google Scholar]
- 6.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79(4):1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med. 1996;2(9):1022–7. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 8.Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(Suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 9.Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22(5):315–29. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- 10.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54(4):459–76. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 11.Leslie-Barbick JE, Moon JJ, West JL. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed. 2009;20(12):1763–79. doi: 10.1163/156856208X386381. [DOI] [PubMed] [Google Scholar]
- 12.Hahn MS, McHale MK, Wang E, Schmedlen RH, West JL. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann Biomed Eng. 2007;35(2):190–200. doi: 10.1007/s10439-006-9099-3. [DOI] [PubMed] [Google Scholar]
- 13.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–34. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 14.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–4. [Google Scholar]
- 15.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9(5):604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 16.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428(6979):138–9. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 17.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141(3):805–14. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Sato M, Doi H, et al. Basic fibroblast growth factor antagonizes transforming growth factor-beta1-induced smooth muscle gene expression through extracellular signal-regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol. 2004;24(8):1384–90. doi: 10.1161/01.ATV.0000136548.17816.07. [DOI] [PubMed] [Google Scholar]
- 19.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12(1):9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 20.Moon JJ, Lee SH, West JL. Synthetic biomimetic hydrogels incorporated with ephrin-A1 for therapeutic angiogenesis. Biomacromolecules. 2007;8(1):42–9. doi: 10.1021/bm060452p. [DOI] [PubMed] [Google Scholar]
- 21.Poche RA, Larina IV, Scott ML, Saik JE, West JL, Dickinson ME. The Flk1-myr::mCherry mouse as a useful reporter to characterize multiple aspects of ocular blood vessel development and disease. Dev Dyn. 2009;238:2318–26. doi: 10.1002/dvdy.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poche RA, Saik JE, West JL, Dickinson ME. The mouse cornea as a transplantation site for live imaging of engineered tissue constructs. Cold Spring Harbor Protoc. 2010;4:pdb prot5416. doi: 10.1101/pdb.prot5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, Dickinson ME. A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anat Rec. 2009;292(3):333–41. doi: 10.1002/ar.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18(1):26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 25.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37(8):1625–32. [PubMed] [Google Scholar]
- 26.De-la-Riva B, Sanchez E, Hernandez A, Reyes R, Tamimi F, Lopez-Cabarcos E, et al. Local controlled release of VEGF and PDGF from a combined brushite–chitosan system enhances bone regeneration. J Control Release. 2009;143:45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Thomopoulos S, Das R, SakiyamaElbert S, Silva MJ, Charlton N, Gelberman RH. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2009;38:225–34. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aizawa Y, Leipzig N, Zahir T, Shoichet M. The effect of immobilized platelet derived growth factor AA on neural stem/progenitor cell differentiation on cell-adhesive hydrogels. Biomaterials. 2008;29(35):4676–83. doi: 10.1016/j.biomaterials.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, He Z, Chen B, Zhao Y, Sun W, Xiao Z, et al. Direct chemical cross-linking of platelet-derived growth factor-BB to the demineralized bone matrix improves cellularization and vascularization. Biomacromolecules. 2009;10(12):3193–8. doi: 10.1021/bm900850q. [DOI] [PubMed] [Google Scholar]
- 30.Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, et al. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15(7):1239–41. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- 31.DiazFlores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24(7):909–69. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 32.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442(7101):453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 33.Lee JG, Kay EP. FGF-2-induced wound healing in corneal endothelial cells requires Cdc42 activation and Rho inactivation through the phosphatidylinositol 3-kinase pathway. Invest Ophthalmol Vis Sci. 2006;47(4):1376–86. doi: 10.1167/iovs.05-1223. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Ouyang J, Wang N, Zhang Y, Bie J. Promotion of PDGF-induced endothelial cell migration by phosphorylated VASP depends on PKA anchoring via AKAP. Mol Cell Biochem. 2010;335(1/2):1–11. doi: 10.1007/s11010-009-0234-y. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y, Cao R, Hedlund EM. Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86(7):785–9. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 36.Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118(Pt. 16):3759–68. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- 37.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200(4):448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.