Abstract
Fluid shear stress regulates gene expression in osteoblasts, in part by activation of the transcription factor NF-κB. We examined whether this process was under control of purinoceptor activation. MC3T3-E1 osteoblasts under static conditions expressed the NF-κB inhibitory protein IκBα and exhibited cytosolic localization of NF-κB. Under fluid shear stress, IκBα levels decreased, and concomitant nuclear localization of NF-κB was observed. Cells exposed to fluid shear stress in ATP-depleted medium exhibited no significant reduction in IκBα, and NF-κB remained within the cytosol. Similar results were found using oxidized ATP or Brilliant Blue G, P2X7 receptor antagonists, indicating that the P2X7 receptor is responsible for fluid shear-stress-induced IκBα degradation and nuclear accumulation of NF-κB. Pharmacologic blockage of the P2Y6 receptor also prevented shear-induced IκBα degradation. These phenomena involved neither ERK1/2 signaling nor autocrine activation by P2X7-generated lysophosphatidic acid. Our results suggest that fluid shear stress regulates NF-κB activity through the P2Y6 and P2X7 receptor.
Keywords: Osteoblast, Mechanotransduction, Purinergic, NF-κB, P2Y6, P2X7, Lysophosphatidic acid, ERK1/2
INTRODUCTION
The mammalian skeleton demonstrates a tremendous capacity for functional adaptation to mechanical forces. For example, conditions of reduced skeletal loads promote bone resorption to minimize unnecessary energy expenditure [1], while increased skeletal loading promotes bone formation to minimize stress and/or strain [2]. Fluid shear stress (FSS) has been presented as a localized biophysical signal generated in response to dynamic loading [3-5], and in vitro data demonstrate that FSS has robust effects on osteoblast proliferation, differentiation, and survival (reviewed in [6]).
The influence of FSS on alterations in osteoblast metabolism involve activation of a variety of mechanosensory protein complexes, such as integrins and focal adhesions [7-9], primary cilia [10], gap junctions and/or gap junction hemichannels [11–13], and mechanosensitive ion channels [14–16]. We have demonstrated that much of the capacity for osteoblastic cells to respond to FSS requires release of ATP and subsequent activation of ATP-binding P2 purinoceptors [13, 17, 18]. P2 receptors are present in nearly all cell types, and induce cellular responses via G-protein activation (in the case of metabotropic P2Y) or ion flux (ionotropic P2X). Within osteoblastic cells, P2Y2 receptors have been implicated in the intracellular Ca2+i response of osteoblastic cells to FSS [17], and mice deficient in P2X7 demonstrate a markedly reduced anabolic response to mechanical loading [19] and impaired fracture healing [20].
We have demonstrated that prostaglandin E2 (PGE2) synthesis or release requires purinergic signaling [21], and that the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is required for maximal cyclooxygenase-2 (COX-2) induction in response to FSS [22]. Cytosolic sequestration of NF-κB is achieved by its binding to the inhibitory protein, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα), which masks the nuclear localization signal of NF-κB. Targeted degradation of IκBα by the 26S proteasome allows for nuclear accumulation and transcriptional activity of NF-κB. In this study, we determined whether NF-κB signaling in osteoblastic cells under FSS is under purinergic control and, if so, which purinoceptors are responsible. As the mechanism of P2X7 action in osteoblasts is partially mediated by the synthesis and autocrine function lysophosphatidic acid (LPA) [23], we also sought the role of LPA in FSS-induced NF-κB activation.
MATERIALS AND METHODS
Cell culture
MC3T3-E1 osteoblasts were cultured as described previously [24]. Because these cells exist as subclones with varying differentiation potential in vitro [25], experiments were performed upon three different subclones; similar results were obtained with each subclone. Cells were seeded at density of 2800 cells/cm2 upon 75x38mm glass slides coated with fibronectin (10μg/mL; Becton Dickson). Two days thereafter, when cells were 75–90% confluent, medium was removed and replaced with reduced serum medium consisting of αMEM, 1% penicillin/streptomycin, 0.5% FBS, and 10mM HEPES, pH 7.2. Fluid shear stress experiments were performed on the following day in the same medium.For static experiments, cells were cultured in 60mm dishes but were otherwise managed in an identical fashion.
Fluid shear stress and static experiments
FSS was applied to cells in a parallel plate flow chamber using a closed flow loop, as described previously [26]. Flow was applied for 45 minutes, after which slides were fixed for immunocytochemical analysis or lysed in 0.1% Triton X-100, 10mM Tris pH 8, 1mM EDTA, 0.2mM Na3VO4, supplemented with a protease inhibitor cocktail (Calbiochem) for Western immunoblotting. For static experiments, cells were collected in lysis buffer after 15 or 45 minutes of agonist treatment.
Pharmacologic agents
The P2X7 antagonists oxidized ATP (oATP, adenosine 5’-triphosphate-2’,3’-dialdehyde; Sigma) [27], which irreversibly inhibits P2X7 function through the formation of a Schiff base with P2X7 after prolonged incubation (>30 minutes), was dissolved in distilled water and added to cells at 300μM for three hours prior to FSS. Another P2X7 antagonist, Brilliant Blue G (Sigma) [28, 29], was added at 30μM for 45 minutes prior to FSS. The selective P2Y6 antagonist MRS2578 (Tocris) [30] was added at 1 or 10μM to cells for 45 minutes prior to FSS. The ATP-degrading enzyme, apyrase (grade III, Sigma), was dissolved in water to a working concentration of 10U/mL and added to cells 10 minutes prior to FSS. The LPA1 and LPA3 receptor antagonist Ki16425 (Cayman Chemicals) was dissolved in DMSO and added at a working concentration of 5μM (0.025% DMSO v/v) to cells 15 minutes prior to FSS. The MEK1/2 antagonist U0126 was dissolved in DMSO and added at a working concentration of 10μM (0.1% DMSO v/v) to cells 15 minutes prior to FSS. All inhibitors were also present within media used during FSS. In a related set of experiments, static cultures were treated with 20ng/mL TNF-α, 1μM LPA, , 100μM of ATP, UTP, or UDP, 300μM 2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate (BzATP) (all from Sigma), or 100μM MRS2578 (Tocris), or appropriate vehicle control for 15 or 45 minutes.
Western immunoblotting
Western immunoblotting was performed as described previously using 10μg of whole cell lysates [13]. Primary antibodies were used at the following ratios: IκBα (1:1000, Santa Cruz Biotechnologies), pERK1/2 (1:1000, Santa Cruz Biotechnologies), ERK1/2 (1:1000, Cell Signaling Technologies), pan-actin (1:3000, Sigma) or α-tubulin (1:2000, Cell Signaling).
Immunocytochemistry
The p65 subunit of NF-κB was imaged as described previously [22]. Briefly, cells were fixed with 2% paraformaldehyde, permeabilized with 0.2% Triton X-100, and treated with 3% donkey serum to reduce non-specific binding. A primary antibody against the p65 subunit of NF-kB was applied for 1 h, followed by incubation with rhodamine-conjugated secondary antibody. Images were recorded using a Nikon Optiphot II microscope through a 60x objective (1.4 numerical aperture).
Statistical analysis
Western blots were scanned into the open-source software GNU Image Manipulation Program, and mean pixel intensities for immunoreactive bands were determined using the Histogram tool. Band intensity for IκBα was normalized to band intensity for α-tubulin or pan-actin, in order to compensate for any inconsistencies in protein loading from lane to lane. Data are presented as fold change±SEM in immunoreactivity compared to static, no-flow samples. ANOVA or Student’s t-test were used where appropriate. P values less than 0.05 were considered statistically significant.
RESULTS
P2 receptor activation is required for IκBα degradation NF-κB translocation
In order to determine whether extracellular ATP and P2 receptors are implicated in NF-κB nuclear translocation in response to fluid shear stress, MC3T3-E1 cells were exposed to 12 dynes/cm2 of laminar flow for 45min in the absence and presence of the ATP disphosphohydrolase, apyrase (5 or 10U/mL). The expression of IκBα relative to pan-actin in cells exposed to fluid shear stress was significantly lower compared to static controls (Fig 1A, B). In contrast, cells exposed to fluid shear stress in the presence of apyrase demonstrated no significant reduction in IκBα levels compared to static controls (Fig 1A, B), indicating that purinergic P2 receptor activation is required for flow-mediated IκBα degradation. Reduced degradation of IκBα in the presence of apyrase correlated with attenuated nuclear translocation of NF-κB: in the absence of FSS, the p65 subunit of NF-κB demonstrated cytosolic localization (Fig 1C, top-left panel), whereas exposure to FSS induced strong nuclear localization of the p65 NF-kB subunit (Fig 1C, top-right panel). Cells treated with apyrase (10U/mL), in the absence (Fig 1C, bottom-left panel) or presence (Fig 1C, bottom-right panel) of FSS, demonstrated cytosolic NF-κB localization. These data indicate that purinergic receptor activation is required for FSS-induced IκBα degradation and subsequent nuclear translocation of NF-κB.
FIGURE 1. IκBα degradation and NF-κB activation requires extracellular nucleotides.


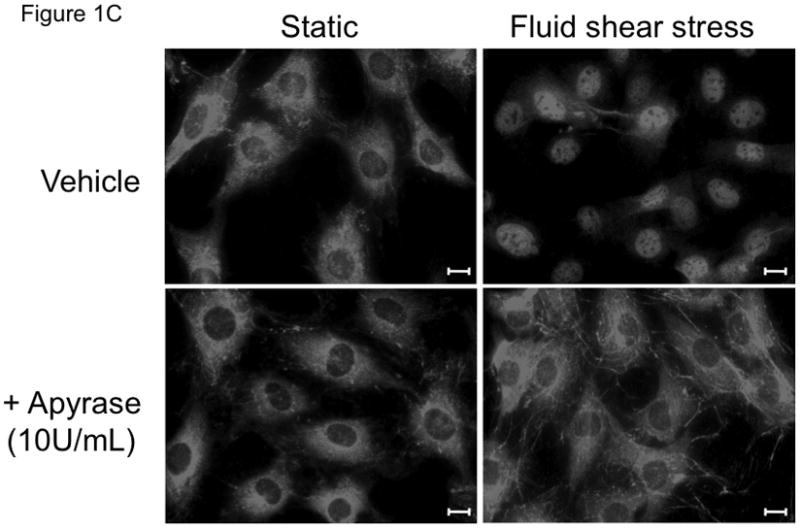
(A) Immunoblotting shows a decrease in IκBα expression in cells exposed to fluid shear stress, whereas the addition of apyrase (10U/mL) to flow media prevented FSS-induced IκBα degradation. (B) Quantitation of IκBα levels in osteoblasts, normalized to fold change compared to appropriate control static cells. Bars represent mean±SEM, n=3–4. a: indicates p<0.05 compared to static control; b: indicates p<0.05 compared to FSS vehicle. (C) Immunostaining of the p65 subunit of NF-κB demonstrates nuclear accumulation of p65 in response to FSS, whereas degradation of extracellular nucleotides with apyrase prevents FSS-induced p65 nuclear translocation. Representative immunocytochemical images are shown from 3 replicates. The white scale bar indicates 10μm.
P2X7R and P2Y6R are responsible for fluid shear stress-induced NF-κB translocation
We have previously shown that osteoblasts derived from that P2rx7−/− mice revealed diminished COX-2 induction in response to fluid shear stress compared to osteoblasts from wild-type mice [19]. We have also demonstrated that NF-κB translocation is required for maximal COX-2 induction in response to fluid shear stress [22]. These data, combined with the findings reported above, suggest that P2X7R may be a pivotal P2 receptor involved in flow-mediated NF-κB activation. To test this hypothesis, cells were treated with P2X7R antagonists oxidized ATP (oATP) or Brilliant Blue G (BBG) [28, 29, 31–33]. Similar to results using apyrase, both P2X7R antagonists oATP (300μM) and BBG (30μM) prevented flow-induced reductions in IκBα levels (Figure 2A and 2B).
FIGURE 2. The P2X7 and P2Y6 receptors mediates FSS-induced IκBα degradation.




(A) IκBα expression was monitored in MC3T3-E1 under static and FSS conditions in the absence or presence of the P2X7 antagonists oATP (300μM) or BBG (30μM). (B) Quantitation of IκBα levels in osteoblasts, normalized to fold change compared to appropriate control static cells. Bars represent mean±SEM, n= 3–4. a: indicates p<0.05 compared to static control; b: indicates p<0.05 compared to FSS vehicle. (C) Quantification of IκBα levels in MC3T3-E1 osteoblasts exposed to FSS in the absence or presence of the P2Y6 antagonist MRS2578 (10μM). (D) NF-κB p65 immunostaining in MC3T3-E1 osteoblasts maintained under static conditions and treated with 0, 10, or 1000μM ATP for 45 minutes reveals cytosolic localization of NF-κB. Representative immunocytochemical images are shown from 6 replicates.
In addition to the P2X7 receptor, the P2Y6 receptor has also been shown to mediate NF-κB activation in rabbit osteoclasts [34]. We have previously demonstrated that MC3T3-E1 osteoblasts express the P2Y6 receptor, the protein expression of which did not significantly change over the course of 21-day osteogenic differentiation [35]. Cells exposed to FSS in the presence of the P2Y6 antagonist MRS 2578 (10μM) also prevented shear-induced IκBα degradation (Figure 2C), indicating that this receptor also plays a role in FSS-induced activation of NF-κB.
We next examined whether addition of ATP or specific purinoceptor agonists to static osteoblasts was sufficient to induce nuclear translocation of NF-κB. Cells were treated for 15 minutes with 20ng/mL TNF-α (positive control for IκBα degradation), 100μM ATP (pan-purinoceptor agonist), 100μM UTP (P2Y agonist), 100μM UDP (P2Y6 agonist), 10μM MRS2693 (P2Y6 agonist), or 300μM BzATP (P2X7 agonist), after which protein lysates were probed for IκBα levels. Whereas significantly TNF-α decreased IκBα expression, no other agonist did (Figure 2D). Similar results were found after 45 minutes, although the decrease in IκBα in response to TNF-α was not as great as at 15 minutes (data not shown), most likely due to NF-κB-induced IκBα expression. Similarly, ATP at 10, 100, or 1000 μM revealed no influence upon nuclear localization of the p65 subunit of NF-κB after 0.5, 1, or 2 hours of treatment (data not shown). These data indicate that purinergic signaling is required, but not sufficient for nuclear translocation of NF-κB.
P2X7R-mediated activation of NF-κB occurs independently of LPA signaling
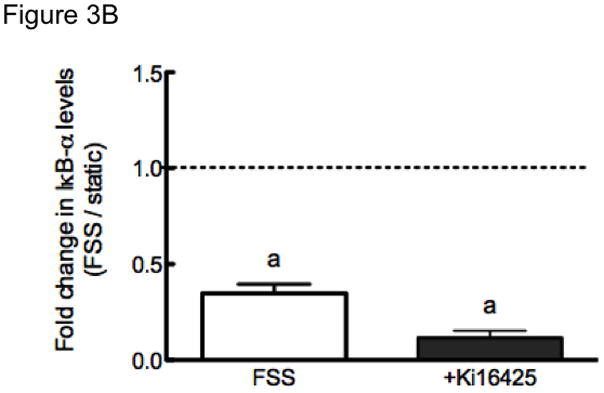
Activation of the P2X7 receptor in osteoblasts generates lysophosphatidic acid (LPA) [23], a potent hydrophilic growth factor that exerts mitogenic and chemotactic effects upon osteoblastic cells [26, 36, 37]. LPA also is linked to NF-κB activation in other cellular phenotypes including fibroblasts [38], endothelial cells [39] and cardiac myocytes [40]. LPA signaling increases Ca2+i within MC3T3-E1 osteoblastic cells through either receptor LPA1 or LPA3 [36]. Because we have previously shown that fluid shear stress-induced NF-κB translocation requires Ca2+i signaling, we hypothesized that LPA1 or LPA3 could be involved in fluid shear stress-induced IκBα degradation. Inhibition of LPA1 and LPA3 receptor with Ki16425 revealed no change in IκBα degradation under conditions of fluid shear stress (Figure 3A and 3B). We next examined ERK1/2 phosphorylation under static conditions to confirm the inhibitory effect of these compounds upon LPA receptor activation (Figure 3C). Consistent with its ability to inhibit LPA-induced Ca2+i transients, 5μM Ki16425 fully inhibited LPA-induced ERK1/2 phosphorylation. These data indicate that signaling via LPA1 or LPA3 is not involved in FSS-induced IκBα degradation and NF-κB signaling.
FIGURE 3. IκBα degradation in response to FSS does not require LPA signaling.


(A) IκBα expression was monitored in MC3T3-E1 under static and FSS conditions in the absence or presence of the LPA1 and LPA3 receptor antagonist Ki16425 (5μM). B) Quantitation of IκBα levels in osteoblasts, normalized to fold change compared to appropriate control static cells. Bars represent mean±SEM, n= 3–4. a: indicates p<0.05 compared to static control. (C) Static MC3T3-E1 cells reveal 1 μM LPA-induced ERK1/2 phosphorylation, and this was inhibited only in cells treated with Ki16425. Representative Western blots are shown from 2 replicates.
MEK1/2 is not involved in fluid shear-induced IκBα degradation
Activation of the P2X7 and an undefined P2Y receptor mediate fluid shear stress-induced ERK1/2 phosphorylation in osteoblasts [41], and ERK1/2 has been implicated in NF-κB activation in macrophage [42], endothelial [43], and epidermal cells [44]. Therefore, we next examined the role of ERK1/2 in IκBα degradation using an antagonist of MEK1/2, a kinase immediately upstream of ERK1/2 [45]. Similar to vehicle controls, cells exposed to FSS in the presence of the MEK1/2 antagonist U0126 (10μM) demonstrated significant reductions in IκBα (Figure 4A and 4B), indicating that fluid shear stress-induced IκBα degradation does not require ERK1/2.
FIGURE 4. MEK signaling is not required for FSS-induced IκBα degradation.
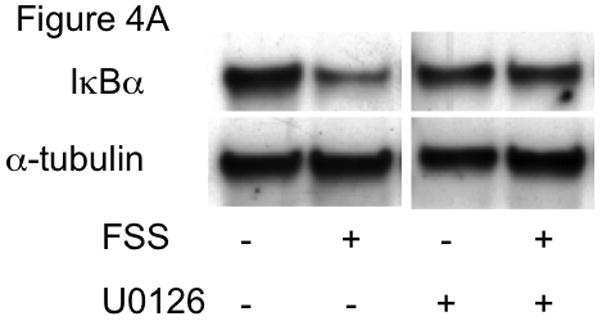

(A) IκBα expression was monitored in MC3T3-E1 under static and FSS conditions in the absence or presence of the MEK1/2 antagonist U0126 (10μM). B) Quantitation of IκBα levels in osteoblasts, normalized to fold change compared to appropriate control static cells. Bars represent mean±SEM, n=6. a: indicates p<0.05 compared to static control. C)
DISCUSSION
While the benefit of mechanical loading upon maintenance of skeletal architecture and bone mineral content is well accepted, the cellular mechanisms whereby application of exogenous loads translates into appropriate skeletal homeostasis or adaptation remain under vigorous study. Multiple cell types release ATP in response to mechanical load or mechanical deformation, underscoring the potential for purinergic receptor signaling to mediate a plethora of cellular responses [46]. In osteoblastic cells, purinergic signaling enhances the ability of parathyroid hormone to stimulate gene expression [47], and is required for PGE2 release [18] and ERK1/2 phosphorylation [41] in response to FSS. In vivo, P2rx7 deletion markedly reduces the relative bone formation rate in response to axial loading of the mouse ulna [19]. These results are similar to data generated from the low-density lipoprotein-related receptor 5 (Lrp5) knockout mouse, although whether there is interaction between the purinergic and Wnt signaling pathways remains to be demonstrated.
Cells within bone, including mesenchymal stem cells [48, 49], osteoblasts [50–54], and osteoclasts [53–57] express purinergic receptors. MC3T3-E1 cells have been shown to express P2X2, P2X4, P2X6 and P2X7 (reviewed in [58]), and P2Y1, P2Y2, P2Y4, P2Y6, and P2Y14 [59]. There are no data to date that reveal which P2 receptor isoforms are expressed by osteocytes or osteocyte-like cells, but we have previously demonstrated that ATP induces PGE2 release in the MLO-Y4 cell line [13], indicating that these cells express functional P2 receptors. Similarly, the P2X7 agonist BzATP has been shown to increase PGE2 release from MLO-Y4 osteocytes [19], indicating that these cells express P2X7 receptor.
Within this work, we demonstrate a requirement for purinergic signaling for FSS-induced IκBα degradation and nuclear localization of NF-κB. This was not dependent upon a single class of P2 receptors, as inhibition of either the ionotropic P2X7 receptor or the metabotropic P2Y6 receptor prevented shear-induced decreases in IκBα. The influence of the P2Y6 receptor upon IκBα levels is likely to be mediated through Gq/phospholipase C-mediated intracellular calcium release, as we previously demonstrated the requirement of such in FSS-induced NF-κB activation [22]. How the P2X7 receptor is involved is less clear. It is unlikely to involve calcium entry from the pericellular space, as we showed that FSS is able to decrease IκBα levels in the presence or absence of extracellular calcium. P2X7 activation is linked to ERK1/2 phosphorylation [41], although ERK1/2 is not required for FSS-induced IκBα degradation (Figure 4A and 4B). Another possible mechanism is by P2X7 activation of Akt, as has been shown in to occur in astrocytes [60]. Alternately, P2X7 activation causes formation of arachidonic acid, which is metabolized by COX enzymes to form prostaglandins. Indeed, the increase in markers of osteogenesis in response to BzATP was abrogated when cells were co-cultured with the general COX antagonist indomethacin [23]. Thus, it is possible that FSS-induced IκBα degradation is mediated directly by prostaglandins, which are themselves synthesized due to activation of the P2X7 receptor.
P2X7 receptor activation leads to the formation of LPA via phospholipase D [23]. LPA has diverse effects upon osteoblast and osteocyte activity, and induces chemotaxis [36, 37], dendrite outgrowth [61], and differentiation [62]. Panupinthu et al. observed that the stimulatory effect of P2X7 upon the osteogenic differentiation of calvarial cells is indeed mediated by LPA working in an autocrine fashion upon LPA1 or LPA3 receptors. Having demonstrated the role of P2X7 in FSS-induced NF-κB activation, we determined whether this involved LPA function. Osteoblasts exposed to FSS in the presence of the LPA1/LPA3 receptor antagonist Ki16425 revealed a similar magnitude of IκBα degradation compared to vehicle-treated cells (Figure 3A and 3B), suggesting that LPA autocrine activity does not mediate NF-kB activation in osteoblastic cells.
Whereas antagonists of P2X7 and P2Y6 prevented FSS-induced IκBα degradation, it is interesting to note that addition of ATP (general P2 agonist), UTP (P2Y agonist), UDP or MRS2693 (P2Y6 agonists), or BzATP (P2X7 agonist) did not promote IκBα degradation in static cells. This suggests that activation of P2X7 or P2Y6 is required, but not sufficient, to induce NF-κB translocation, and that another signaling cascade that is induced by FSS is also involved. A possible candidate for this additional signaling cascade is the integrin/focal adhesion kinase (FAK) pathway. Integrins have been identified as mechanosensory molecules in osteoblasts and osteocytes [7, 63–65]. Similarly, FAK is required for FSS-induced COX-2 and osteopontin expression [66]. Deletion of FAK prevented FSS-induced NF-κB activation in osteoblasts [67], suggesting that purinergic signaling may interplay with integrin/FAK in regulation of NF-κB translocation in osteoblasts.
Figure 5 represents an integrative model for the work described within and our previous efforts. Fluid shear stress promotes the vesicular release of ATP from pre-osteoblastic cells, where it subsequently functions in an autocrine fashion to modulate prostaglandin synthesis and release [18]. We have further demonstrated that both P2X7 and an undefined P2Y (although not P2Y2) receptor are required for maximal ERK1/2 phosphorylation in response to FSS [41]. Data presented in Figure 1 demonstrate that purinoceptor activation is required for IκBα degradation and NF-kB nuclear localization, while use of the P2X7 antagonists oATP and BBG confirm that this is an obligate receptor for FSS-induced NF-κB activation (Figure 2) in a process that does not ERK1/2 (Figure 4). Although P2X7 receptor activation promotes synthesis of the bioactive lipid LPA [23], use of an LPA receptor antagonist did not modulate the suppressive influence of FSS upon IκBα degradation (Figure 3).
FIGURE 5. Mechanistic model for P2X7 and P2Y6 signaling under FSS in osteoblastic cells.

FSS promotes the vesicular release of ATP, where it binds to metabotropic P2Y or ionotropic P2X receptors; activation of P2X7 causes ERK1/2 phosphorylation, although this is not involved in IκBα degradation, and nuclear localization of NF-κB. Activation of P2X7 promotes LPA formation through PLA2 or PLD, but subsequent activation of LPA1 or LPA3 does not promote IκBα degradation. Activation of P2Y6 promotes the release of intracellular calcium from the endoplasmic reticulum. Purinoceptor activation alone is not sufficient for NF-κB translocation, suggesting that another FSS-activated signaling pathway (indicated by ?) is involved in this process.
CONCLUSION
Our data show that FSS induces NF-κB degradation in osteoblastic cells by a mechanism involving P2X7 and P2Y6 receptors but not ERK1/2 or autocrine LPA signaling in osteoblastic cells. These results expand the framework for purinergic regulation of mechanotransduction in osteoblasts, yet also suggest that another signaling pathway activated by sher stress is necessary to interact with P2 receptors to elicit cellular responses.
Acknowledgments
NIH NIAMS AR051901 (RLD), NIA AG13087 (HJD), and NIAMS AR057547 (DCG) supported the work described within.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uhthoff HK, Jaworski ZFG. Bone loss in response to long-term immobilization. J Bone and Joint Surg- British. 1978;60B:420–429. doi: 10.1302/0301-620X.60B3.681422. [DOI] [PubMed] [Google Scholar]
- 2.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone and Joint Surg- American. 1984;66A:397–402. [PubMed] [Google Scholar]
- 3.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 4.Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Annals of biomedical engineering. 2002;30:693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- 5.Kwon RY, Meays DR, Tang WJ, Frangos JA. Microfluidic enhancement of intramedullary pressure increases intersitital fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Miner Res. 2010 doi: 10.1002/jbmr.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeletal-integrin interactions. Am J Physiol. 1998;275:C1591–1601. [PubMed] [Google Scholar]
- 8.Kim JB, Leucht P, Luppen CA, Park YJ, Beggs HE, Damsky CH, Helms JA. Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone. 2007;41:39–51. doi: 10.1016/j.bone.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leucht P, Kim JB, Currey JA, Brunski J, Helms JA. FAK-Mediated mechanotransduction in skeletal regeneration. PLoS ONE. 2007;2:e390. doi: 10.1371/journal.pone.0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142:3464–3473. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 12.Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 13.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan RL, Misler S. Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR-106) FEBS Lett. 1989;251:17–21. doi: 10.1016/0014-5793(89)81420-6. [DOI] [PubMed] [Google Scholar]
- 15.Duncan RL, Hruska KA. Chronic, intermittent loading alters mechanosensitive channel characteristics in osteoblast-like cells. Am J Physiol. 1994;267:F909–916. doi: 10.1152/ajprenal.1994.267.6.F909. [DOI] [PubMed] [Google Scholar]
- 16.Ryder KD, Duncan RL. Parathyroid hormone enhances fluid shear-induced [Ca2+]i signaling in osteoblastic cells through activation of mechanosensitive and voltage- sensitive Ca2+ channels. J Bone Miner Res. 2001;16:240–248. doi: 10.1359/jbmr.2001.16.2.240. [DOI] [PubMed] [Google Scholar]
- 17.You J, Jacobs CR, Steinberg TH, Donahue HJ. P2Y purinoceptors are responsible for oscillatory fluid flow-induced intracellular calcium mobilization in osteoblastic cells. J Biol Chem. 2002;277:48724–48729. doi: 10.1074/jbc.M209245200. [DOI] [PubMed] [Google Scholar]
- 18.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Meyer R, Duncan RL, Turner CH. P2X7 nucleotide receptor plays an important role in callus remodeling during fracture repair. Calcif Tissue Int. 2009;84:405–412. doi: 10.1007/s00223-009-9237-7. [DOI] [PubMed] [Google Scholar]
- 21.Genetos DC, Donahue HJ. Intercellular communication and mechanotransduction in bone. Curr Opin Orthop. 2005;16:311–315. [Google Scholar]
- 22.Chen NX, Geist DJ, Genetos DC, Pavalko FM, Duncan RL. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33:399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 23.Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, Dixon SJ. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181:859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CM, Genetos DC, You Z, Yellowley CE. Hypoxia regulates PGE(2) release and EP1 receptor expression in osteoblastic cells. J Cell Physiol. 2007;212:182–188. doi: 10.1002/jcp.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 26.Waters KM, Tan R, Genetos DC, Verma S, Yellowley CE, Karin NJ. DNA microarray analysis reveals a role for lysophosphatidic acid in the regulation of anti-inflammatory genes in MC3T3-E1 cells. Bone. 2007 doi: 10.1016/j.bone.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- 28.Hibell AD, Thompson KM, Xing M, Humphrey PP, Michel AD. Complexities of measuring antagonist potency at P2X(7) receptor orthologs. J Pharmacol Exp Ther. 2001;296:947–957. [PubMed] [Google Scholar]
- 29.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- 30.Lambrecht G, Braun K, Damer M, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P. Structure-activity relationships of suramin and pyridoxal-5'-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des. 2002;8:2371–2399. doi: 10.2174/1381612023392973. [DOI] [PubMed] [Google Scholar]
- 31.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 32.Vassort G. Adenosine 5'-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 33.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 34.Korcok J, Raimundo LN, Du X, Sims SM, Dixon SJ. P2Y6 nucleotide receptors activate NF-kappaB and increase survival of osteoclasts. J Biol Chem. 2005;280:16909–16915. doi: 10.1074/jbc.M410764200. [DOI] [PubMed] [Google Scholar]
- 35.Strohbach CA, Genetos DC, Taylor AF, Donahue HJ. Differentiation Affects MC3T3-E1 Mechanoresponsiveness and P2Y2 Expression. J Bone Miner Res. 2004;19:SU217. [Google Scholar]
- 36.Masiello LM, Fotos JS, Galileo DS, Karin NJ. Lysophosphatidic acid induces chemotaxis in MC3T3-E1 osteoblastic cells. Bone. 2006;39:72–82. doi: 10.1016/j.bone.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Karagiosis SA, Chrisler WB, Bollinger N, Karin NJ. Lysophosphatidic acid-induced ERK activation and chemotaxis in MC3T3-E1 preosteoblasts are independent of EGF receptor transactivation. J Cell Physiol. 2009;219:716–723. doi: 10.1002/jcp.21720. [DOI] [PubMed] [Google Scholar]
- 38.Shahrestanifar M, Fan X, Manning DR. Lysophosphatidic acid activates NF-kappaB in fibroblasts. A requirement for multiple inputs. J Biol Chem. 1999;274:3828–3833. doi: 10.1074/jbc.274.6.3828. [DOI] [PubMed] [Google Scholar]
- 39.Palmetshofer A, Robson SC, Nehls V. Lysophosphatidic acid activates nuclear factor kappa B and induces proinflammatory gene expression in endothelial cells. Thromb Haemost. 1999;82:1532–1537. [PubMed] [Google Scholar]
- 40.Chen J, Chen Y, Zhu W, Han Y, Han B, Xu R, Deng L, Cai Y, Cong X, Yang Y, Hu S, Chen X. Specific LPA receptor subtype mediation of LPA-induced hypertrophy of cardiac myocytes and involvement of Akt and NFkappaB signal pathways. J Cell Biochem. 2008;103:1718–1731. doi: 10.1002/jcb.21564. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42:644–652. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen BC, Lin WW. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petzold T, Orr AW, Hahn C, Jhaveri KA, Parsons JT, Schwartz MA. Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. Am J Physiol Cell Physiol. 2009;297:C814–822. doi: 10.1152/ajpcell.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu J, Haseebuddin M, Young M, Colburn NH. Suppression of p65 phosphorylation coincides with inhibition of IkappaBalpha polyubiquitination and degradation. Mol Carcinog. 2005;44:274–284. doi: 10.1002/mc.20142. [DOI] [PubMed] [Google Scholar]
- 45.DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- 46.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 47.Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA. Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001;28:507–512. doi: 10.1016/s8756-3282(01)00430-6. [DOI] [PubMed] [Google Scholar]
- 48.Riddle RC, Taylor AF, Rogers JR, Donahue HJ. ATP release mediates fluid flow-induced proliferation of human bone marrow stromal cells. J Bone Miner Res. 2007;22:589–600. doi: 10.1359/jbmr.070113. [DOI] [PubMed] [Google Scholar]
- 49.Coppi E, Pugliese AM, Urbani S, Melani A, Cerbai E, Mazzanti B, Bosi A, Saccardi R, Pedata F. ATP modulates cell proliferation and elicits two different electrophysiological responses in human mesenchymal stem cells. Stem Cells. 2007;25:1840–1849. doi: 10.1634/stemcells.2006-0669. [DOI] [PubMed] [Google Scholar]
- 50.Kumagai H, Sacktor B, Filburn CR. Purinergic regulation of cytosolic calcium and phosphoinositide metabolism in rat osteoblast-like osteosarcoma cells. J Bone Miner Res. 1991;6:697–708. doi: 10.1002/jbmr.5650060707. [DOI] [PubMed] [Google Scholar]
- 51.Reimer WJ, Dixon SJ. Extracellular nucleotides elevate [Ca2+]i in rat osteoblastic cells by interaction with two receptor subtypes. Am J Physiol. 1992;263:C1040–1048. doi: 10.1152/ajpcell.1992.263.5.C1040. [DOI] [PubMed] [Google Scholar]
- 52.Schofl C, Cuthbertson KS, Walsh CA, Mayne C, Cobbold P, von zur Muhlen A, Hesch RD, Gallagher JA. Evidence for P2-purinoceptors on human osteoblast-like cells. J Bone Miner Res. 1992;7:485–491. doi: 10.1002/jbmr.5650070504. [DOI] [PubMed] [Google Scholar]
- 53.Bowler WB, Birch MA, Gallagher JA, Bilbe G. Identification and cloning of human P2U purinoceptor present in osteoclastoma, bone, and osteoblasts. J Bone Miner Res. 1995;10:1137–1145. doi: 10.1002/jbmr.5650100720. [DOI] [PubMed] [Google Scholar]
- 54.Luo LC, Yu H, Ferrier J. Differential purinergic receptor signalling in osteoclasts and osteoblastic cells. Cell Signal. 1997;9:603–607. doi: 10.1016/s0898-6568(97)00052-1. [DOI] [PubMed] [Google Scholar]
- 55.Yu H, Ferrier J. ATP induces an intracellular calcium pulse in osteoclasts. Biochem Biophys Res Commun. 1993;191:357–363. doi: 10.1006/bbrc.1993.1225. [DOI] [PubMed] [Google Scholar]
- 56.Yu H, Ferrier J. Mechanisms of ATP-induced Ca2+ signaling in osteoclasts. Cell Signal. 1994;6:905–914. doi: 10.1016/0898-6568(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 57.Arnett TR, King BF. ATP as an osteoclast regulator? J Physiol. 1997;503(Pt 2):236. doi: 10.1111/j.1469-7793.1997.236bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10:322–330. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Qi J, Chi L, Faber J, Koller B, Banes AJ. ATP reduces gel compaction in osteoblast-populated collagen gels. J Appl Physiol. 2007;102:1152–1160. doi: 10.1152/japplphysiol.00535.2006. [DOI] [PubMed] [Google Scholar]
- 60.Jacques-Silva MC, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, Kang Y, Gonzalez FA, Weisman GA, Neary JT. P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol. 2004;141:1106–1117. doi: 10.1038/sj.bjp.0705685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karagiosis SA, Karin NJ. Lysophosphatidic acid induces osteocyte dendrite outgrowth. Biochem Biophys Res Commun. 2007;357:194–199. doi: 10.1016/j.bbrc.2007.03.121. [DOI] [PubMed] [Google Scholar]
- 62.Liu YB, Kharode Y, Bodine PV, Yaworsky PJ, Robinson JA, Billiard J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem. 2010;109:794–800. doi: 10.1002/jcb.22471. [DOI] [PubMed] [Google Scholar]
- 63.Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- 64.Ponik SM, Pavalko FM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J Appl Physiol. 2004;97:135–142. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- 65.Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107:13648–13653. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young SR, Gerard-O'Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24:411–424. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young SR, Gerard-O'Riley R, Harrington M, Pavalko FM. Activation of NF-kappaB by fluid shear stress, but not TNF-alpha, requires focal adhesion kinase in osteoblasts. Bone. 2010;47:74–82. doi: 10.1016/j.bone.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


